Abstract
Group B Streptococcus (GBS) is an opportunistic organism that can harmlessly colonize the human gut, vagina, and rectum but can also cause pneumonia, sepsis, and meningitis in neonates born to colonized mothers. We have shown previously that growth rate and oxygen level regulate the ability of GBS to invade eukaryotic cells in vitro. Herein we extend and expand on these observations to show that GBS type V, an emergent serotype, grown in a chemostat at a cell mass-doubling time (td) of 1.8 h with oxygen invaded human ME-180 cervical epithelial cells in large numbers compared with those grown at the same td without oxygen or at a slower td of 11.0 h. The fact that several GBS type V cell wall-associated and membrane proteins were expressed exclusively under the invasive growth condition prompted an investigation, using genomics and proteomics, of all upregulated genes and proteins. Several proteins with potential roles in adherence were identified, including an undefined surface antigen (SAG1350), a lipoprotein (SAG0971), penicillin-binding protein 2b (SAG0765), glyceraldehyde-3-phosphate dehydrogenase (SAG0823), and an iron-binding protein (SAG1007). Mouse antisera to these five proteins inhibited binding of GBS type V to ME-180 cells by ≥85%. Recombinant undefined surface antigen (SAG1350), lipoprotein (SAG0971), and penicillin-binding protein 2b (SAG0765) each bound to ME-180 cells in a dose-dependent fashion, confirming their ability to act as ligands. Collectively, these data increase the number of potential GBS adherence factors and also suggest a role for these surface-associated proteins in initial pathogenic events.
Group B Streptococcus (GBS) is a facultative anaerobe that can persist in both poorly and well-oxygenated tissues in humans. It commonly colonizes the anaerobic environments of the rectum and the vagina but can also colonize, grow, and disseminate from the oxygen-rich lungs of a newborn and cause pneumonia, life-threatening sepsis, and meningitis. That the presence of oxygen during growth is positively associated with the ability of GBS to invade human cells was recently demonstrated in vitro with GBS grown in a chemostat, where nutrient conditions are precisely controlled (17). In addition to oxygen, the rate of growth also influences invasiveness, as shown by the fact that GBS grown in a chemostat at a cell mass-doubling time (td) of 1.8 h invaded respiratory epithelial cells in significantly greater numbers than did those grown at a relatively slower td of 11.0 h (25).
Newly described genes such as those encoding the penicillin-binding protein (PBP) (ponA) (19), superoxide dismutase (sodA) (33), the delta subunit of RNA polymerase (18), glutamine transporter (glnQ) (40), and RogB, a transcriptional regulator of GBS (12), add to a growing list of GBS virulence factors. Indeed, the sequencing of GBS genomes from several serotypes (41), including types V and III (8, 42), and technologies such as DNA microarray and proteomic analysis now allow global approaches to revealing the complex nature of GBS pathogenicity (22) as well as new approaches to vaccine development (24).
In this study, we sought to compare the genes upregulated and cell wall-associated and membrane proteins expressed by GBS grown in a chemostat in conditions that resulted in high-level in vitro invasiveness with those of GBS held at conditions that resulted in low-level invasion. We hypothesized that novel proteins would be expressed by GBS under growth conditions that support high-level invasion. We chose to study GBS type V because of its recent emergence and current prevalence in adult human disease (34, 47). To the best of our knowledge, this is the first study that integrates bacterial physiology (controlled and balanced growth in a chemostat) with proteomics and genomics to investigate factors involved with bacterium-host cell interactions.
MATERIALS AND METHODS
Bacterial strain and human cell line.
Clinically derived GBS type V strain 2603V/R (24) was used in this study. Human cervical epithelial ME-180 cells (ATCC HTB-33) were maintained in McCoy's 5a cell culture medium with 10% fetal bovine serum and incubated at 37°C with 5% CO2. Spent medium was replaced every 2 to 3 days and 1 day before use.
DIVAS.
GBS was cultured with a chemically defined medium in a chemostat (17) and held at steady state with a td of 1.8 h with 0% or 12% dissolved oxygen (DO2) and at a td of 11 h with 12% DO2. The DO2 value ranged between 12% and 15%. For the purpose of presentation, the 12% dissolved oxygen set point value of the biocontroller, which monitors and regulates the chemostat culture, was used. A dynamic in vitro attachment and invasion system (DIVAS) was assembled and operated as described previously (25), except that 60- by 15-mm culture dishes replaced tissue culture flasks. ME-180 monolayers were washed once with phosphate-buffered saline (PBS), and 3 ml of PBS was added to each dish before perfusion of GBS. Perfusion of GBS over monolayers of ME-180 cells proceeded for 2 h. Antibiotics were used to kill extracellular GBS, and invaded GBS was quantified by standard plate counts as described in detail previously (25).
GBS surface-associated proteins and sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
GBS cells (450 ml) held at a steady-state high growth rate at a td of 1.8 h with 0% or 12% DO2 and at a low growth rate (td, 11.0 h; DO2, 12%) were harvested from the chemostat, pelleted by centrifugation (8,281 × g; 4°C; 20 min), and washed once in PBS, pH 7.3. Cell pellets were suspended in 4 ml of distilled water (dH2O), and a protease inhibitor cocktail (Sigma Chemical Co.) was added according to the manufacturer's protocol. GBS cells were lysed by the addition of hydrated glass beads (3 ml, 0.1-mm diameter; Biospec Products, Bartlesville, OK) to the suspended cell pellets and mixed with a vortex mixer for 10 min at 4°C in 8-ml glass vials. Cell debris was pelleted by centrifugation (500 × g; 3 min), and the supernatant fluid was collected in Eppendorf tubes and subjected to centrifugation (15,300 × g; 5 min; 4°C). The resulting pellet was dissolved in 3 ml dH2O and again mixed with glass beads and vortexed as described above. Supernatant fluids, containing both cell wall-associated and membrane proteins (GBS proteins), were pelleted by ultracentrifugation (81,446 × g; 4°C; 2 h). Pellets were washed once with 1 ml of dH2O, suspended in 250 μl of dH2O, and transferred to an Eppendorf tube containing 30 μl of wet glass beads, and the suspension was amalgamated for 30 s. The whole mixture was centrifuged for 5 s, and the upper layer was transferred to an Eppendorf tube. Protein measurements were performed with the BCA protein assay kit (Pierce, Rockford, IL), resolved on a 10% Tris-glycine precast polyacrylamide gel, and visualized by staining with colloidal Coomassie brilliant blue.
Reversed-phase microcapillary liquid chromatography electrospray ionization and tandem mass spectrometry and Sequest analysis.
GBS proteins were separated by electrophoresis for only 3 cm of a 10-cm gel. Each gel lane, containing the same amount of protein, was divided into six regions. Each region was excised and digested in gel with trypsin following standard procedures (36). Samples were loaded on a fused-silica capillary column (13-cm length, 75-μm inner diameter) packed with Magic C18 AQ 200 reversed-phase material (Microm Biosources, Auburn, CA). Peptides were separated using a linear gradient elution from 10% mobile phase A (95% H2O, 5% acetonitrile, 0.45% formic acid, 0.005% heptafluorobutyric acid) to 40% mobile phase B (95% acetonitrile, 5% H2O, 0.45% formic acid, 0.005% heptafluorobutyric acid) in 70 min. The eluting peptides were subjected to nanoelectrospray ionization, and their mass was analyzed by use of an LCQ Deca system (Thermo Finnigan, San Jose, CA). Peptide ions meeting certain abundance criteria were automatically selected for sequence analysis by tandem mass spectrometry. Peptide sequences were searched against a GBS type V database (GenBank accession no. AE009948) (42) and assigned by use of the Sequest algorithm (7). Only proteins meeting certain search criteria were considered (two or more unique peptides per protein; each peptide must be fully tryptic and have an Xcorr value of >2 [for charge z equal to 1 or 2] or >3 [for z equal to 3] as well as a dCorr value of >0.08) (31). The peptide hits from all regions of a given gel lane were combined by self-programmed scripts, and a single protein hit list was generated per gel lane. A given protein was considered to be more abundant in one lane than in the other if the number of identified unique peptides differed by a factor of ≥1.8. Prediction of surface proteins was performed with bioinformatic tools as described previously (24).
RNA extraction and labeling.
A 20-ml volume of GBS held at a steady state of growth as described above was harvested from the chemostat vessel, RNAprotect bacterial reagent (40 ml; QIAGEN) was immediately added, and the sample was incubated for 5 min at room temperature. GBS cells were then pelleted by centrifugation and suspended in 1 ml of lysozyme (30 mg/ml) in Tris-EDTA buffer and 2,000 U of mutanolysin, and the mixture was incubated for 15 min at 37°C. RTL buffer (3 ml; QIAGEN) and 30 μl of β-mercaptoethanol were added, and the contents were mixed and stored at −80°C.
Total RNA was extracted from GBS pellets by use of RNeasy spin columns (QIAGEN, Chatsworth, CA). For RNA labeling, 2 μg was reverse transcribed with Superscript II reverse transcriptase (Life Technologies, Paisley, Scotland), random 9-mer primers, and the fluorochromes Cy-3 dCTP and Cy-5 dCTP (Amersham Pharmacia Biotech, Inc.). Cy-3- and Cy-5-labeled cDNAs were copurified on Qia-Quick spin columns (QIAGEN, Chatsworth, CA). The hybridization probe was constituted by a mixture of the differently labeled cDNAs derived from bacteria grown under two different conditions (invasive and noninvasive). Probe hybridization and washings were performed as recommended by the slide supplier (Amersham Pharmacia Biotech, Inc.).
DNA microarrays.
Microarrays were prepared with DNA fragments of 2,099 annotated open reading frames (ORFs) in the GBS 2603V/R genome (98%), as previously described by Tettelin et al. (42). PCR primers were selected from a MULTIFASTA file of the genomic ORFs using Primer 3 software, and locally developed PERL scripts supported the handling of multiple nucleotide sequence sets. The PCR primer pairs (23 to 28 nucleotides long) were selected within the ORF sequences so as to have an average annealing temperature between 58°C and 74°C and produce amplified products of 200 to 400 bp. Amplification reactions were performed on 2603V/R genomic DNA with Gene Amp PCR system 9700 (PE Applied Biosystems, Foster City, CA), using Taq polymerase (ROCHE Diagnostic, Mannheim, Germany). Single-band PCR products were purified with Qia-Quick spin columns (QIAGEN, Chatsworth, CA) and quantified spectrophotometrically and expressed as optical density at 260 nm.
Array printing was performed using a Gen III spotter (Amersham Pharmacia Biotech, Inc.) on type VII aluminum-coated slides (Amersham Pharmacia Biotech, Inc.) according to the manufacturer's protocol. Thirty-seven different eukaryotic and prokaryotic genes were included in the chips as positive and negative controls. To establish the stringency of hybridization conditions, six sequences in the range of 81 to 100% homology to a spiked control RNA were also included as controls.
Image processing.
Slides were scanned with a GIII scanner (Amersham Pharmacia Biotech, Inc.) at a resolution of 10 μm per pixel. In each experiment the two RNA samples were labeled in direct (Cy3-Cy5) and reverse (Cy5-Cy3) labeling reactions to correct for dye-dependent variation of labeling efficiency. The resulting 16-bit images were processed by use of the Autogene program (version 2.5; BioDiscovery, Inc., Los Angeles, CA). For each image, the signal value of each spot was normalized to the median of all spot signals, determined after subtraction of the local background value from the mean pixel intensity of each spot. The spots that gave negative values after background subtraction were arbitrarily assigned the standard deviation value of negative controls. Data resulting from direct and reverse labeling were averaged for each spot. Expression ratios at each time point were obtained by the direct comparison of RNA from GBS obtained from highly invasive conditions (td of 1.8 h and 12% DO2) with RNA from GBS grown either at a td of 11.0 h or with 0% DO2. Data for each time point represent averages from at least two independent experiments.
Recombinant GBS proteins and antisera.
Cloning and expression of GBS proteins and the immunization protocol used to raise antiserum in mice to purified proteins have previously been described in detail (24). Briefly, ORFs coding for selected surface proteins were amplified from GBS type V strain 2603V/R genomic DNA. PCR primers were designed to obtain genes without predicted signal peptide coding sequences. PCR products were introduced into plasmid expression vectors so as to generate recombinant proteins fused with either 6-His or glutathione S-transferase. His-tagged proteins were obtained by cloning in pET21b+ vectors (Novagen), and Escherichia coli BL21(DE3) cells (Novagen) were used as the recipient. Glutathione S-transferase proteins were obtained by using pGEX-NN plasmids and E. coli BL21SI (Novagen) as the recipient.
Purified recombinant GBS proteins were used for intraperitoneal immunization of groups of 6- to 8-week-old CD-1 outbred mice (four mice per group; Charles River Laboratories, Calco, Italy). The proteins (20 μg of each) were administered to mice on days 1 (emulsified in complete Freund's adjuvant), 21, and 35 (in incomplete Freund's adjuvant). Serum from each group of mice was collected on days 0 and 49, and the protein-specific immune response (total immunoglobulin) in pooled serum was measured by enzyme-linked immunosorbent assay. Antisera were chosen for use in inhibition studies based on availability.
Inhibition of GBS adherence.
Five milliliters of GBS held under the invasive-growth condition (td of 1.8 h with 12% DO2) was harvested from the chemostat and adjusted to an optical density at 650 nm of 0.44 and diluted 1:10 with RPMI 1640 to achieve a concentration of 4 × 106 CFU/ml. Diluted GBS (49 μl) was combined with 25 μl of mouse serum (final assay dilution of 1:100) raised to a recombinant protein, and the final volume was adjusted to 2.5 ml with RPMI 1640. After incubation for 1 h at 4°C, 500 μl of this mixture was added to confluent ME-180 cells (4 × 105 cells/well) grown in 24-well plates to achieve a multiplicity of infection (GBS:ME-180 cells) of 0.1:1. Untreated GBS served as the negative control. Infected monolayers were incubated at 37°C for 2 h in a 5% CO2 incubator. Nonadherent GBS was removed by washing the monolayer five times with PBS. Parallel monolayers (four wells in total) were used to measure quantitatively both total (adhered and invaded) associated GBS and invaded GBS. To determine the total number of associated GBS cells (adhered and invaded), ME-180 cells (two wells) were detached from the well by addition of 0.2 ml of trypsin-EDTA and lysed with 0.8 ml of 0.025% Triton X-100. Lysed cells were transferred quantitatively to Eppendorf tubes and vortexed for 1 min, and diluted aliquots were plated on blood agar plates. Plates were incubated at 37°C overnight, and GBS CFU were quantified by standard plate counts. To determine the number of invaded GBS cells, ME-180 cells (two wells) were incubated at 37°C for 2 h with 0.5 ml of RPMI 1640 containing penicillin and gentamicin to kill extracellular GBS (17). Antibiotic-treated ME-180 cells were lysed as described above, and the number of GBS cells was quantified by standard plate counts. The number of adherent GBS cells was calculated by subtracting the number of GBS cells that invaded from the total number of associated GBS cells (adhered and invaded). Two quantitative GBS measurements per each of two wells were determined, and the four values were averaged. Percent inhibition was calculated as follows: 1 − (number of adherent GBS cells treated with test serum/number of adherent GBS cells treated without serum) × 100.
Binding of GBS proteins to ME-180 cells.
Confluent ME-180 cells were nonenzymatically detached from wells with a cell dissociation solution (Sigma), harvested, and suspended in 1% bovine serum albumin (BSA) in PBS (buffer 1) or in the same buffer containing a recombinant GBS protein at different concentrations. The reaction mixture contained approximately 2 × 105 cells in 100 μl and was incubated for 60 min at 4°C. After being washed twice with 500 μl of buffer 1, cells were blocked with 20 μl of 20% normal rabbit serum for 30 min at 4°C and washed again with buffer 1. Cells were then resuspended in 100 μl mouse polyclonal serum against recombinant GBS proteins (diluted 1:500 in 10% fetal calf serum and 1% BSA in PBS) and incubated for 1 h at 4°C, followed by washing with buffer 1 and further incubation in 50 μl R-phycoerythrin-conjugated goat F(ab)2 antibody to mouse immunoglobulin G (diluted 1:100 in 5% fetal calf serum and 1% BSA in RPMI 1640; Jackson ImmunoResearch Laboratories) for 45 min at 4°C. After being washed again in buffer 1, cells were resuspended in 200 μl of PBS and analyzed with a FACScan flow cytometer (Beckton-Dickinson). The delta mean fluorescence intensity between samples incubated with protein and with buffer alone was calculated for each experiment with Cell Quest software.
Statistics.
The significance of GBS invasion was determined by unpaired t testing with the Welch correction. P values of <0.05 were considered significant. These statistical analyses were performed with Instat (version 3.0a; GraphPad, San Diego, CA). Accuracy and statistical significance of the expression ratios were determined by applying the test analysis as described by Grifantini and coworkers (11). Genes whose expression ratios changed ≥1.8-fold and had P values of <0.01 were considered up- or downregulated. Genes with P values of >0.01 were not considered regulated, regardless of their differential expression level. The obtained P value represents the probability of a specific gene to be differentially expressed under the two experimental conditions. The false discovery rates for each regulated gene (see Tables 4 and 5) were calculated according to the procedure described at http://lgsun.grc.nia.nih.gov/ANOVA/glossary.html#fdr. False discovery rate values ranged from 3 × 10−2 to 7.3 × 10−15.
TABLE 4.
Genes (n = 16) for predicted surface-associated proteins upregulated when group B Streptococcus was grown at td of 1.8 h with 12% DO2 compared to td of 1.8 h with 0% DO2a
| Function and TIGR locus | Putative identification | Gene | Fold increase |
|---|---|---|---|
| Biosynthesis of cofactors, prosthetic groups, and carriers | |||
| SAG1744 | Prenyltransferase, UbiA family | 1.9 | |
| Cell envelope formation | |||
| SAG2143 | Membrane protein, putative | 2.2 | |
| Energy metabolism | |||
| SAG1742 | Cytochrome d oxidase, subunit I | cydA | 2.0 |
| Protein fate | |||
| SAG0031 | Peptidase, M23/M37 family | 2.5 | |
| Transcription | |||
| SAG0777 | ATP-dependent RNA helicase, DEAD/DEAH box family | 4.3 | |
| Transport and binding | |||
| SAG0715 | Amino acid ABC transporter, permease protein | 1.8 | |
| SAG0716 | Amino acid ABC transporter, permease protein | 1.9 | |
| SAG0717 | Amino acid ABC transporter, amino acid-binding protein | 2.0 | |
| SAG0769 | Oxalate:formate antiporter | 2.4 | |
| SAG1007 | Iron compound ABC transporter, iron compound-binding protein | 1.9 | |
| SAG1085 | Xanthine permease | pbuX | 3.3 |
| SAG1394 | Iron compound ABC transporter, permease protein | 2.4 | |
| SAG1466 | Glutamine ABC transporter, glutamine-binding protein/permease protein | glnP | 2.5 |
| SAG1739 | ABC transporter, ATP-binding protein CydC | cydC | 1.8 |
| SAG1796 | Amino acid ABC transporter, permease protein | 2.1 | |
| Unknown function | |||
| SAG0776 | YaeC family protein | 1.9 |
Genes (n = 3) whose encoded proteins were also revealed by proteomics are shown in boldface.
TABLE 5.
Genes (n = 33) for predicted surface-associated proteins upregulated when group B Streptococcus was grown at td of 1.8 h with 12% DO compared with td of 11 h with 12% DO2a
| Function or description and TIGR locus | Putative identification | Gene | Fold increase |
|---|---|---|---|
| Cell envelope formation | |||
| SAG0288 | Phospho-N-acetylmuramoyl-pentapeptide transferase | mraY | 2.1 |
| SAG0405 | Protein of unknown function/lipoprotein, putative | 2.0 | |
| SAG0971 | Protein of unknown function/lipoprotein, putative | 1.9 | |
| SAG1410 | Glycosyl transferase, group 1 family protein | 2.2 | |
| SAG1412 | Polysaccharide biosynthesis protein | 1.9 | |
| SAG2143 | Membrane protein, putative | 2.3 | |
| Energy metabolism | |||
| SAG0764 | Phosphoglycerate mutase family protein | 2.7 | |
| SAG0823 | Glyceraldehyde-3-phosphate dehydrogenase, NADP dependent | gapN | 2.0 |
| SAG0859 | ATP synthase Fo, beta subunit | atpF | 2.1 |
| SAG0940 | 6-Phosphofructokinase | pfk | 3.0 |
| SAG0941 | Pyruvate kinase | pyk | 3.2 |
| SAG0959 | l-Lactate dehydrogenase | ldh | 3.0 |
| SAG1768 | Glyceraldehyde 3-phosphate dehydrogenase | gap | 2.0 |
| Conserved hypothetical proteins | |||
| SAG0303 | 1.9 | ||
| SAG0304 | 1.9 | ||
| SAG0785 | 2.1 | ||
| SAG0963 | 2.0 | ||
| SAG1830 | 1.9 | ||
| Protein fate | |||
| SAG0808 | Protease maturation protein, putative | 2.0 | |
| SAG1530 | Peptidyl-prolyl cis-trans isomerase, cyclophilin type | 1.8 | |
| SAG1828 | ATP-dependent Clp protease, ATP-binding subunit | 1.9 | |
| Purines, pyrimidines, nucleosides, and nucleotides | |||
| SAG0079 | Adenylate kinase | adk | 2.0 |
| Transcription | |||
| SAG0777 | ATP-dependent RNA helicase, DEAD/DEAH box family | 4.4 | |
| Transport and binding | |||
| SAG0365 | Xanthine/uracil permease family protein | 3.3 | |
| SAG0715 | Amino acid ABC transporter, permease protein | 1.8 | |
| SAG1085 | Xanthine permease | pbuX | 3.3 |
| SAG1466 | Glutamine ABC transporter, glutamine-binding protein/permease protein | glnP | 2.6 |
| SAG1732 | Glycerol uptake facilitator protein, putative | 2.2 | |
| SAG1796 | Amino acid ABC transporter, permease protein | 2.1 | |
| SAG2149 | Cobalt transport family protein | 1.8 | |
| Unknown function | |||
| SAG0021 | Protease, putative | 1.9 | |
| SAG0780 | Acyltransferase family protein | 2.2 | |
| SAG1939 | Protein of unknown function, TIGR00256 | 2.4 |
Genes (n = 8) whose encoded proteins were also revealed by proteomics are shown in boldface.
RESULTS
Evidence for different protein expression profiles in GBS grown under different conditions.
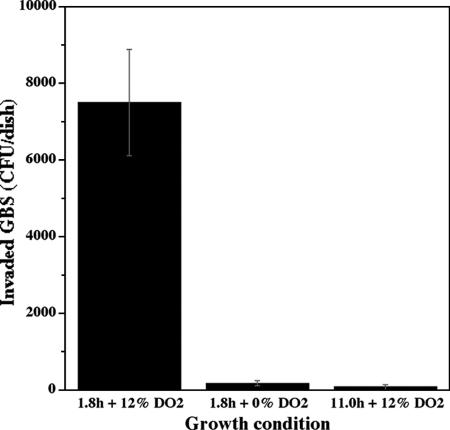
GBS type V strain 2603V/R invaded ME-180 cells in larger numbers when cultured at a high growth rate (td of 1.8 h) with 12% DO2 than at the same growth rate with 0% DO2 (Fig. 1). GBS also invaded poorly when cultured at a lower growth rate (td of 11.0 h) despite a DO2 level of 12% (Fig. 1).
FIG. 1.
Invasion of human cervical epithelial cells by group B Streptococcus type V strain 2603V/R under different growth and dissolved oxygen conditions. Significantly (P = 0.03; two-tailed nonparametric Mann-Whitney test) more GBS cells invaded these epithelial cells when grown at a td of 1.8 h with 12% DO2 than under the other growth conditions tested.
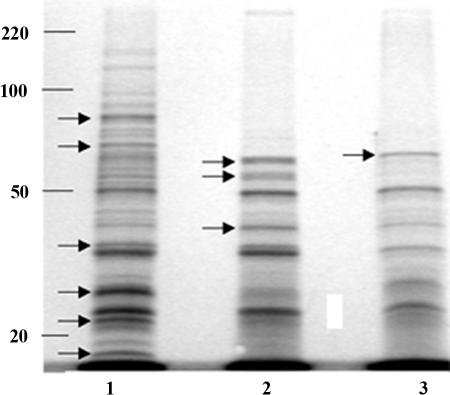
Because of the differences in invasiveness dictated by growth rate, we investigated GBS proteins expressed when cells were held under the different growth conditions. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of GBS cell wall-associated and membrane proteins revealed significant differences in expression depending on the growth condition used (Fig. 2). Several GBS proteins were expressed solely or predominantly under growth conditions conducive to high-level in vitro invasiveness (td of 1.8 h and 12% DO2), and, while the expression of some proteins were influenced by oxygen, others appeared to be influenced by growth rate.
FIG. 2.
Protein profiles of cell wall-associated and membrane proteins from group B Streptococcus type V strain 2603V/R grown in continuous culture at a td of 1.8 h with 12% DO2 (lane 1), a td of 1.8 h with 0% DO2 (lane 2), and a td of 11.0 h with 12% DO2 (lane 3). Molecular weights are shown on the left. Arrows indicate the positions of proteins expressed exclusively or in higher abundance under each set of conditions.
Proteomic analyses.
To identify all type V GBS proteins differentially expressed at each growth condition, we sought an inclusive proteomics approach. Tandem mass spectrometry-based peptide sequencing identified the GBS proteins expressed when cells were held at a steady state of growth at each of the three different growth conditions described above. Among the 130 proteins expressed in increased abundance (Table 1), 78 (60%) were detected exclusively and 52 (40%) were more abundant when GBS was grown at a td of 1.8 h with 12% DO2 (highly invasive condition) than at a td of 1.8 h with 0% DO2 (poorly invasive condition). Among these 130 proteins, 48 were predicted to be surface associated based on bioinformatic analysis; their putative identities are described in Table 2.
TABLE 1.
Comparison of the number and distribution of group B Streptococcus type V cell wall-associated and membrane proteins and genes upregulated under three different sets of growth conditionsa
| Growth conditions | Total no. of proteins detected in abundance | No. of proteins detected exclusively under high-level invasive condition | No. of proteins detected in abundance under high-level invasive condition | No. of genes | No. of proteins and genes in common |
|---|---|---|---|---|---|
| td = 1.8 h, 12% DO2 (vs. td = 1.8 h, 0% DO2) | 130 (48) | 78 (30) | 52 (18) | 43 (16) | 10 (3) |
| td = 1.8 h, 12% DO2 (vs. td = 11 h, 12% DO2) | 158 (48) | 111 (30) | 47 (18) | 149 (33) | 36 (8) |
Numbers were derived based on abundance, two or more peptide match criteria, and genes with expression ratios of ≥1.8-fold. Values in parentheses are numbers of predicted surface-associated proteins and/or genes.
TABLE 2.
Predicted group B Streptococcus surface-associated proteins (n = 48) detected exclusively and in greater abundance under highly invasive conditions (td of 1.8 h and 12% DO2) than under poorly invasive conditions (td of 1.8 h and 0% DO2)a
| Function or description and TIGR locus | Putative identification | Gene | Average Sequest Xcorr value | Ratio of identified peptides |
|---|---|---|---|---|
| Cell envelope formation | ||||
| SAG0159 | Penicillin-binding protein 1B, putative | 3.2 | ||
| SAG0287 | Penicillin-binding protein 2X | pbpX | 3.4 | |
| SAG0757 | Protein of unknown function/lipoprotein, putative | 3.9 | ||
| SAG0765 | Penicillin-binding protein 2b | 3.4 | ||
| SAG1419 | Lipoprotein, putative | 2.9 | ||
| SAG2066 | Penicillin-binding protein 2A | pbp2A | 3.5 | |
| SAG0298 | Penicillin-binding protein 1A | pbp1A | 3.4 | 3.0 |
| SAG0405 | Protein of unknown function/lipoprotein, putative | 3.1 | 7.0 | |
| SAG1173 | CpsC protein | cpsC | 2.3 | 4.0 |
| SAG1787 | DltD protein | dltD | 3.6 | 6.0 |
| Cellular processes | ||||
| SAG0017 | PcsB protein | pscB | 4.1 | |
| SAG0016 | Cell division protein FtsH | ftsH | 3.4 | 2.8 |
| SAG0478 | Cell division protein FtsA | ftsA | 3.5 | 4.0 |
| SAG0479 | Cell division protein FtsZ | ftsZ | 3.4 | 5.0 |
| Energy metabolism | ||||
| SAG0859 | ATP synthase Fo, beta subunit | atpF | 3.4 | |
| SAG0940 | 6-Phosphofructokinase | pfk | 2.7 | |
| SAG0941 | Pyruvate kinase | pyk | 3.5 | 4.5 |
| Fatty acid and phospholipid metabolism | ||||
| SAG0342 | Enoyl-coenzyme A hydratase/isomerase family protein | 3.1 | ||
| Conserved hypothetical proteins | ||||
| SAG0304 | 3.4 | |||
| SAG0455 | 3.0 | |||
| SAG1361 | 4.3 | |||
| SAG1774 | 3.8 | |||
| SAG2068 | 2.9 | |||
| SAG1139 | 3.2 | 3.0 | ||
| SAG1613 | 2.8 | 8.0 | ||
| Regulatory functions | ||||
| SAG0319 | Serine/threonine protein kinase | 3.7 | 2.3 | |
| SAG0624 | Septation ring formation regulator ExrA, putative | 3.5 | 19 | |
| Protein fate | ||||
| SAG1828 | ATP-dependent Clp protease, ATP-binding subunit | 2.7 | ||
| SAG1914 | Membrane-associated zinc metalloprotease, putative | 4.0 | ||
| SAG2174 | Serine protease | 3.5 | ||
| Signal transduction | ||||
| SAG0394 | Sensor histidine kinase | 2.9 | ||
| Transcription | ||||
| SAG0777 | ATP-dependent RNA helicase, DEAD/DEAH box family | 3.3 | ||
| Transport and binding | ||||
| SAG0514 | Cation-transporting ATPase, E1-E2 family | 3.7 | ||
| SAG1108 | Spermidine/putrescine ABC transporter, spermidine/putrescine-binding protein | potD | 3.4 | |
| SAG1257 | Cation-transporting ATPase, E1-E2 family | 3.3 | ||
| SAG1262 | Cation-transporting ATPase, E1-E2 family | 2.6 | ||
| SAG1337 | ABC transporter, ATP-binding/permease protein | 4.2 | ||
| SAG1359 | Permease, putative | 2.7 | ||
| SAG1732 | Glycerol uptake facilitator protein, putative | 4.0 | ||
| SAG1007 | Iron-compound ABC transporter, iron-compound-binding protein | 3.0 | 2.3 | |
| SAG1393 | Iron compound ABC transporter, substrate-binding protein | 3.5 | 3.5 | |
| SAG1466 | Glutamine ABC transporter, glutamine-binding protein/permease | glnP | 3.0 | 5.5 |
| SAG1796 | Amino acid ABC transporter, permease protein | 3.4 | 7.0 | |
| Unknown function | ||||
| SAG0019 | Aminotransferase, class I | 3.2 | ||
| SAG0306 | KH domain protein | 2.9 | ||
| SAG1186 | Metallo-beta-lactamase superfamily protein | 2.5 | ||
| SAG1381 | Sulfatase | 3.1 | 7.0 | |
| SAG1628 | LemA protein | lemA | 2.9 | 5.0 |
Surface-associated proteins (n = 3) whose genes were also revealed by genomics are shown in boldface.
When the effect of growth rate on protein expression was investigated, 111 (70%) of the 158 total proteins were found exclusively when GBS was held at a td of 1.8 h compared with a td of 11.0 h (Table 1); 47 proteins were detected in abundance. Of the 111 GBS proteins that were differentially expressed under highly invasive conditions, 48 were predicted to be surface associated. The putative identities of these proteins are shown in Table 3.
TABLE 3.
Predicted group B Streptococcus surface-associated proteins (n = 48) detected exclusively and in greater abundance under high-level invasive conditions (td = 1.8 h and 12% DO2) than under poorly invasive conditions (td of 11 h and 12% DO2)a
| Function or description and TIGR locus | Putative identification | Gene | Average Sequest Xcorr value | Ratio of identified peptides |
|---|---|---|---|---|
| Cell envelope formation | ||||
| SAG0765 | Penicillin-binding protein 2b | 3.4 | ||
| SAG1419 | Lipoprotein, putative | 2.9 | ||
| SAG1683 | Immunogenic secreted protein, putative | 3.3 | ||
| SAG2066 | Penicillin-binding protein 2A | pbp2A | 3.5 | |
| SAG0159 | Penicillin-binding protein 1B, putative | 3.1 | 8.0 | |
| SAG1173 | CpsC protein | cpsC | 2.3 | 4.0 |
| SAG2131 | Membrane protein, putative | 3.2 | 3.0 | |
| Cellular processes | ||||
| SAG0017 | PcsB protein | pscB | 4.1 | |
| SAG0478 | Cell division protein FtsA | ftsA | 3.5 | |
| SAG0479 | Cell division protein FtsZ | ftsZ | 3.4 | |
| SAG0016 | Cell division protein FtsH | ftsH | 3.4 | 5.5 |
| SAG0508 | Beta-lactam resistance factor | fibB | 3.0 | 4.0 |
| Energy metabolism | ||||
| SAG0859 | ATP synthase Fo, beta subunit | atpF | 3.4 | |
| SAG0878 | Acetoin dehydrogenase, thymine PPi dependent, E1 component, alpha subunit | 2.8 | ||
| SAG0940 | 6-Phosphofructokinase | pfk | 2.7 | |
| SAG0959 | l-Lactate dehydrogenase | ldh | 3.8 | |
| SAG1121 | Polysaccharide deacetylase family protein | 2.2 | ||
| SAG0881 | Acetoin dehydrogenase, thymine PPi dependent, E3 component, dihydrolipoamide dehydrogenase | 3.6 | 7.2 | |
| SAG0941 | Pyruvate kinase | pyk | 3.5 | 9.0 |
| Conserved hypothetical proteins | ||||
| SAG0455 | 3.0 | |||
| SAG1774 | 3.8 | |||
| SAG1613 | 2.8 | 8.0 | ||
| Protein fate | ||||
| SAG1828 | ATP-dependent Clp protease, ATP-binding subunit | 2.7 | ||
| SAG1914 | Membrane-associated zinc metalloprotease, putative | 4.0 | ||
| SAG2174 | Serine protease | 3.5 | 4.5 | |
| Regulatory functions | ||||
| SAG0624 | Septation ring formation regulator EzrA, putative | 3.5 | ||
| SAG0319 | Serine/threonine protein kinase | 3.7 | 7.0 | |
| Signal transduction | ||||
| SAG0394 | Sensor histidine kinase | 2.9 | ||
| Transcription | ||||
| SAG0777 | ATP-dependent RNA helicase, DEAD/DEAH box family | 3.3 | ||
| Transport and binding | ||||
| SAG0151 | Oligopeptide ABC transporter, ATP-binding protein | 3.5 | ||
| SAG0290 | ABC transporter substrate-binding protein | 3.1 | ||
| SAG1108 | Spermidine/putrescine ABC transporter, spermidine/putrescine-binding protein | potD | 3.4 | |
| SAG1257 | Cation-transporting ATPase, E1-E2 family | 3.3 | ||
| SAG1262 | Cation-transporting ATPase, E1-E2 family | 2.6 | ||
| SAG1359 | Permease, putative | 2.7 | ||
| SAG0514 | Cation-transporting ATPase, E1-E2 family | 3.7 | 19.0 | |
| SAG1007 | Iron compound ABC transporter, iron compound-binding protein | 3.0 | 2.3 | |
| SAG1441 | Maltose/maltodextrin ABC transporter, maltose/maltodextrin-binding protein | 3.8 | 3.0 | |
| SAG1466 | Glutamine ABC transporter, glutamine-binding protein/permease protein | glnP | 3.0 | 2.2 |
| SAG1610 | Amino acid ABC transporter, substrate-binding protein | 3.0 | 3.7 | |
| SAG1796 | Amino acid ABC transporter, permease protein | 3.4 | 2.8 | |
| Unknown function | ||||
| SAG0019 | Aminotransferase, class I | 3.2 | ||
| SAG0306 | KH domain protein | 2.9 | ||
| SAG0886 | Protein of unknown function | 3.1 | ||
| SAG1186 | Metallo-beta-lactamase superfamily protein | 2.5 | ||
| SAG1350 | Surface antigen-related protein | 2.9 | ||
| SAG1126 | Protein of unknown function | 3.5 | 3.5 | |
| SAG1381 | Sulfatase | 3.1 | 2.3 |
Surface-associated proteins (n = 8) whose genes were also revealed by genomics are shown in boldface.
Transcriptional analyses.
We also sought to determine which of the GBS ORFs were regulated at the transcriptional level in highly invasive growth conditions. Microarray analysis showed that 43 genes were upregulated (Table 1) by GBS when grown at a td of 1.8 h with 12% DO2 (highly invasive condition) compared with genes expressed at a td of 1.8 h with 0% DO2 (poorly invasive condition). Of these 43 genes, 16 were predicted to encode surface-associated proteins whose putative identities are described in Table 4. A more pronounced number of genes were upregulated when growth rate was varied; 149 genes were upregulated by GBS when held under the invasive growth condition, 33 of which were predicted to encode for surface-associated proteins (Table 5). Many of these genes encoded proteins involved not only in transport and binding functions but also in cell envelope synthesis and energy metabolism.
Proteins and genes regulated under the highly invasive growth condition.
Two variables, growth rate and oxygen, regulated the ability of GBS to invade human cells in vitro. Combined genomic and proteomic analyses revealed a total of 10 genes and proteins expressed exclusively or in abundance under the highly invasive growth condition. Of these 10, 3 were predicted to be surface-associated proteins whose putative identities are shown in Tables 2 and 4. Thirty-six genes and proteins (Table 1) were expressed only under the high-level invasive growth condition. Of these 36, 8 were predicted to be surface-associated proteins whose putative identities are shown in Tables 3 and 5.
Inhibition of GBS adherence to ME-180 cells by antiserum specific to selected recombinant GBS proteins.
An earlier investigation of GBS antigens with vaccine potential led to the cloning and purification of 312 surface-associated proteins with antisera subsequently raised in mice to all of these proteins (24). Because some of these proteins were also identified in the present study, specific antisera were used to further investigate their role in GBS pathogenesis. Antisera to 24 GBS proteins were tested for their ability to block adherence of chemostat-grown (td of 1.8 h with 12% DO2) GBS to ME-180 cells. Seven of 24 antisera tested inhibited adherence by ≥57%, with 5 antisera abrogating adherence by >85% (Table 6). These protein targets, which include a surface antigen-related protein (SAG1350), a putative lipoprotein (SAG0971), penicillin-binding protein 2b (SAG0765), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SAG0823), and an iron-binding protein (SAG1007), were expressed by GBS exclusively or in greater abundance under the invasive growth conditions regulated either by growth rate or by oxygen level (Table 6). The positive control, antiserum to recombinant Lmb, a known GBS adhesin (37), inhibited >90% of binding of GBS to ME-180 cells.
TABLE 6.
Inhibition of group B Streptococcus type V adherence to ME-180 cells by antiserum to selected surface-associated proteins and the regulation of expression of these proteins
| TIGR locus | Ratio chip | Average Xcorr value | Annotation | Gene | Inhibition of adherence ± SD (%) | Prediction assay, regulationa |
|---|---|---|---|---|---|---|
| SAG1350 | NAb | 2.9 | Surface antigen-related protein | 96.3 ± 1.4 | P, high growth rate | |
| SAG0971 | 1.9 | NA | Lipoprotein of unknown function/lipoprotein, putative | 93.5 ± 1.8 | G, high growth rate | |
| SAG0765 | 1.7 | 3.4 | Penicillin-binding protein 2b | 90.4 ± 2.0 | G, high growth rate; P, high growth rate and oxygen | |
| SAG0823 | 2.0 | NA | Glyceraldehyde-3-phosphate dehydrogenase, NADP dependent | gapN | 89.6 ± 1.0 | G, high growth rate |
| SAG1007 | 2.0 | 3.0 | Iron compound ABC transporter, iron compound-binding protein | 85.5 ± 4.2 | G, oxygen; P, high growth rate and oxygen | |
| SAG0017 | NA | 4.1 | PcsB protein | pscB | 66.5 ± 8.0 | P, high growth rate and oxygen |
| SAG1466 | 2.3 | 3.0 | Glutamine ABC transporter, glutamine-binding protein/permease protein | glnP | 57.3 ± 9.6 | G, high growth rate and oxygen; P, high growth rate and oxygen |
| SAG1938 | NA | NA | Positive control, LRA family, Lmb (59%) | 92.0 ± 1.0 |
G, genomics; P, proteomics; high growth rate, td = 1.8 h; oxygen, 12% DO2.
NA, not applicable.
Binding of recombinant GBS proteins to ME-180 cells.
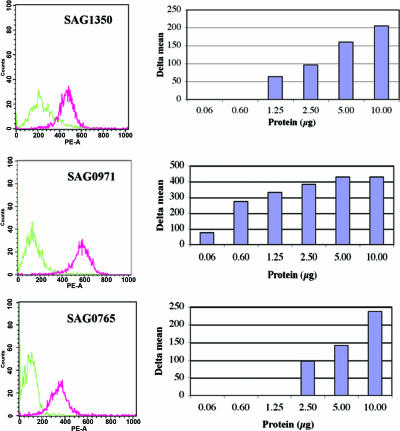
We sought to determine whether the recombinant GBS proteins identified by the inhibition of adherence study bound directly to ME-180 cervical epithelial cells. Surface antigen-related protein (SAG1350), a putative lipoprotein (SAG0971), penicillin-binding protein 2b (SAG0765), and glyceraldehyde-3-phosphate dehydrogenase (SAG0823) were chosen because antisera to these proteins inhibited ≥85% of binding of GBS to ME-180 cells. Recombinant surface antigen-related protein (SAG1350), putative lipoprotein (SAG0971), and penicillin-binding protein 2b (SAG0765) bound to ME-180 cells in a dose-dependent manner (Fig. 3). On the contrary, recombinant glyceraldehyde-3-phosphate dehydrogenase (SAG0823) did not bind to ME-180 cells at any concentration tested despite good inhibition of GBS binding to ME-180 cells by specific antisera to this protein (data not shown).
FIG. 3.
Dose-dependent binding of purified recombinant group B Streptococcus proteins to ME-180 cervical epithelial cells. Fluorescence-activated cell sorting analyses were performed with the use of immune (pink) and preimmunization (green) mouse sera raised to each protein. (Left) Fluorescence intensity values plotted against numbers of cells, obtained with 20 μg of surface antigen-related protein (SAG1350), 10 μg of a putative lipoprotein (SAG0971), and 10 μg of penicillin-binding protein 2b (SAG0765). (Right) Delta mean values between immune and preimmunization sera measured at different protein concentratfions.
DISCUSSION
This is the first study to examine the pathogenicity of S. agalactiae using a combination of DIVAS technology (25) with newly available genomic information (42)-based microarray and proteomics to reveal a molecular portrait of GBS when it is held at a constant growth rate in a highly invasive state.
The ability of GBS type V strain 2603V/R to invade cervical epithelial cells was regulated by growth rate and oxygen, with the highest-level invasion measured when growth was rapid and oxygen was not limiting. This pattern of regulation, also observed with type III strains (17), may be common among GBS independent of strain or serotype. Invasiveness was associated with the appearance of several cell wall-associated and membrane proteins detected exclusively or in larger amounts, making them potentially important in initial attachment and/or invasion events. Separation of GBS proteins by electrophoresis in a short gel, followed by digestion and peptide sequencing through microcapillary liquid chromatography electrospray ionization and tandem mass spectrometry, enabled the identification of several hundred proteins under each growth condition. In addition to identification, a rough estimate of relative abundances has been carried out, based on the number of identified peptides. More-elaborate abundance measurements using isotope-labeling techniques that reveal more-precise and more-subtle differences are planned for subsequent studies (20). Parallel microarray analysis revealed differential gene expression between highly invasive and poorly invasive conditions. Abundance measurements by genomic and proteomic techniques did not result in identical lists of upregulated genes/proteins; instead, only a fraction of genes/proteins have been identified by both techniques (Table 1). Some experimental limitations may contribute to this finding, e.g., some of the upregulated gene products may not be detected by the proteomics approach at all. Sample preparation was carried out in different experiments for the three growth conditions following the same protocol. Slight, unintentional variations in sample treatment leading to a failure to detect single peptides cannot be excluded. On the other hand, a limited correlation between RNA and protein expression has been observed in various studies using different experimental approaches (2, 10, 13, 16, 23, 28, 43). It therefore seems that a limited correlation of gene and protein level (monitored at one given time point) is a common biological phenomenon, rather than a sole experimental artifact. The key biological factors responsible for the deviations are considered to be translational regulation and differences in protein half-lives in vivo (2, 10). The data set presented here serves to highlight the most pronounced differences in upregulated protein expression. In addition, use of a bioassay (inhibition of GBS binding to ME-180 cells by specific antibody) further reduced the rather large number of proteins potentially involved in GBS interaction with epithelial cells to seven. Of these seven proteins, three bound directly to ME-180 cells, suggesting a role for these proteins in GBS attachment.
A family of PBPs (SAG0159, SAG0287, SAG0765, and SAG2066) were expressed exclusively by GBS under the invasive growth condition. PBPs have been proposed as virulence factors in several gram-positive bacteria (9, 21, 26). More importantly, Pbp1a has been shown to be critical for GBS pathogenesis in a neonatal rat model of infection and thus is an important virulence factor (19). The fact that GBS Pbp2b binds to ME-180 cells and that antibody to it inhibits GBS attachment to these cervical epithelial cells is novel and suggests that it is a new virulence factor. Its expression was revealed by both proteomics and microarray analysis.
A putative lipoprotein revealed by genomics (SAG0971) was upregulated in GBS in response to increased growth rate. Sutcliffe and Harrington (39) previously identified this protein by searching the GBS genomes with a taxon-specific G+LLP pattern for bacterial lipoproteins (38). In the present study, recombinant SAG0971 bound to epithelial cells in vitro, a result not inconsistent with the role of other bacterial lipoproteins as adhesins, including Lmb in GBS (37).
SAG1350, which was annotated only as a surface antigen-related protein, was upregulated when GBS was in the rapid growth stage and its expression was not regulated by the level of available oxygen. Full characterization of SAG1350 is currently under way.
Until recently, the mechanism for acquisition of iron, which is an essential nutrient for most living organisms, was not defined for GBS. Clancy et al. (4) have now reported a siderophore (hydroxamate)-mediated iron transport system in GBS characterized by a FhuD siderophore receptor localized to the cell membrane. A polycistronic operon of four genes (fhuCDBG) putatively encodes an ATPase, cell surface receptor, and two transmembrane proteins, respectively (4). It is tempting to speculate that the iron compound ABC transporter-binding protein (SAG1007), which is regulated by both growth rate and oxygen levels and revealed by both proteomics and genomics in our study, is also involved with iron uptake in a manner similar to that described by Clancy and coworkers.
Glutamine has a central role in amino acid biosynthesis. Tamura and coworkers (40) demonstrated that GBS mutant strain COH1-GT1 with a Tn917 insertion in glnQ, a glutamine transport gene, showed less adherence to fibronectin and less adherence to and invasion of respiratory epithelial cells in vitro and was less virulent in rat pups than the parent strain. They also showed that glnQ and glnP (encoding a putative glutamine permease) are cotranscribed. Interestingly, glnQ has been identified as a virulence gene in Streptococcus pneumoniae in two different animal models (21, 32). Analysis of our data revealed both growth rate- and oxygen-regulated expression of a glutamine ABC tranporter/binding/permease protein (SAG1466, encoded by glnP). Transcriptional profiling revealed two- to threefold-higher mRNA levels of both glnP and glnQ in GBS under the highly invasive conditions than under the less invasive growth conditions.
Microarray analysis also revealed a 1.9-fold increase in the two genes encoding GAPDH (SAG0823 and SAG1768) when GBS was cultured at the high as opposed to the low growth rate. GAPDH is a major outer surface protein of group A Streptococcus, is a ligand for many mammalian proteins, and has ADP-ribosylating activity (29). Staphylococcal GAPDH binds both plasmin and transferrin, properties thought to be important for the ability of staphylococci to penetrate host tissues (27). Although GAPDH has been shown to be surface associated in GBS (15), its function has not been defined.
Whether the newly identified GBS genes or proteins described herein do indeed play a role in pathogenesis awaits further experimentation, namely by creating isogenic mutants and testing whether adherence, invasion, or virulence is altered compared with the parent strain.
Only a few GBS proteins, including ScpB, a C5a peptidase that binds to immobilized fibronectin (1); Lmb, a homologue of the Lra1 adhesin proteins with specificity to human laminin (37); and FbsA, a fibrinogen-binding protein (35), have demonstrated specificities for binding to host epithelial surface components. Some proteins revealed in this study, e.g., iron-binding protein (SAG1007) or glutamine-binding protein (SAG1466), intuitively would be expected to have a role in metabolism as opposed to adherence. Perhaps, however, some proteins have dual functions depending on the growth environment and types of nutrients encountered. For example, Yamamoto et al. (45, 46) previously located a gene, cydA, that encodes a subunit of cytochrome bd oxidase required for respiratory metabolism and showed enhanced oxygen consumption by GBS when the aerobic growth environment included heme and menaquinone. Interestingly, GBS lacking cydA was highly attenuated in virulence (45), suggesting that this protein, putatively part of an electron transport chain, also has a key role for GBS survival in the host. Although genes for cytochrome bd (cyd family) exist in GBS, spectral and/or protein evidence has not been achieved, perhaps due to inadequate growth conditions to optimally express cytochromes or inadequate preparation of the membrane fractions.
Striking among the genes that were downregulated when GBS was held under the invasive growth condition were virtually all genes required for hemolysin expression, including cylE (data not shown). Hemolysin has been shown to be required for GBS invasion of lung epithelial cells in vitro (5) and is also critical to the development of GBS pneumonia in a neonatal rabbit model of pulmonary infection (14). The association between high-level invasion and the downregulation of this particular operon is surprising and warrants further investigation.
The fact that apparently healthy neonates can succumb to GBS disease within 24 h of acquiring the pathogen during birth from a colonized mother suggests a high rate of growth and efficient dissemination. While exact in vivo bacterial growth environments are difficult if not impossible to duplicate in vitro, certain host niches can support rapid GBS growth. For example, GBS proliferation in human amniotic fluid in vitro was previously shown to be rapid (6), with a calculated cell generation time of 16 min during exponential growth. A td of 0.8 h has been achieved by GBS in continuous culture with a chemically defined medium (30), and potentially faster tds are theoretically achievable with complex media. Thus, the td of 1.8 h used in this study is likely to mimic in vivo growth in specific host environments and is well within the range of domain 1 growth rates (3).
In summary, the physiopathology of GBS infections suggests that this opportunistic pathogen can adapt to various environmental factors, including pH, osmolarity, temperature, and the presence of nutrients such as oxygen. DIVAS was developed to control environmental and nutritional variables during growth and to explore the unique impact of these variables on bacterial pathogenesis. The use of controlled growth and nutrient conditions, coupled with genomic and proteomic techniques, revealed novel GBS genes and proteins that may have important roles in transition of GBS from a harmless commensal to a frank pathogen.
Acknowledgments
This work was supported by NIH contract AI-25495 and grant AI-053191. We thank Vilas Patwardhan for technical assistance.
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsements by the U.S. government.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyer, A., J. Hollunder, H. P. Nasheuer, and T. Wilhelm. 2004. Post-transcriptional expression regulation in the yeast Saccharomyces cerevisiae on a genomic scale. Mol. Cell Proteomics 3:1083-1092. [DOI] [PubMed] [Google Scholar]
- 3.Chesbro, W., M. Arbige, and R. Eifert. 1990. When nutrient limitation places bacteria in the domains of slow growth: metabolic, morphologic, and cell cycle behavior. FEMS Microbiol. Ecol. 74:103-120. [Google Scholar]
- 4.Clancy, A., J. W. Loar, C. D. Speziali, D. E. Heinrichs, and C. E. Rubens. 2006. Evidence for siderophore-dependent iron acquisition in group B Streptococcus. Mol. Microbiol. 59:707-721. [DOI] [PubMed] [Google Scholar]
- 5.Doran, K. S., J. C. Chang, V. M. Benoit, L. Eckmann, and V. Nizet. 2002. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J. Infect. Dis. 185:196-203. [DOI] [PubMed] [Google Scholar]
- 6.Eidelman, A. I., A. Nevet, B. Rudensky, R. Rabinowitz, C. Hammerman, D. Raveh, and M. S. Schimmel. 2002. The effect of meconium staining of amniotic fluid on the growth of Escherichia coli and group B Streptococcus. J. Perinatol. 22:467-471. [DOI] [PubMed] [Google Scholar]
- 7.Eng, J., A. McCormick, and J. R. Yates. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 8.Glaser, P., C. Rusniok, C. Buchrieser, F. Chevalier, L. Frangeul, T. Msadek, M. Zouine, E. Couve, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499-1513. [DOI] [PubMed] [Google Scholar]
- 9.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenbaum, D., C. Colangelo, K. Williams, and M. Gerstein. 2003. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grifantini, R., S. Sebastian, E. Frigimelica, M. Draghi, E. Bartolini, A. Muzzi, R. Rappuoli, G. Grandi, and C. A. Genco. 2003. Identification of iron-activated and -repressed Fur-dependent genes by transcriptome analysis of Neisseria meningitidis group B. Proc. Natl. Acad. Sci. USA 100:9542-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2003. Analysis of RogB-controlled virulence mechanisms and gene repression in Streptococcus agalactiae. Infect. Immun. 71:5056-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gygi, S. P., Y. Rochon, B. R. Franza, and R. Aebersold. 1999. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 19:1720-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensler, M. E., G. Y. Liu, S. Sobczak, K. Benirschke, V. Nizet, and G. P. Heldt. 2005. Virulence role of group B Streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J. Infect. Dis. 191:1287-1291. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, M. J., J. C. Moore, J. D. Lane, R. Wilson, P. K. Pribul, Z. N. Younes, R. J. Dobson, P. Everest, A. J. Reason, J. M. Redfern, F. M. Greer, T. Paxton, M. Panico, H. R. Morris, R. G. Feldman, and J. D. Santangelo. 2002. Identification of major outer surface proteins of Streptococcus agalactiae. Infect. Immun. 70:1254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ideker, T., V. Thorsson, J. A. Ranish, R. Christmas, J. Buhler, J. K. Eng, R. Bumgarner, D. R. Goodlett, R. Aebersold, and L. Hood. 2001. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292:929-934. [DOI] [PubMed] [Google Scholar]
- 17.Johri, A. K., J. Padilla, G. Malin, and L. C. Paoletti. 2003. Oxygen regulates invasiveness and virulence of group B Streptococcus. Infect. Immun. 17:6707-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, A. L., K. M. Knoll, and C. E. Rubens. 2000. Identification of Streptococcus agalactiae virulence genes in the neonatal rat sepsis model using signature-tagged mutagenesis. Mol. Microbiol. 37:1444-1455. [DOI] [PubMed] [Google Scholar]
- 19.Jones, A. L., R. H. Needham, A. Clancy, K. M. Knoll, and C. E. Rubens. 2003. Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol. Microbiol. 47:247-256. [DOI] [PubMed] [Google Scholar]
- 20.Julka, S., and F. Regnier. 2004. Quantification in proteomics through stable isotope coding: a review. J. Proteome Res. 3:350-363. [DOI] [PubMed] [Google Scholar]
- 21.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555-571. [DOI] [PubMed] [Google Scholar]
- 22.Lauer, P., C. D. Rinaudo, M. Soriani, I. Margarit, D. Maione, R. Rosini, A. R. Taddei, M. Mora, R. Rappuoli, G. Grandi, and J. L. Telford. 2005. Genome analysis reveals pili in group B Streptococcus. Science 309:105. [DOI] [PubMed] [Google Scholar]
- 23.Li, S., and S. T. Carmichael. 2006. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol. Dis. 23:362-373. [DOI] [PubMed] [Google Scholar]
- 24.Maione, D., I. Margarit, C. D. Rinaudo, V. Masignani, M. Mora, M. Scarselli, H. Tettelin, C. Brettoni, E. T. Iacobini, R. Rosini, N. D'Agostino, L. Miorin, S. Buccato, M. Mariani, G. Galli, R. Nogarotto, V. N. Dei, F. Vegni, C. Fraser, G. Mancuso, G. Teti, L. C. Madoff, L. C. Paoletti, R. Rappuoli, D. L. Kasper, J. L. Telford, and G. Grandi. 2005. Identification of a universal group B Streptococcus vaccine by multiple genome screen. Science 309:148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malin, G., and L. C. Paoletti. 2001. Use of a dynamic in vitro attachment and invasion system (DIVAS) to determine influence of growth rate on invasion of respiratory epithelial cells by group B Streptococcus. Proc. Natl. Acad. Sci. USA 98:13335-13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 27.Modun, B., and P. Williams. 1999. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect. Immun. 67:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie, L., G. Wu, and W. Zhang. 2006. Correlation between mRNA and protein abundance in Desulfovibrio vulgaris: a multiple regression to identify sources of variations. Biochem. Biophys. Res. Commun. 339:603-610. [DOI] [PubMed] [Google Scholar]
- 29.Pancholi, V., and V. A. Fischetti. 1993. Glyceraldehyde-3-phosphate dehydrogenase on the surface of group A streptococci is also an ADP-ribosylating enzyme. Proc. Natl. Acad. Sci. USA 90:8154-8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paoletti, L. C., R. A. Ross, and K. D. Johnson. 1996. Cell growth rate regulates expression of group B Streptococcus type III capsular polysaccharide. Infect. Immun. 64:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng, J., J. E. Elias, C. C. Thoreen, L. J. Licklider, and S. P. Gygi. 2003. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2:43-50. [DOI] [PubMed] [Google Scholar]
- 32.Polissi, A., A. Pontiggia, G. Feger, M. Altieri, H. Mottl, L. Ferrari, and D. Simon. 1998. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect. Immun. 66:5620-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 69:5098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rench, M. A., and C. J. Baker. 1993. Neonatal sepsis caused by a new group B streptococcal serotype. J. Pediatr. 122:638-640. [DOI] [PubMed] [Google Scholar]
- 35.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557-569. [DOI] [PubMed] [Google Scholar]
- 36.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 37.Spellerberg, B., E. Rozdzinski, S. Martin, J. Weber-Heynemann, N. Schnitzler, R. Lutticken, and A. Podbielski. 1999. Lmb, a protein with similarities to the LraI adhesin family, mediates attachment of Streptococcus agalactiae to human laminin. Infect. Immun. 67:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutcliffe, I. C., and D. J. Harrington. 2002. Pattern searches for the identification of putative lipoprotein genes in gram-positive bacterial genomes. Microbiology 148:2065-2077. [DOI] [PubMed] [Google Scholar]
- 39.Sutcliffe, I. C., and D. J. Harrington. 2004. Putative lipoproteins of Streptococcus agalactiae identified by bioinformatic genome analysis. Antonie Leeuwenhoek 85:305-315. [DOI] [PubMed] [Google Scholar]
- 40.Tamura, G. S., A. Nittayajarn, and D. L. Schoentag. 2002. A glutamine transport gene, glnQ, is required for fibronectin adherence and virulence of group B streptococci. Infect. Immun. 70:2877-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tettelin, H., V. Masignani, M. J. Cieslewicz, C. Donati, D. Medini, N. L. Ward, S. V. Angiuoli, J. Crabtree, A. L. Jones, A. S. Durkin, R. T. Deboy, T. M. Davidsen, M. Mora, M. Scarselli, I. Margarit y Ros, J. D. Peterson, C. R. Hauser, J. P. Sundaram, W. C. Nelson, R. Madupu, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, S. A. Sullivan, S. C. Daugherty, D. H. Haft, J. Selengut, M. L. Gwinn, L. Zhou, N. Zafar, H. Khouri, D. Radune, G. Dimitrov, K. Watkins, K. J. O'Connor, S. Smith, T. R. Utterback, O. White, C. E. Rubens, G. Grandi, L. C. Madoff, D. L. Kasper, J. L. Telford, M. R. Wessels, R. Rappuoli, and C. M. Fraser. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome.” Proc. Natl. Acad. Sci. USA 102:13950-13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 99:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washburn, M. P., A. Koller, G. Oshiro, R. R. Ulaszek, D. Plouffe, C. Deciu, E. Winzeler, and J. R. Yates III. 2003. Protein pathway and complex clustering of correlated mRNA and protein expression analyses in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 100:3107-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reference deleted.
- 45.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2005. Respiration metabolism of group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol. Microbiol. 56:525-534. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2006. Roles of environmental heme, and menaquinone, in streptococcus agalactiae. Biometals 19:205-210. [DOI] [PubMed] [Google Scholar]
- 47.Zaleznik, D. F., M. A. Rench, S. Hillier, M. A. Krohn, R. Platt, M. L. Lee, A. E. Flores, P. Ferrieri, and C. J. Baker. 2000. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30:276-281. [DOI] [PubMed] [Google Scholar]