Abstract
Susceptibility to bacterial pneumonia in cattle is enhanced by stressors such as transportation, weaning, and commingling, which trigger a physiologic stress response resulting in elevated levels of endogenous corticosteroids and catecholamines. To determine the effect of neuroendocrine mediators on the expression of innate defense peptides in the lung, bovine tracheal epithelial cells were exposed to dexamethasone, catecholamines, acetylcholine, or substance P, and then β-defensin expression was quantified using real-time reverse transcription-PCR. Basal expression of tracheal antimicrobial peptide (TAP) mRNA was not affected by any of the mediators tested. However, induction of TAP expression by lipopolysaccharide was significantly inhibited by pretreatment with dexamethasone. Bronchial biopsy specimens from dexamethasone-treated calves had significantly lower expression of TAP and lingual antimicrobial peptide (LAP) mRNA than saline-treated controls following 48 h of treatment. Lipopolysaccharide-elicited neutrophil recruitment was enhanced in the lungs of dexamethasone-treated calves compared to saline-treated controls. These findings indicate that modulation of epithelial antimicrobial peptide expression is one mechanism through which corticosteroids and stress may impair innate pulmonary defenses.
The respiratory epithelium and its secretions create a necessary physical and chemical barrier to inhaled opportunistic pathogens. The airway surface fluid, produced by submucosal glands and lining epithelial cells, contains multiple factors which are directly bactericidal or prevent microbial adhesion and proliferation during clearance by the mucociliary apparatus (3, 14). Defensins, an important component of this epithelial host defense system, are a family of low-molecular-weight (3 to 6 kDa) cationic peptides with broad-spectrum antimicrobial activity (42). Based on the distribution of their six conserved cysteine residues and the resulting intramolecular disulfide bonds, vertebrate defensins have been grouped into α-, β-, and θ-defensin subfamilies, with β-defensins expressed at epithelial barriers such as the respiratory, gastrointestinal, and genitourinary tracts. While some defensins are constitutively expressed, the prototypic epithelial β-defensin, tracheal antimicrobial peptide (TAP), is induced by proinflammatory stimuli such as tumor necrosis factor alpha, interleukin-1β (IL-1β), lipopolysaccharide (LPS), lipoteichoic acid, muramyl dipeptide, and heat-killed bacteria (13, 15). Expression of TAP and a related bovine β-defensin, lingual antimicrobial peptide (LAP), are induced by infection with Mannheimia haemolytica (9, 47). The LPS-induced upregulation of TAP is mediated by CD14 through an NF-κB-dependent pathway (13), presumably associated with TLR-4 (Toll-like receptor 4) activation analogous to that demonstrated for induction of hBD-2 (human β-defensin 2) expression by lipoproteins via TLR-2 (21).
Bacterial pneumonia, caused by opportunistic pathogens such as Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, is the leading cause of morbidity and mortality in feedlot beef cattle (30). A myriad of factors predispose cattle to this disease, including transportation, weaning, overcrowding, disrupted social structures, and other stressors in addition to precipitation, fluctuating environmental temperatures, and viral infection with bovine viral diarrhea virus, infectious bovine rhinotracheitis virus, bovine respiratory syncytial virus, and parainfluenza 3 virus (20). These factors are thought to compromise respiratory defenses, thus allowing opportunistic pathogens to proliferate, invade, and colonize the lung and incite the inflammatory reaction responsible for the clinical signs of the disease (54). The interactions between these multiple host, agent, and environmental factors are complex, and the importance of the innate pulmonary defense system and how stressors alter its function are the subject of current investigation.
In response to social disruption, adverse environmental conditions, or a multitude of other stressors, animals initiate a cascade of physiologic processes aimed at nullifying danger through confrontation or evasion. A growing body of evidence is clarifying complex interactions between the central nervous system, which qualifies and quantifies stress, and the immune system, which defends against infection and facilitates tissue repair (46, 49, 52). Optimally timed activation of specific components of the immunoinflammatory response is critical to defend against impending infection, as deficiencies or excesses of the inflammatory response lead to morbidity. The mammalian stress response is classically orchestrated by two systems: the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system, acting via corticosteroids and catecholamines, respectively. Stress has been shown to affect multiple components of the immune system, with responses dependent on the type and duration of stimulus (52).
Due to the importance of the innate immune system as a first line of defense against infectious disease and the well-recognized role of stress in predisposing cattle to shipping fever pneumonia, we sought to determine the effects of several stress-associated neuroendocrine mediators on expression of antimicrobial peptide mRNA in tracheal epithelial cells. Since corticosteroids inhibit NF-κB signaling (2, 41) and susceptibility to respiratory disease is enhanced during periods of high endogenous corticosteroids (37), reduced expression of epithelial defensins may be one mechanism through which stress predisposes cattle to bacterial pneumonia. Furthermore, other neuroendocrine mediators may also influence antimicrobial peptide expression; for example, cholinergic (51) and adrenergic (18) agonists have been shown to prevent activation of the NF-κB pathway in a number of cell populations, substance P is an important proinflammatory mediator in the airways (5, 31), and the catecholamines epinephrine and norepinephrine have pleiotropic effects on airway epithelial cells (39). Here we examine the effect of dexamethasone on TAP and LAP expression in vitro and in vivo and investigate the modulation of β-defensin expression by other neuroendocrine mediators in cultured tracheal epithelial cells.
MATERIALS AND METHODS
General reagents.
All reagents for cell isolation, media, and supplements were purchased from Invitrogen Canada unless otherwise stated. Primers for real-time reverse transcription (RT)-PCR and LPS from Pseudomonas aeruginosa serotype 10 (no. L8643) were obtained from Sigma Chemical Co., St. Louis, MO.
Tracheal epithelial cell culture.
Two culture methods were employed to produce either a monolayer of freshly isolated tracheal epithelial cells adherent to a collagen matrix or a pseudostratified epithelium of mature differentiated tracheal epithelial cells grown at an air-liquid interface. Common to both methods, fresh bovine tracheas were obtained from a slaughterhouse and placed in ice-cold phosphate-buffered saline (PBS) containing 0.1 mg/ml penicillin-streptomycin, 0.5 mg/ml gentamicin, and 10 μg/ml amphotericin B. The mucosa was carefully dissected from the cartilage and washed five times in PBS before being placed in PBS with 2% protease (Dispase; Invitrogen), penicillin-streptomycin, gentamicin, and amphotericin B overnight at 4°C. Fetal bovine serum (2% final volume) was added to stop protease activity, and epithelial cells were mechanically agitated and dispersed in PBS. Cells were pelleted at 500 × g for 5 min and resuspended in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F-12 containing 5% fetal bovine serum, 0.1 mg/ml penicillin-streptomycin, 5 μg/ml amphotericin B, 25 μg/ml bovine pituitary extract, 25 ng/ml epidermal growth factor, and 1% insulin-transferrin-selenium. Cell viability was greater than 95% based on Trypan blue exclusion.
To obtain cells in a monolayer, 106 cells/ml were seeded onto 24-well plates coated overnight with collagen type I (Sigma, St. Louis, MO) and incubated at 37°C in 5% CO2 for 24 h before being washed with PBS and resubmerged in fresh culture medium. The medium was changed daily, and monolayers were used in experiments between 4 to 6 days of culture, at 85 to 95% confluence. Immunohistochemistry performed by the Animal Health Laboratory, University of Guelph, Canada, revealed cells were 98% positive for pancytokeratin (an epithelial cell specific marker, AE1/AE3, no. M3515; Dako, ON, Canada) and 99% negative for vimentin (a smooth muscle cell marker, no. M0725; Dako) and maintained their epithelial morphology.
To obtain a pseudostratified epithelium cultured at an air-liquid interface, methods previously described (35, 53) were followed with minimal modification. Briefly, freshly isolated tracheal epithelial cells were seeded onto collagen type I-coated semipermeable membranes (0.6 cm2, Millicell-PCF; Millipore, Bedford, MA) at a density of 5 × 105 cells/ml in culture medium. After 4 to 6 days in culture, the apical culture medium was removed until cell layers were confluent, as evidenced by their ability to block fluid movement toward the apical surface. Culture medium, now added only to the basolateral surface, was then changed to Dulbecco's modified Eagle's medium-Ham's F-12 containing 2% Ultroser G (Biosepra, Clergy-Sainte-Christophe, France), 0.1 mg/ml penicillin-streptomycin, and 2.5 μg/ml amphotericin B, and the medium was changed every 2 to 3 days. Experiments and histological analyses were performed between 14 and 21 days in culture. Cells were treated by adding dexamethasone to the basolateral media for 24 h and then adding LPS to the apical surface for 16 h. For each experimental condition, replicates were performed in triplicate from primary cultures obtained from at least three different animals. The upregulation of TAP expression induced by LPS varied between different animals from 3- to 20-fold.
In vivo study.
Eight, 8-month-old male castrated Holstein calves (weight range, 226 to 279 kg) were housed as a large group indoors in a large straw-bedded pen at a University of Guelph research station. Four days prior to treatment, all calves received 40 mg/kg of body weight of subcutaneous florfenicol (Nuflor; Schering-Plough Animal Health, Point-Claire, Quebec, Canada). Calves were randomly assigned to two groups of four, with group 1 serving as saline-treated controls and group 2 receiving 0.1 mg/kg of body weight of dexamethasone 21 disodium phosphate (UNI-DEX; UNIVET Pharmaceuticals, ON, Canada) by intramuscular injection every 24 h at 0900 h beginning on day 0. Prior to bronchoscopy, calves were physically restrained in a chute and sedated by intravenous delivery of 2 mg/kg sodium pentobarbital (Somnotol; MTC Pharmaceuticals, ON, Canada) and 0.08 mg/kg butorphanol (Torbugesic; Wyeth Animal Health, ON, Canada). The bronchoscope was directed to sample a different region of lung each day. On days 0, 2, 6, and 7 (i.e., before and 48, 144, and 168 h after the initial administration of saline or dexamethasone), the right caudal, right middle, left cranial, and left caudal lobes, respectively, were lavaged with PBS and biopsied. Following lavage and biopsy on day 6, 500 μg of LPS in 10 ml of PBS was instilled into the left caudal lobes of all calves. Biopsy specimens of the mucosa of second- to third-generation bronchi were harvested using disposable biopsy forceps with a fenestrated oval cup (United States Endoscopy Group Inc., Mentor, OH). Histological analysis of typical tracheal biopsy specimens revealed that the majority of tissue retrieved was ciliated pseudostratified columnar epithelium, with occasional small amounts of submucosal connective tissue. One or two bronchial mucosal biopsy specimens were flash frozen in liquid nitrogen and stored at −80°C for up to 1 month prior to total RNA isolation. The Animal Health Laboratory, University of Guelph, Canada, performed bacterial cultures of the bronchoalveolar lavage fluid (BALF) and serum cortisol measurements. Total and differential cell counts of BALF and differential cell counts of blood smears were manually performed on preparations stained with Wright's Giemsa.
RNA isolation and synthesis of cDNA.
Total RNA was isolated from cultured cells or tracheal biopsy specimens using the GenElute mammalian total RNA kit (Sigma) according to the manufacturer's instructions. Trace genomic DNA was removed by treatment with DNase I (QIAGEN) and RNA eluted in RNase-free water. RNA quality and quantity was assessed by capillary electrophoresis (BioAnalyser 2100; Agilent Technologies) for biopsy RNA and by capillary electrophoresis or spectrophotometry (absorbance at 240 nm) for RNA from cultured cells. Five hundred nanograms of total RNA was reverse transcribed using SuperScript II RNase H− reverse transcriptase (Invitrogen) per the manufacturer's protocol, using anchor dT as a primer. Following heat inactivation, cDNA products were diluted 1:10 in molecular grade water and stored at −20°C or used immediately in real-time RT-PCRs.
Real-time RT-PCR quantification.
Real-time RT-PCR was performed using a Light Cycler (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Primers for TAP, LAP, TLR-4, and the housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were designed using Primer3 software to amplify 100- to 200-bp products. Primer sequences and products sizes were as follows: TAP forward, 5′-TCTTCCTGGTCCTGTCTGCT-3′; reverse, 5′-GCTGTGTCTTGGCCTTCTTT-3′, 183 bp; LAP forward, 5′-AATTCTCAAAGCTGCCGT-3′; reverse 5′-CACAGTTTCTGACTCCGC-3′, 164 bp; TLR-4 forward. 5′-ATCTCTACAAAATCCCCGACAA-3′; reverse, 5′-TATCAAGGTGGAGAGGTGGTTT-3′, 194 bp; GAPDH forward, 5′-GGCGTGAACCACGAGAAGTATAA-3′; reverse 5′-CCCTCCACGATGCCAAAGT-3′, 120 bp (28). For each primer set, standard curves were generated to quantify gene expression relative to GAPDH. Standard curves were accepted if the slope fell between −3.2 and −3.4, which corresponded to an exponential amplification of approximately 2.0, which approaches a reaction efficiency of 100%. Specific coefficient files were generated from the standard curve for each gene (LightCycler Relative Quantification Software, version 1.0; Roche). Each reaction mixture consisted of 2 μl of 1:10 diluted cDNA, 0.5 μM concentrations of each primer, water, and 10 μl of Quantitect SYBR green I reaction mix (QIAGEN). Samples were denatured for 10 min at 95°C, followed by 45 cycles of 95°C for 5 s, 58°C for 5 s, and 72°C for 15 s. Melting curve analysis was performed for each run, and periodic samples were examined by gel electrophoresis to confirm an appropriately sized amplification product. Amplicons were purified (QIAquick PCR purification kit; QIAGEN) and sequences determined using an Applied Biosystems 3730 DNA analyzer (Robarts Research Institute, University of Western Ontario, London, ON, Canada).
The initial numbers of TAP or LAP template copies present in 500 ng of total RNA obtained from all biopsy specimens taken on day 0 (n = 8) and untreated monolayer cultures (n = 9) were calculated using the formula No = 1/2n, where No is the initial number of TAP or LAP amplicons, and n is the crossing point threshold (26). The mean (± standard error of the meant [SEM]) relative difference was then calculated, with the mean initial number of amplicons in monolayer cultures equal to 1.
Mass spectrometry.
Cytoplasmic protein extracts were obtained from cultured cells as previously described (24). Briefly, treated cells (1 × 106) were harvested in trypsin-EDTA and washed twice in PBS, and cell pellets were resuspended and incubated in a 5× cell pellet volume of lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 μM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride) for 15 min on ice. Cells were centrifuged for 5 min at 420 × g at 4°C, and cell pellets were resuspended in a 2× cell pellet volume of lysis buffer. Cells were passed 10 times through a 27-gauge needle, and lysates were centrifuged at 11,000 × g at 4°C for 20 min. Supernatants (cytoplasmic extracts) were transferred to siliconized tubes and stored at −80°C. Samples were concentrated by C18 TopTip (Glygen Corp., Columbia, MD) and mixed in a 1:1 (vol/vol) ratio with a saturated solution of α-cyano-4-hydroxycinnamic acid in acetonitrile (50%) and trifluoroacetic acid (0.1%) prior to analysis by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) (mass spectrometry) using a Bruker Reflex III mass spectrometer (mass accuracy, ±0.1%). The instrument was calibrated externally using adrenocorticotrophin (ACTH, 18-39) and cytochrome C, and spectra were acquired in positive-ion, linear mode detection. The molecular masses (average) of TAP (entry P25068) and LAP (entry Q28880), as listed in the Swiss-Prot/UniProtKB database, are 4,091.04 Da and 4,954.89 Da, respectively.
Statistical analysis.
Data are presented as means ± SEM. Real-time RT-PCR data were analyzed by one-way analysis of variance (ANOVA) and post hoc Tukey's multiple comparison test. In vivo data were analyzed by repeated-measures ANOVA and Bonferroni post tests to compare replicate means, with biopsy real-time RT-PCR data normalized by log transformation prior to analysis. The difference in TAP and LAP mRNA expression between cultured and biopsied cells was analyzed by Student's t test. Statistical analyses were performed using GraphPad Prism software (San Diego, CA) with a P value of <0.05 considered significant.
RESULTS
Dexamethasone inhibits the LPS-induced upregulation of TAP mRNA in cultured tracheal epithelial cells.
To examine the effects of corticosteroids on β-defensin mRNA expression, bovine tracheal epithelial cells were cultured under two different serum-free conditions and then exposed to varying concentrations of dexamethasone. Dexamethasone had no significant effect on either TAP mRNA expression relative to GAPDH expression or GAPDH mRNA expression alone over a range of dexamethasone concentrations (10−4 to 10−8 M) and treatment times (6, 12, 24, and 36 h) (data not shown). Since TAP is known to be induced by proinflammatory cytokines and certain bacterial products, we examined the effects of corticosteroids on the LPS-induced upregulation of TAP mRNA expression in tracheal epithelial cells. Treatment of submerged, monolayer tracheal epithelial cells with LPS alone resulted in a dose- and time-dependent enhancement of TAP mRNA expression relative to GAPDH. TAP mRNA was significantly elevated (P < 0.05) following 6 h of LPS (1 μg/ml) treatment, reaching a maximum level of induction (averaging 10-fold higher than unstimulated cells) at 16 h, and maintained similar levels of expression up to 36 h (data not shown). Maximal responsiveness was observed when cells were treated for 16 h with 1 μg/ml of LPS, with 100 ng/ml LPS causing a half-maximal (average, fivefold) induction (data not shown). Tracheal epithelial cells grown at an air-liquid interface formed a well-differentiated, pseudostratified, partially ciliated layer of cytokeratin-positive and vimentin-negative epithelial cells. Treatment of the apical surface of these differentiated cells with LPS for 16 h resulted in a dose-dependent increase in TAP mRNA expression similar to that observed for the monolayer cultured cells (data not shown).
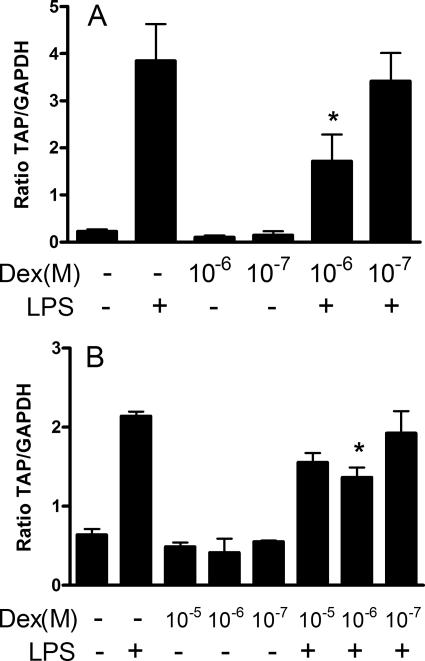
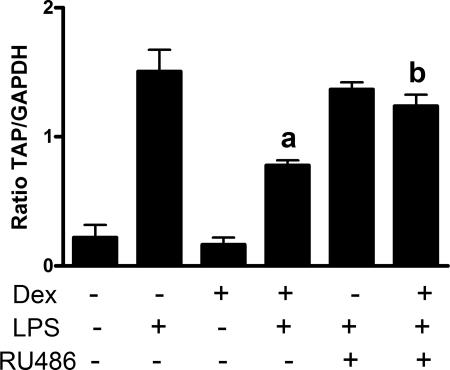
Treatment of cells with dexamethasone 24 h prior to LPS stimulation significantly inhibited (P < 0.05) the LPS-induced upregulation of TAP mRNA expression in tracheal epithelial cells grown to differentiation at an air-liquid interface (Fig. 1a) and in cells grown as a submerged monolayer (Fig. 1b). Dexamethasone pretreatment inhibited the LPS-induced induction of TAP mRNA expression by 58.2% and 51.0% in tracheal epithelial cells grown at an air-liquid interface and as a monolayer, respectively. Similar effects were observed in repeat experiments using three additional calves as sources of monolayer-cultured epithelial cells; in these experimental replicates, dexamethasone (10−6 M) significantly (P < 0.05) inhibited the LPS upregulation of TAP by 41.1%, 52.6%, and 62.2%, while replicates of air-liquid interface cultures from two additional animals gave inhibition levels of 79.1% and 45.3%. To determine if dexamethasone acts through the glucocorticoid receptor, cells were concurrently treated with the glucocorticoid receptor antagonist mifepristone (RU486), which binds the glucocorticoid receptor with high affinity, reversibly inducing a transconformation of the hormone binding domain. RU486 did not affect the LPS-induced induction of TAP but significantly (P < 0.05) inhibited the effects of dexamethasone (Fig. 2).
FIG. 1.
Dexamethasone (Dex) inhibits the LPS-induced upregulation of TAP mRNA levels in primary cultures of TECs. (A) TECs were grown to a differentiated pseudostratified columnar epithelium on collagen-coated, porous inserts at an air-liquid interface and then treated in the presence or absence of 10−6 or 10−7 M dexamethasone for 24 h on their basolateral surfaces, followed by 16 h with (+) or without (−) LPS (100 ng/ml) applied to their apical surfaces. (B) TECs were grown submerged in media on collagen-coated plates and treated in the presence or absence of 10−5, 10−6, or 10−7 M dexamethasone for 24 h, followed by 16 h with or without LPS (100 ng/ml). Data represent the mean ± SEM ratios of TAP mRNA expression relative to GAPDH mRNA expression from triplicate primary cultures. Representative data from three (A) or four (B) separate animals are shown (*, P < 0.05 versus LPS alone, one-way ANOVA with post hoc Tukey's).
FIG. 2.
RU486 abrogates the effect of dexamethasone (Dex) on the LPS induction of TAP mRNA expression. Cultured TECs were treated with dexamethasone (10−6 M) with (+) or without (−) RU486 (10−6 M) for 24 h prior to the addition of LPS (100 ng/ml) for 16 h. Data represent the pooled mean (± SEM) ratios of TAP mRNA expression relative to GAPDH mRNA expression from triplicate primary cultures from three separate animals (a, P < 0.05 versus LPS alone; b, P < 0.05 versus Dex plus LPS, one-way ANOVA with post hoc Tukey's).
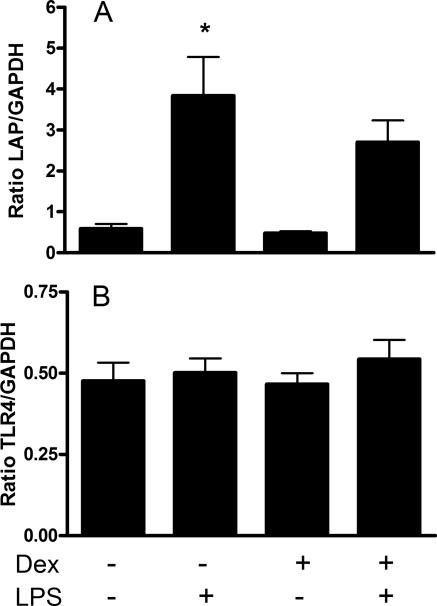
Dexamethasone did not alter LAP or TLR-4 mRNA.
To determine if dexamethasone inhibits the expression of other LPS-inducible β-defensins in tracheal epithelial cells (TECs), LAP mRNA expression was quantified relative to GAPDH. LPS treatment significantly (P < 0.05) induced expression of LAP mRNA (Fig. 3a). Although LAP mRNA expression in LPS-treated cells was lower in cells pretreated with dexamethasone, this effect was not significant. Since the level of TLR-4 expression on TECs may influence their ability to respond to LPS, TLR-4 mRNA was quantified relative to GAPDH in dexamethasone-treated cells. There was no change in TLR-4 mRNA expression observed following LPS or dexamethasone treatment (Fig. 3b).
FIG. 3.
Dexamethasone (Dex) does not inhibit LPS-induced LAP mRNA expression, nor does LPS or dexamethasone alter TLR-4 mRNA expression. (A) Cultured TECs were treated with (+) or without (−) dexamethasone (10−6 M) for 24 h prior to the addition of LPS (100 ng/ml) for 16 h. Data represent the pooled mean (± SEM) ratios of LAP mRNA expression relative to GAPDH mRNA from triplicate primary cultures from three separate animals (*, P < 0.05 versus untreated cells). (B) Cells were treated as described for panel A, but data represent the pooled mean (± SEM) ratios of TLR-4 mRNA expression relative to GAPDH mRNA from triplicate primary cultures from three separate animals.
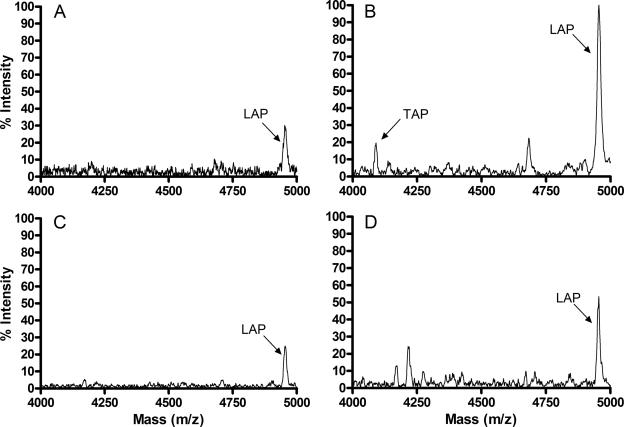
Dexamethasone inhibits the LPS-induced upregulation of TAP in cultured tracheal epithelial cells.
To determine if the inhibition of LPS-induced TAP mRNA expression by dexamethasone correlates with reduced peptide expression, cytoplasmic extracts of cultured tracheal epithelial cells were analyzed semiquantitatively by mass spectrometry. Data using triplicate cultures of cells obtained from three separate animals consistently revealed a peak corresponding to TAP (4,090.045 Da) in cells treated with LPS (100 ng/ml) for 16 h (Fig. 4b) but not in cytoplasmic extracts of untreated cells (Fig. 4a) or cells treated with dexamethasone alone (Fig. 4c). Furthermore, TAP was not detectable in cells treated with dexamethasone (10−6 M) and LPS (100 ng/ml) (Fig. 4d) from 2 of the 3 animals, and the third animal showed a consistent decrease in relative signal intensity of the TAP peak. A peak corresponding to LAP (4,954.184 Da) was present in cells from all treatment groups, with an increased relative signal intensity in cells treated with LPS compared to untreated or dexamethasone-treated cells (Fig. 4). In 2 of the 3 animals, the relative signal intensity of the LAP peak was reduced in cells treated with dexamethasone and LPS compared to cells treated with LPS alone.
FIG. 4.
Dexamethasone inhibits the LPS-induced upregulation of TAP expression in cultured TECs. Cells were treated with or without dexamethasone (10−6 M) for 24 h prior to the addition of LPS (100 ng/ml) for 16 h. MALDI-TOF mass spectra of cytoplasmic extracts were obtained from untreated (A), LPS-treated (B), dexamethasone-treated (C), or dexamethasone- and LPS-treated (D) cells. Representative data from cells isolated from three separate animals are shown.
Other neuroendocrine mediators tested do not affect TAP mRNA expression.
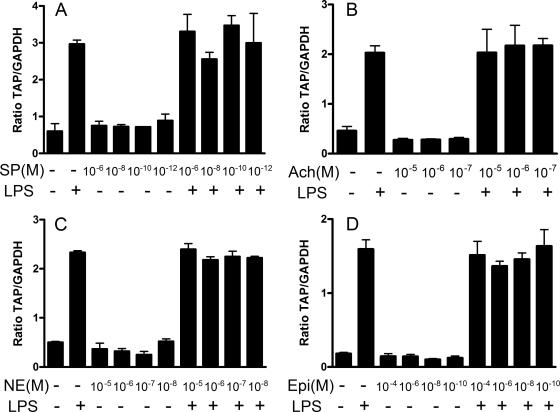
A panel of neuroendocrine mediators were tested for their ability to modulate basal or LPS-induced TAP expression (Fig. 5). A range of concentrations of norepinephrine, epinephrine, acetylcholine, or substance P were added to submerged monolayer cultures of tracheal epithelial cells for 3 h or 30 min prior to the addition of LPS (100 ng/ml). There was no consistent effect of any of these four mediators on the basal or LPS-induced expression of TAP mRNA.
FIG. 5.
Substance P, acetylcholine, norepinephrine, and epinephrine do not alter TAP mRNA expression in cultured TECs. (A) TECs were exposed to substance P (SP) (10−6, 10−8, 10−10, or 10−12 M) or vehicle for 30 min prior to the addition of LPS (100 ng/ml) for 16 h. (B) Acetylcholine (Ach) (10−5, 10−6, or 10−7 M) or vehicle was added to TECs for 3 h prior to the addition of LPS (100 ng/ml) for 16 h. TECs were treated with norepinephrine (NE) (10−5, 10−6, 10−7, or 10−8 M) (C) or epinephrine (Epi) (10−4, 10−6, 10−8, or 10−10 M) (D) for 30 min prior to the addition of LPS (100 ng/ml) for 16 h. Data represent the mean ± SEM ratios of TAP mRNA expression relative to GAPDH mRNA from triplicate primary cultures. Representative data from three separate animals are shown. +, with; −, without.
Dexamethasone reduces antimicrobial peptide mRNA expression in bronchial biopsy specimens.
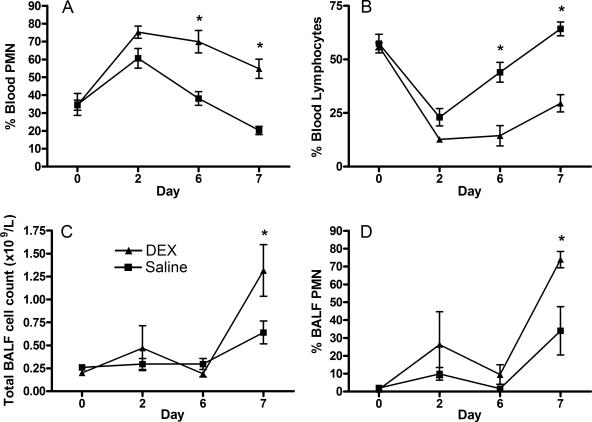
To investigate the effect of elevated systemic glucocorticoids on epithelial β-defensin mRNA expression in vivo, calves were treated with intramuscular injections of saline or dexamethasone. Clinical parameters including heart rate, respiration rate, mentation, appetite, and rectal temperature did not deviate from normal limits throughout the experiment. However, serum cortisol was markedly reduced to below detectable levels (<5.5 nmol/liter) by day 6 in all dexamethasone-treated calves, while cortisol remained within the reference interval (30 to 220 nmol/liter) in saline-treated calves (72.00 ± 23.01 nmol/liter). Furthermore, the percentage of blood neutrophils from dexamethasone-treated calves was increased compared to saline-treated controls from day 2 until the end of the experiment, whereas saline-treated animals had a transient neutrophilia on day 2 that returned to within normal limits by day 6 (Fig. 6a). Trends observed for the percentages of blood lymphocytes were inversely related to percentages of blood neutrophils, with dexamethasone-treated animals showing significantly reduced lymphocyte percentages on days 6 and 7 (Fig. 6b).
FIG. 6.
Dexamethasone and LPS alter proportions of peripheral blood and BALF neutrophils. Calves were injected with dexamethasone (0.1 mg/kg) or saline every 24 h for 6 days, and then LPS (500 μg in 10 ml PBS) was infused into a bronchus in the caudal lung lobe. Peripheral blood and BALF were sampled prior to treatment (day 0) and on days 2, 6, and 7 (24 h after LPS infusion). Percentages of neutrophils (A) and lymphocytes (B) in whole blood, the total cell count in BALF (C), and the percentage of neutrophils in BALF (D) were measured. Data represent the mean ± SEM counts from saline- or dexamethasone-treated calves (4 calves per group) on 4 separate days. Significance is based on repeated-measures ANOVA with post hoc Bonferroni (*, P < 0.05 for saline versus dexamethasone treated on each day).
BALF was recovered on days 0, 2, 6, and 7 of dexamethasone treatment; the day 7 samples were collected 24 h after infusion of LPS into the same bronchus. The total number of cells and the percentage of neutrophils in the BALF did not differ prior to infusion of LPS in saline compared to dexamethasone-treated calves. At 24 h after instillation of LPS, the mean number of cells in BALF increased from 0.30 × 109 to 0.64 × 109 cells/liter, and the mean percentage of neutrophils in BALF rose from 1.6 to 34.0 in saline-treated animals. In contrast, over the same 24-h period, dexamethasone-treated calves showed significantly higher (P < 0.05) increases in mean BALF cells from 0.19 × 109 to 1.32 × 109 cells/liter and in the mean percentage of BALF neutrophils from 9.5 to 73.8 (Fig. 6c and d). Endoscopic evaluation of airways revealed no evidence of inflammation or exudates, and bacterial culture of BALF was negative for all samples.
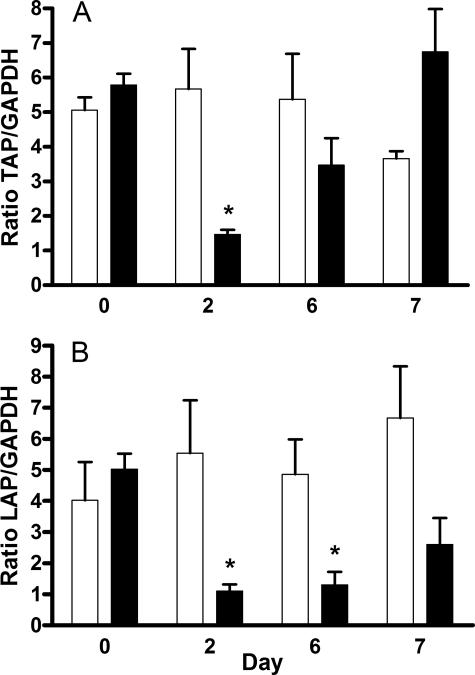
Bronchial biopsy specimens of were taken at days 0, 2, and 6 of saline or dexamethasone treatment and on day 7, after both groups had received LPS for 24 h. TAP and LAP mRNA expression was quantified relative to GAPDH expression using real-time RT-PCR. Gene-specific primers for bovine TAP, LAP, and GAPDH amplified specific PCR products from biopsy-derived RNA, confirmed by sequencing. The mean ratios of TAP to GAPDH mRNA in bronchial biopsy specimens were similar in both groups on days 0 and 6 but significantly reduced in the dexamethasone-treated group on day 2 (Fig. 7a). Similarly, the mean ratio of LAP to GAPDH mRNA was significantly reduced in dexamethasone-treated calves on day 2 and also on day 6 (Fig. 7b). LPS treatment did not have a significant effect on TAP or LAP expression in bronchial biopsy specimens harvested at day 7.
FIG. 7.
Dexamethasone reduces β-defensin mRNA expression in bronchial biopsy specimens from dexamethasone-treated calves. Calves were injected with 0.1 mg/kg dexamethasone (black bars) or saline (white bars) every 24 h for 6 days, and then LPS (500 μg in 10 ml PBS) was infused into a bronchus in the caudal lung lobe. Total mRNA extracted from bronchial biopsy specimens was then used to quantify TAP (A) or LAP (B) mRNA expression using real-time RT-PCR. Data represent the mean ± SEM ratios of TAP or LAP mRNA expression relative to GAPDH mRNA from four separate animals. Significance is based on repeated-measures ANOVA with post hoc Bonferroni on log-transformed data (*, P < 0.05 for saline- versus dexamethasone-treated calves each day).
To compare the basal level of TAP and LAP mRNA expression in biopsy specimens to their expression in monolayer cultures, initial amplicon numbers were calculated as outlined in Materials and Methods. A 64.68- ± 15.95-fold (P < 0.0001)-higher basal level of TAP and a 67.98- ± 11.63-fold (P < 0.0001)-higher basal level of LAP mRNA expression were observed in biopsy specimens compared to monolayer cultures. Direct comparison of the basal TAP and LAP expression relative to GAPDH in cultured cells versus cells obtained by biopsy was confounded by differing mean crossing points for GAPDH between the two sample types (27.28 ± 0.22 in biopsy specimens versus 22.83 ± 0.52 in monolayer cultures).
Putative glucocorticoid response elements identified in TAP promoter.
Analysis of transcription factor binding sites within 2 kbp of the 5′ flanking region of the TAP gene was performed using MatInspector (version 7.4.2). A previously reported NF-κB consensus site was identified at 177 bp upstream from the transcription start site, and a novel glucocorticoid response element (5′-AACCGTCGTTGACACGACG-3′) was revealed at 1,718 bp upstream.
DISCUSSION
The epithelial lining fluid of the respiratory tract contains antimicrobial peptides, which prevent growth and colonization of the airways by inhaled opportunistic pathogens. Alterations in the composition of this fluid during periods of stress may predispose animals to bacterial pneumonia. We investigated bovine β-defensin mRNA expression in bronchial biopsy specimens from dexamethasone-treated calves and cultured tracheal epithelial cells using real-time RT-PCR. This study confirms the LPS-inducible expression of TAP and LAP mRNA in the tracheal epithelium (9, 12) and demonstrates that dexamethasone significantly inhibits the upregulation of TAP mRNA and protein expression in vitro; the upregulation of LAP mRNA was not similarly affected. Furthermore, treatment of calves with dexamethasone reduces the basal expression of TAP and LAP mRNA in bronchial biopsy specimens. These findings suggest a mechanism by which elevated glucocorticoid concentrations enhance susceptibility to respiratory disease.
Cultured tracheal epithelial cells were used to explore the ability of a panel of neuroendocrine mediators to modulate β-defensin expression. Dexamethasone, but not norepinephrine, epinephrine, acetylcholine, or substance P, was shown to influence β-defensin mRNA expression induced by LPS, using both freshly isolated, submerged tracheal epithelial cells and tracheal epithelial cells grown to differentiation at an air-liquid interface. Furthermore, changes in TAP mRNA expression correlated well with changes in protein abundance, which others, quantifying TAP (24, 27) and hBD-2 (50), have similarly demonstrated. Our findings regarding the effects of dexamethasone are in agreement with those of Tomita et al. who found that LPS-induced upregulation of hBD-2 mRNA was inhibited by dexamethasone in a human airway cell line (48), which is relevant due to the high homology between hBD-2 and TAP genes (4) and their similar regulation by inflammatory mediators (13). Conversely, Duits et al. reported no effect of dexamethasone on hBD-1 or hBD-2 expression, whereas basal and induced hBD-3 mRNA expression was inhibited by dexamethasone in primary bronchial epithelial cells (16). Reasons for this discrepancy are not clear, but considering our finding that dexamethasone did not significantly affect the LPS-induced upregulation of LAP, it seems likely that expression of each β-defensin is regulated somewhat independently, as has been shown for their pathogen-specific induction in humans (34).
Although the effects of catecholamines, acetylcholine, and substance P on airway smooth muscle and tracheobronchial gland function have been well studied (19), their ability to alter defensin expression in airway epithelial cells was unknown. Several time points and concentrations of these neuropeptides were investigated, but no consistent effects on β-defensin expression were found. We cannot rule out the possibility that receptors or signaling pathways for these neuropeptides are somehow downregulated or desensitized in our culture system. However, previous studies indicate that adrenergic (10) and cholinergic (40) receptors and the substance P receptor neurokinin-1 (23) maintain functionality in cultured tracheal epithelial cells.
The molecular mechanism through which dexamethasone inhibits the LPS-induced upregulation of TAP mRNA involves the glucocorticoid receptor, since RU486 significantly inhibited the dexamethasone effect (Fig. 2). Glucocorticoids exert their effects on target cells by binding to cytosolic glucocorticoid receptors which translocate to the nucleus, bind DNA, and activate or repress gene transcription. Additionally, glucocorticoids can alter gene expression via DNA-independent mechanisms such as direct protein-protein interactions (11). The ability of glucocorticoids to inhibit NF-κB activation depends on their ability to prolong the activity of IκB, the inhibitor of NF-κB (2, 41). Although this seems the most likely pathway by which glucocorticoids inhibit the LPS-induced upregulation of TAP, glucocorticoids might also directly influence defensin gene expression through alternate direct or indirect mechanisms, such as posttranslational modifications, or by affecting mRNA stability. Since the TAP promoter region also contains AP-1 binding sites and glucocorticoids can interfere with AP-1 signaling (11), we cannot rule out involvement of this pathway as well. Furthermore, analysis of the 5′-flanking region of the TAP gene identified a potential glucocorticoid receptor-binding site in the TAP promoter (1,718 bp upstream of the initiation of transcription site), suggesting that the activated glucocorticoid receptor may directly influence TAP transcription. Promoter analysis studies are required to determine the involvement of transrepression in the dexamethasone-mediated inhibition of TAP expression.
Investigations into the ability of glucocorticoids to modulate the innate defenses of cattle in vivo have focused on altered gene and protein expression of neutrophils (8, 29) and TLR expression in blood and lung leukocytes (17). The effectiveness of our treatment regimen was confirmed by suppression of endogenous cortisol, induction of both blood neutrophilia and lymphopenia, and the resultant increased ratio of neutrophils to lymphocytes in dexamethasone-treated calves (1). The inducibility of TAP and LAP mRNA expression by proinflammatory cytokines and bacterial components had the potential to complicate our in vivo dexamethasone study if calves developed bronchopneumonia. Antibiotics were administered prophylactically, and BALF was analyzed for bacterial growth and leukocyte counts. Endoscopic examination during BALF retrieval revealed no gross evidence of airway inflammation and there was no bacterial growth from BALF samples.
Examination of β-defensin expression in bronchial biopsy specimens revealed a significant reduction of both TAP and LAP mRNA in dexamethasone- versus saline-treated calves 48 h after the first treatment. This contrasts with our in vitro finding that dexamethasone reduces LPS-induced but not baseline levels of TAP. We have demonstrated that baseline TAP and LAP mRNA expression exists at a higher level in vivo, perhaps as a result of constant exposure to inhaled bacteria, and speculate that inhibition is possible at this higher level of expression. In contrast, cultured cells display a lower basal level of expression, below which inhibition is not possible unless previously induced by LPS. Our in vivo observations are consistent with a study on human fetal lung explants, which demonstrated that hBD-2 mRNA expression was reduced by dexamethasone treatment, while expression of hBD-1 mRNA was increased (45). Similarly, frogs treated with corticosteroids had reduced expression of antimicrobial peptides and failed to reduce the number of bacteria in an experimental oral infection compared to untreated frogs (43, 44). The reduction of antimicrobial peptide expression by glucocorticoids seems counterintuitive, as circumstances that elevate endogenous glucocorticoids, such as stress or infection, would likely benefit from the induction of epithelial antimicrobial activity. Perhaps, under these circumstances, glucocorticoids are acting as anti-inflammatory agents, since defensins have been shown to be chemoattractant for cells of the innate and adaptive immune systems (32) and can initiate cytokine release (6, 33).
LPS was deposited into the left caudal lung lobes of calves following 6 days of saline or dexamethasone injections in an attempt to examine the effects of glucocorticoids on the LPS-induced β-defensin gene expression. A marked increase in total cell number and percentage of neutrophils in BALF was observed following LPS treatment, indicating that the dose was sufficient to incite inflammation, at least in the small bronchioles and alveoli. However, inspection of the larger bronchi, accessible to the bronchoscope, revealed no discernible evidence of inflammation, suggesting LPS-induced inflammation was restricted to more distal areas of the lung. It is likely for this reason that we observed no induction of β-defensin gene expression in bronchial tissue biopsy specimens following infusion of LPS, even though β-defensin induction has been previously demonstrated in vivo during bacterial challenge (47). We speculate that the bronchial mucus may have prevented the LPS from accessing surface receptors on the bronchial epithelial cells.
Dexamethasone-treated calves had greater neutrophil recruitment to the lung following LPS stimulation than did saline-treated calves, based on both the total number of cells and the percentage of neutrophils in BALF. The effects of dexamethasone on bovine neutrophil function are generally thought to be immunosuppressive: reducing phagocytosis (36), antibody-dependent cell-mediated cytotoxicity (36, 38), and generation of reactive-oxygen species (25, 36, 38). In particular, dexamethasone downregulates neutrophil cell surface expression of the adhesion molecule l-selectin (CD62L) (7, 25) and the β2-integrin CD18 (7), which should intuitively reduce neutrophil extravasation. Conversely, there is evidence that dexamethasone enhances chemotactic ability, as neutrophils taken from dexamethasone-treated cattle show increased migration to C5a and IL-8 (1). Furthermore, treatment of cattle with dexamethasone was recently shown to enhance IL-8-elicited neutrophil migration into the uterus (25). An alternative explanation for our results relates to the established ability of dexamethasone to decrease bovine neutrophil apoptosis (8), thereby prolonging the life span of neutrophils in the lungs of treated animals. Since neutrophils entering tissues in response to inflammatory stimuli can delay apoptosis for up to 24 or 48 h (22), this mechanism is unlikely, as we sampled lavage fluid at 24 h post-LPS infusion. Our finding of increased LPS-induced recruitment of neutrophils to the lung of dexamethasone-treated animals lends support to the notion that glucocorticoids may not only mobilize blood neutrophils but also potentiate neutrophil migration to areas of inflammation.
In conclusion, we have demonstrated that dexamethasone impairs β-defensin expression in tracheal biopsy specimens from treated animals and in isolated, cultured tracheal epithelial cells. This may influence susceptibility to respiratory disease by reducing airway surface antimicrobial activity or by blunting the ability of defensins to recruit and activate inflammatory cells. Our finding that dexamethasone promotes neutrophil extravasation into the lungs in response to LPS argues against the latter and suggests that further mechanistic studies would be of value.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada, the Beef Cattle Research Council (Canadian Cattlemen's Association), the Ontario Ministry of Agriculture and Food, and the Canadian Institutes of Health Research.
We thank Dyanne Brewer at the Biological Mass Spectrometry Facility (University of Guelph) for MALDI-TOF analysis.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Anderson, B. H., D. L. Watson, and I. G. Colditz. 1999. The effect of dexamethasone on some immunological parameters in cattle. Vet. Res. Commun. 23:399-413. [DOI] [PubMed] [Google Scholar]
- 2.Auphan, N., J. A. DiDonato, C. Rosette, A. Helmberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270:286-290. [DOI] [PubMed] [Google Scholar]
- 3.Bals, R., and P. S. Hiemstra. 2004. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23:327-333. [DOI] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, P. J. 2001. Neurogenic inflammation in the airways. Respir. Physiol. 125:145-154. [DOI] [PubMed] [Google Scholar]
- 6.Boniotto, M., W. J. Jordan, J. Eskdale, A. Tossi, N. Antcheva, S. Crovella, N. D. Connell, and G. Gallagher. 2006. Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 50:1433-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton, J. L., M. E. Kehrli, Jr., S. Kapil, and R. L. Horst. 1995. Regulation of L-selectin and CD18 on bovine neutrophils by glucocorticoids: effects of cortisol and dexamethasone. J. Leukoc. Biol. 57:317-325. [DOI] [PubMed] [Google Scholar]
- 8.Burton, J. L., S. A. Madsen, L. C. Chang, P. S. Weber, K. R. Buckham, R. van Dorp, M. C. Hickey, and B. Earley. 2005. Gene expression signatures in neutrophils exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet. Immunol. Immunopathol. 105:197-219. [DOI] [PubMed] [Google Scholar]
- 9.Caverly, J. M., G. Diamond, J. M. Gallup, K. A. Brogden, R. A. Dixon, and M. R. Ackermann. 2003. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect. Immun. 71:2950-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloutier, M. M., C. M. Schramm, and L. Guernsey. 1998. Tannin inhibits the cAMP-beta-adrenergic receptor pathway in bovine tracheal epithelium. Am. J. Physiol. 274:L252-L257. [DOI] [PubMed] [Google Scholar]
- 11.De Bosscher, K., W. Vanden Berghe, and G. Haegeman. 2000. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: negative interference of activated glucocorticoid receptor with transcription factors. J. Neuroimmunol. 109:16-22. [DOI] [PubMed] [Google Scholar]
- 12.Diamond, G., D. E. Jones, and C. L. Bevins. 1993. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc. Natl. Acad. Sci. USA 90:4596-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond, G., V. Kaiser, J. Rhodes, J. P. Russell, and C. L. Bevins. 2000. Transcriptional regulation of beta-defensin gene expression in tracheal epithelial cells. Infect. Immun. 68:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38.:27-38. [DOI] [PubMed] [Google Scholar]
- 15.Diamond, G., J. P. Russell, and C. L. Bevins. 1996. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc. Natl. Acad. Sci. USA 93:5156-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duits, L. A., M. Rademaker, B. Ravensbergen, M. A. van Sterkenburg, E. van Strijen, P. S. Hiemstra, and P. H. Nibbering. 2001. Inhibition of hBD-3, but not hBD-1 and hBD-2, mRNA expression by corticosteroids. Biochem. Biophys. Res. Commun. 19:522-525. [DOI] [PubMed] [Google Scholar]
- 17.Eicher, S. D., K. A. McMunn, H. M. Hammon, and S. S. Donkin. 2004. Toll-like receptors 2 and 4, and acute phase cytokine gene expression in dexamethasone and growth hormone treated dairy calves. Vet. Immunol. Immunopathol. 98:115-125. [DOI] [PubMed] [Google Scholar]
- 18.Farmer, P., and J. Pugin. 2000. Beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L675-L682. [DOI] [PubMed] [Google Scholar]
- 19.Finkbeiner, W. E. 1999. Physiology and pathology of tracheobronchial glands. Respir. Physiol. 118:77-83. [DOI] [PubMed] [Google Scholar]
- 20.Frank, G. H., R. E. Briggs, R. W. Loan, C. W. Purdy, and E. S. Zehr. 1996. Respiratory tract disease and mucosal colonization by Pasteurella haemolytica in transported cattle. Am. J. Vet. Res. 57:1317-1320. [PubMed] [Google Scholar]
- 21.Hertz, C. J., Q. Wu, E. M. Porter, Y. J. Zhang, K. H. Weismuller, P. J. Godowski, T. Ganz, S. H. Randell, and R. L. Modlin. 2003. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J. Immunol. 171:6820-6826. [DOI] [PubMed] [Google Scholar]
- 22.Homburg, C. H., and D. Roos. 1996. Apoptosis of neutrophils. Curr. Opin. Hematol. 3:94-99. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. S., K. F. Rabe, H. Magnussen, J. M. Green, and S. R. White. 1995. Migration and proliferation of guinea pig and human airway epithelial cells in response to tachykinins. Am. J. Physiol. 269:L119-L126. [DOI] [PubMed] [Google Scholar]
- 24.Klein-Patel, M. E., G. Diamond, M. Boniotto, S. Saad, and L. K. Ryan. 2006. Inhibition of beta-defensin gene expression in airway epithelial cells by low doses of residual oil fly ash is mediated by vanadium. Toxicol. Sci. 92:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konig, T., H. J. Schuberth, W. Leibold, and H. Zerbe. 2006. Dexamethasone depresses the expression of l-selectin but not the in vivo migration of bovine neutrophils into the uterus. Theriogenology 65:1227-1241. [DOI] [PubMed] [Google Scholar]
- 26.Kubista, M., J. M. Andrade, M. Bengtsson, A. Forootan, J. Jonak, K. Lind, R. Sindelka, R. Sjoback, B. Sjogreen, L. Strombom, A. Stahlberg, and N. Zoric. 2006. The real-time polymerase chain reaction. Mol. Aspects Med. 27:95-125. [DOI] [PubMed] [Google Scholar]
- 27.Legarda, D., M. E. Klein-Patel, S. Yim, M. H. Yuk, and G. Diamond. 2005. Suppression of NF-kappaB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system. Cell. Microbiol. 7:489-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leutenegger, C. M., A. M. Alluwaimi, W. L. Smith, L. Perani, and J. S. Cullor. 2000. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan polymerase chain reaction. Vet. Immunol. Immunopathol. 77:275-287. [DOI] [PubMed] [Google Scholar]
- 29.Lippolis, J. D., B. D. Peterson-Burch, and T. A. Reinhardt. 2006. Differential expression analysis of proteins from neutrophils in the periparturient period and neutrophils from dexamethasone-treated dairy cows. Vet. Immunol. Immunopathol. 111:149-164. [DOI] [PubMed] [Google Scholar]
- 30.Loneragan, G. H., D. A. Dargatz, P. S. Morley, and M. A. Smith. 2001. Trends in mortality ratios among cattle in US feedlots. J. Am. Vet. Med. Assoc. 219:1122-1127. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor, T. M., J. O'Connell, D. I. O'Brien, T. Goode, C. P. Bredin, and F. Shanahan. 2004. The role of substance P in inflammatory disease. J. Cell. Physiol. 201:167-180. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheim, J. J., and D. Yang. 2005. Alarmins: chemotactic activators of immune responses. Curr. Opin. Immunol. 17:359-365. [DOI] [PubMed] [Google Scholar]
- 33.Perregaux, D. G., K. Bhavsar, L. Contillo, J. Shi, and C. A. Gabel. 2002. Antimicrobial peptides initiate IL-1 beta posttranslational processing: a novel role beyond innate immunity. J. Immunol. 168:3024-3032. [DOI] [PubMed] [Google Scholar]
- 34.Porter, E., H. Yang, S. Yavagal, G. C. Preza, O. Murillo, H. Lima, S. Greene, L. Mahoozi, M. Klein-Patel, G. Diamond, S. Gulati, T. Ganz, P. A. Rice, and A. J. Quayle. 2005. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect. Immun. 73:4823-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radi, Z. A., and M. R. Ackermann. 2004. Growth of differentiated ovine tracheal epithelial cells in vitro. J. Vet. Med. A 51:167-170. [DOI] [PubMed] [Google Scholar]
- 36.Reddy, P. G., D. S. McVey, M. M. Chengappa, F. Blecha, H. C. Minocha, and P. E. Baker. 1990. Bovine recombinant granulocyte-macrophage colony-stimulating factor enhancement of bovine neutrophil functions in vitro. Am. J. Vet. Res. 51:1395-1399. [PubMed] [Google Scholar]
- 37.Roth, J. A., and M. L. Kaeberle. 1982. Effect of glucocorticoids on the bovine immune system. J. Am. Vet. Med. Assoc. 180:894-901. [PubMed] [Google Scholar]
- 38.Roth, J. A., and M. L. Kaeberle. 1985. In vivo effect of ascorbic acid on neutrophil function in healthy and dexamethasone-treated cattle. Am. J. Vet. Res. 46:2434-2436. [PubMed] [Google Scholar]
- 39.Salathe, M. 2002. Effects of beta-agonists on airway epithelial cells. J. Allergy Clin. Immunol. 110:S275-S281. [DOI] [PubMed] [Google Scholar]
- 40.Salathe, M., T. Lieb, and R. J. Bookman. 2000. Lack of nitric oxide involvement in cholinergic modulation of ovine ciliary beat frequency. J. Aerosol Med. 13:219-229. [DOI] [PubMed] [Google Scholar]
- 41.Scheinman, R. I., P. C. Cogswell, A. K. Lofquist, and A. S. Baldwin, Jr. 1995. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270:283-286. [DOI] [PubMed] [Google Scholar]
- 42.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6:551-557. [DOI] [PubMed] [Google Scholar]
- 43.Simmaco, M., A. Boman, M. L. Mangoni, G. Mignogna, R. Miele, D. Barra, and H. G. Boman. 1997. Effect of glucocorticoids on the synthesis of antimicrobial peptides in amphibian skin. FEBS Lett. 416:273-275. [DOI] [PubMed] [Google Scholar]
- 44.Simmaco, M., M. L. Mangoni, A. Boman, D. Barra, and H. G. Boman. 1998. Experimental infections of Rana esculenta with Aeromonas hydrophila: a molecular mechanism for the control of the normal flora. Scand. J. Immunol. 48:357-363. [DOI] [PubMed] [Google Scholar]
- 45.Starner, T. D., B. Agerberth, G. H. Gudmundsson, and P. B. McCray, Jr. 2005. Expression and activity of beta-defensins and LL-37 in the developing human lung. J. Immunol. 174:1608-1615. [DOI] [PubMed] [Google Scholar]
- 46.Sternberg, E. M. 2006. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6:318-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolzenberg, E. D., G. M. Anderson, M. R. Ackermann, R. H. Whitlock, and M. Zasloff. 1997. Epithelial antibiotic induced in states of disease. Proc. Natl. Acad. Sci. USA 94:8686-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomita, T., T. Nagase, E. Ohga, Y. Yamaguchi, M. Yoshizumi, and Y. Ouchi. 2002. Molecular mechanisms underlying human beta-defensin-2 gene expression in a human airway cell line (LC2/ad). Respirology 7:305-310. [DOI] [PubMed] [Google Scholar]
- 49.Tracey, K. J. 2002. The inflammatory reflex. Nature 420:853-859. [DOI] [PubMed] [Google Scholar]
- 50.Vora, P., A. Youdim, L. S. Thomas, M. Fukata, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, A. Wada, T. Hirayama, M. Arditi, and M. T. Abreu. 2004. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 173:5398-5405. [DOI] [PubMed] [Google Scholar]
- 51.Wang, H., H. Liao, M. Ochani, M. Justiniani, X. Lin, L. Yang, Y. Al Abed, H. Wang, C. Metz, E. J. Miller, K. J. Tracey, and L. Ulloa. 2004. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10:1216-1221. [DOI] [PubMed] [Google Scholar]
- 52.Webster, J. I., L. Tonelli, and E. M. Sternberg. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20:125-163. [DOI] [PubMed] [Google Scholar]
- 53.Yamaya, M., W. E. Finkbeiner, S. Y. Chun, and J. H. Widdicombe. 1992. Differentiated structure and function of cultures from human tracheal epithelium. Am. J. Physiol. 262:L713-L724. [DOI] [PubMed] [Google Scholar]
- 54.Zecchinon, L., T. Fett, and D. Desmecht. 2005. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 36:133-156. [DOI] [PubMed] [Google Scholar]