Abstract
A successful vaccine against Plasmodium vivax malaria would significantly improve the health and quality of the lives of more than 1 billion people around the world. A subunit vaccine is the only option in the absence of long-term culture of P. vivax parasites. The circumsporozoite protein that covers the surface of Plasmodium sporozoites is one of the best-studied malarial antigens and the most promising vaccine in clinical trials. We report here the development of a novel “immunologically optimal” recombinant vaccine expressed in Escherichia coli that encodes a chimeric CS protein encompassing repeats from the two major alleles, VK210 and VK247. This molecule is widely recognized by sera from patients naturally exposed to P. vivax infection and induces a highly potent immune response in genetically disparate strains of mice. Antibodies from immunized animals recognize both VK210 and VK247 sporozoites. Furthermore, these antibodies appear to be protective in nature since they cause the agglutination of live sporozoites, an in vitro surrogate of sporozoite infectivity. These results strongly suggest that recombinant CS is biologically active and highly immunogenic across major histocompatibility complex strains and raises the prospect that in humans this vaccine may induce protective immune responses.
Outside of sub-Saharan Africa, Plasmodium vivax is the most prevalent of all human malarias. In addition to being present in tropical and subtropical regions, the ability of the parasite to complete its mosquito cycle at temperatures as low as 15°C has also allowed it to be spread in temperate climates. A unique feature of P. vivax is that some strains are capable of causing delayed infection by remaining latent for several months in the liver before emerging into the circulation to manifest clinical symptoms. Such individuals have been known to maintain transmission of malaria in areas where it is no longer naturally transmitted (41). Although P. vivax is usually not fatal, it is responsible for ca. 50% of all malaria cases worldwide (20). The large number of clinical cases and the severe morbidity this type of malaria causes contributes to a serious economic impact in developing countries. Recently, reports of severe forms of malaria caused by P. vivax infection have begun to appear (42). However, due to the fact that the disease caused by P. vivax is less lethal than P. falciparum, investments made to develop a vaccine against this parasite are lagging far behind. There is a need for concerted efforts toward developing vaccines to control the global transmission of P. vivax infections.
Malaria parasites, while developing within hepatocytes, do not cause clinical illness and therefore are ideal targets for designing vaccines to protect children and malaria-naive adults against infection. Immunization with irradiation-attenuated malaria sporozoites has long been shown to induce protection against experimental sporozoite challenge in animal models and in humans (13, 25), and currently efforts are ongoing to develop good manufacturing practices methods to produce sufficient quantities of P. falciparum sporozoites for large-scale vaccination with irradiation-attenuated (28), as well as genetically attenuated vaccines (18, 35). A sporozoite-based vaccine is not an option for P. vivax, which is as yet not amenable to in vitro culturing. Thus, a subunit-based approach remains the only suitable approach for vaccines against vivax malaria.
The circumsporozoite (CS) protein present on the sporozoites of all Plasmodium is the most abundant sporozoite protein. CS protein is involved in the motility and invasion of the sporozoite during its passage from the site of inoculation into circulation, from where it migrates to the liver and enters the hepatocyte (27, 34). Recombinant and synthetic CS constructs were the first prototype vaccines developed and tested for malaria. Although less efficacious in humans, such vaccines have been shown to induce high levels of protection in animal models (40, 43). The most advanced malaria vaccine for humans, RTS,S, is based on the CS protein of P. falciparum (21). In several clinical trials, the RTS,S vaccine has been shown to confer 40 to 60% protection for a short duration, and vaccination with RTS,S has shown a beneficial effect against both clinical uncomplicated malaria and severe malaria in children from Mozambique, Africa (1).
A limited number of attempts have been made to develop a CS protein-based vaccine for P. vivax. Vaccination studies with a recombinant P. vivax CS protein expressed in yeast (6) in the late 1980s induced a limited degree of immunity in monkeys (15) and very poor immune responses in humans (24). Subsequently, due to limitations in immunogenicity and difficulties in production, synthetic peptide-based vaccines were developed either as multiple antigenic peptides (MAPs) or as linear peptides and tested in nonhuman primates (23, 49) and humans (22).
We designed a novel synthetic “immunologically optimal” chimeric, codon-modulated CS gene construct that incorporates the major domains of the CS protein but is distinct from the native molecule. This synthetic CS construct includes the N- and C-terminal parts of the CS protein and a truncated repeat region that contains repeat sequences from both the VK210 (type 1) and the VK247 (type 2) parasites. The type 1 amino acid repeat sequence from a South Korean isolate was used to encompass the amino acid heterogeneity found within the VK210 repeat motif. In order to make a vaccine for global use, we also included a single copy of the VK247 repeat and a 12-amino-acid insert that is present in some field isolates in the synthetic protein. To optimize expression levels and produce an immunogen that closely mimics its native form, the nucleotide sequence of the CS gene was synthetically constructed based on Escherichia coli codons. This hybrid molecule has been designated vivax malaria protein 1 (VMP 001) as part of the ongoing malaria vaccine development program of the Walter Reed Army Institute of Research.
We present data here on the synthetic design, construction, expression, process development, biological characterization, and immunogenicity of VMP 001. A high-level expression of recombinant protein with >95% purity and low endotoxin levels has been achieved. Recombinant VPM 001 formulated in Montanide ISA adjuvant induced high levels of humoral immune responses in all strains of inbred and one outbred strain of mice tested. Fine-specificity analysis demonstrates that VMP 001 induced high levels of immune response against both type 1 and type 2 CS repeats and against the 12-amino-acid insert. In addition, we were able to detect antibodies to the Ala-Gly-Asp-Arg (AGDR) sequence, an epitope recognized by a protective monoclonal antibody. Anti-VMP 001 antibodies recognized both VK210 and VK247 sporozoites and were able to agglutinate live sporozoites. VMP 001 was also recognized by sera from acutely infected individuals from a region where malaria is endemic.
MATERIALS AND METHODS
Design and construction of chimeric CS.
The synthetic CS gene construct encoding for VMP 001 was made based on the amino acid sequence of a Korean isolate of P. vivax isolated from an individual that contracted malaria after returning from the Republic of Korea. The synthetic CS gene designed in the present study started immediately after the signal sequence, with amino acids THCGH, and was identical to the Korean isolate through region I (KLKQP). The wild-type Korean isolates have 20 repeats, only two of which are identical to the classical (VK210) GDRA[A/D]GQPA, sequence. The remaining repeats are minor variants of this classical repeat. The synthetic construct was made to represent at least one copy of each of the repeats present in the wild-type isolates. The two “classical” repeats were represented twice. In addition, a single copy of the classical VK247 repeat, ANGAGNGPG, was included in the construct. This was followed by the C-terminal region and ended at amino acids TDVCT, just before the anchor region.
The codon usage frequencies for E. coli and P. vivax were calculated by using the information available from the online database at www.kazusa.or.jp. A manual evaluation of the P. vivax CS gene sequence was performed and, when needed, P. vivax codons were modified for optimal expression in E. coli. Overall, 65% (167 of 257) of the codons were altered. Synthetic genes were constructed and assembled by BlueHeron Biotech, Inc. (Bothell, WA), using GeneMaker, a proprietary gene synthesis platform. The synthetic genes were cloned into BlueHeron Bio pUC minus MCS vector.
Cloning and expression. (i) Cloning of VMP 001 in expression vector.
The synthetic gene was subcloned into the pQE60-AKI vector (52) in frame with a 3′ His6 tag. The resultant plasmid, designated AKI-ePvCS1-2, was used to transform BL21(DE3) host cells for expression. Expression was confirmed by using anti-His6 antibodies (Clontech, Palo Alto, CA) and monoclonal antibodies against both VK210 and VK247 isolates that were kindly provided by the Department of Entomology, Walter Reed Army Institute of Research.
(ii) VMP 001 expression in E. coli.
Since the recombinant P. vivax CS protein (VMP 001) produced in the present study is ultimately intended for human use, BL21(DE3) cells carrying the AKI-ePvCS1-2 plasmid were adapted to alternate protein source (APS) medium that is free of animal protein. After we optimized expression conditions at a small scale, clones were grown in APS broth with 0.8% glycerol, 1% glucose, and 25 μg of kanamycin/ml in a BioFlow3000 10L fermentor (New Brunswick Scientific, Edison, NJ). Cells were grown at 37°C, induced at an A600 between 4 and 6 with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and harvested 2 h postinduction, and the cell paste was frozen at −70°C until processing. The process was scaled up and a 300L GMP fermentation was performed to produce paste for large-scale protein purification.
Protein purification.
The E. coli cell paste was resuspended in cracking buffer (1 M NaCl, 50 mM Imidazole, 20 mM sodium phosphate [pH 6.2]) and disrupted by microfluidization in a 110s microfluidizer (Microfluidics Corp., Newton, MA). After centrifugation, CS protein was purified from the soluble fraction. Briefly, the supernatant was incubated with 1% N-lauryl sarcosine (Sigma, St. Louis, MO) and then loaded onto Ni-NTA Superflow columns (QIAGEN, California). After extensive washes, the protein was eluted with 500 mM imidazole, diluted fourfold, and passed over Q anion-exchange resin. The flowthrough of the Q column was passed over SP cation-exchange resin. Protein was eluted from the SP resin by using 300 mM NaCl in 20 mM sodium phosphate (pH 6.2). The sample was dialyzed into phosphate-buffered saline (PBS; pH 7.2), and the protein concentration was estimated by using BCA (Pierce, Rockford, IL).
Antibodies. (i) Mice.
Six- to twelve-week-old female outbred CD1 mice and inbred C57BL/6 (H-2b), B10.BR (H-2k), and BALB/c (H-2d) mice were obtained from Jackson Laboratories (Bar Harbor, ME) for assessing the immune response to VMP 001. All studies were performed under IACUC guidelines.
(ii) Immunization.
Mice were immunized with 10 μg of recombinant VMP 001 in complete Freund's adjuvant (Sigma), Montanide ISA 720, or Montanide ISA 51 (Seppic, New Jersey) and boosted twice at 3-week intervals with 10 μg of protein emulsified in incomplete Freund's adjuvant or Montanide, respectively. For a potency study, mice were immunized with 10, 1, or 0.1 μg of protein in Freund's adjuvant. Blood samples were collected 2 weeks after each immunization, and sera were stored at −20°C until use.
ELISA. (i) Mouse ELISA.
Immulon 2HB plates (Dynatech, Alexandria, VA) were coated overnight at 4°C with 100 μl per well of 1 μg of VMP 001/ml in PBS (pH 7.4). After a blocking step with PBS-Casein (Pierce, Rockford, IL), plates were incubated with serum diluted in PBS-T for 2 h at room temperature, followed by horseradish peroxidase (HRP)-labeled anti-mouse immunoglobulin G (IgG; Promega, Madison, WI) for an hour at room temperature. The reaction was developed with ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] and read after 60 min at A405. Enzyme-linked immunosorbent assay (ELISA) titers are defined as serum dilution that gives an optical density (OD) of 1.
(ii) Human ELISA.
To assess the reactivity of humans to VMP 001 after natural P. vivax infection, sera from symptomatic, smear-positive patients from Mae Sod, Thailand, were drawn after we obtained informed consent and then tested in an ELISA. VMP 001-coated plates were blocked and then incubated with sera diluted 1:100 in PBS-T for 2 h at room temperature, followed by HRP-labeled anti-human IgG diluted 1:2,000 for 1 h at room temperature. Plates were developed with ABTS and read after 45 min at A405. A sample was considered positive when the OD was >3 standard deviations over the mean of 14 normal human sera from the United States.
Heparan sulfate binding.
Immulon 4HB plates (Dynatech) were coated with 100 μl/well of a 10-μg/ml solution of heparan sulfate from bovine kidney (Sigma) diluted in water. The plates were left uncovered and incubated overnight at 37°C. Control wells were incubated with 10 μg of bovine serum albumin (BSA)/ml. The plate was washed and blocked with a 1% solution of BSA in PBS-T for an hour at room temperature. Recombinant CS protein was diluted in PBS-T starting at 5 μg/ml, and plates were incubated for 2 h at room temperature. Control wells were incubated with liver stage antigen −1 from P. falciparum or with BSA. Binding of VMP 001 to heparan sulfate was detected by addition of mouse anti-CS antibody diluted 1:20,000 for 2 h, followed by anti-mouse IgG-HRP for an hour. Plates were incubated at room temperature and washed in between steps. The reaction was developed by using ABTS, and plates were read at A405 after 45 min.
Western blot.
Recombinant CS protein was electrophoresed on 4 to 20% Bis-Tris sodium dodecyl sulfate (SDS)-polyacrylamide gels (Invitrogen) and run under reducing or nonreducing conditions using the MES buffer system. Samples were transferred electrophoretically onto nitrocellulose membranes (Invitrogen). The membranes were blocked for up to 1 h by using nonfat milk in PBS containing 0.1% Tween 20 (PBS-T). After being washed with PBS-T the blots were incubated for 1 h at room temperature with primary antibody diluted 1:10,000 in PBS-T. Alkaline phosphatase-labeled anti-mouse (or anti-rabbit) IgG (Promega) was added after the blots were washed. The blots were washed and developed with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) solution (NBT/BCIP; Promega).
Immunofluorescence.
Anopheles dirus mosquitoes were fed with blood collected from P. vivax-infected patients. Sporozoites were obtained from the salivary glands approximately 17 to 21 days after the blood meal and typed for the strain of P. vivax (P. vivax strains 210 and 247). Sporozoites were coated onto multiwell slides, air dried, and fixed with acetone. Slides were blocked with BSA diluted to 1% in PBS (PBS-BSA) for 30 min. Anti-VPM 001 serum, diluted in PBS-BSA, was added to the wells, and the slides were incubated in a humidified chamber for 1 h at room temperature. The slides were washed with PBS and fluorescein isothiocyanate-labeled goat anti-mouse antibody (Promega) was added for 30 min at room temperature. Slides were washed, mounted in Fluromount, and viewed on an Olympus microscope at ×100 magnification.
For live immunofluorescence assay, P. vivax strain 210 sporozoites isolated from the salivary glands of infected mosquitoes were washed with PBS and then incubated with mouse anti-VMP 001 serum for 30 min. Anti-mouse immunoglobulin-fluorescein isothiocyanate or TRITC (tetramethyl rhodamine isothiocyanate) (Kirkegaard and Perry, Gaithersburg, MD; Dako Laboratories) diluted 1:40 in PBS-0.1% BSA was added to the slide, and after 30 min the slides were observed under the microscope at ×40 magnification.
RESULTS AND DISCUSSION
Rationale and design of vaccine construct.
Although the CS molecule from all malaria parasites is similar in structure (30), the CS sequences obtained from different Plasmodium species show a wide range of sequence diversity, with no broad sequence conservation with the exception of two sequence motifs: region I, a five-amino-acid sequence immediately preceding the repeat region, and region II, located within the C-terminal region. Previous studies suggest that both region I and region II plus are involved in binding to heparan sulfate (53), which serves as a sporozoite receptor on the hepatocytes. Thus, an immune response against these conserved motifs could block a receptor-ligand interaction, which is critical for the establishment of infection. Also, the N-terminal region (between the signal sequence and region I) of the CS protein of P. falciparum has been shown to be important in hepatocyte binding (39), and antibodies against this region prevent sporozoite invasion of liver cells (38). In addition, CD4 epitopes have been mapped to the first 60 amino acids in the N-terminal domain (2, 48).
In addition to antibodies, cellular immunity is also believed to play an important role in protection against the pre-erythrocytic stages of malaria (16, 32). Studies in humans and mice have identified T-cell epitopes on the N- and C-terminal regions of the CS protein in P. vivax (2, 4). Thus, inclusion of the N- and C-terminal regions of the molecule in a vaccine is likely to provide help for the cellular arm of the immune response.
The central region of the CS molecule is comprised of repeating amino acid motifs that vary for each Plasmodium species. Although all sequenced strains of P. falciparum have a common and highly conserved repeat sequence, P. vivax has two distinct forms of the CS protein designated VK210 (type 1) and VK247 (type 2), which differ in the sequence of the central repeat region. The majority of the field infections caused by P. vivax are attributed to VK210-type sporozoites; however, a significant number of VK247-type parasites either as single or mixed (along with VK210) infections have been observed (48, 54).
There is increased sequence diversity in the repeat region of VK210 isolates from Central Asian and Far Eastern isolates (29) that is not observed in the traditional Sal 1 vaccine strain of P. vivax, which has two major amino acid variations (5, 31); the isolates from the Far East and Central Asia have these two sequence variations and five additional repeat motifs (29). Our primary objective was to produce a recombinant P. vivax vaccine that encompasses the predominant antigenic forms of the CS protein present in the different geographical regions. Thus, the core vaccine sequence is based on a Korean isolate to deal with the known antigenic diversity. The final construct has a truncated repeat region that incorporates the reported variant repeat sequence combinations of the VK210 strain and also contains the VK247 repeat sequence.
The VK210 isolates from the Far East, as well as those isolated from Somalia and Iran, show the presence of a 12-amino-acid insert at the end of the repeat region (29, 54). Although the function of this region is not known, parasites from these regions have a high preponderance of delayed infections. Although the presence of this 12-amino-acid insert may not be causal, this region could be an important immune target, and we have therefore included it in our vaccine construct.
Both repeat (11, 36, 49) and nonrepeat (7, 33, 47) regions of CS have been implicated in protection. Monoclonal antibodies directed against the repeat sequences are known to passively transfer protection against sporozoite challenge in experimental models (14, 49) and inhibit sporozoite invasion in liver cell cultures (10). Furthermore, unlike in P. falciparum, where natural infection leads to an almost entirely anti-repeat response, responses to P. vivax infection have shown to induce antibody responses to nonrepeat regions as well (3, 7, 17, 47), a phenomenon also observed in murine malaria (12). Although the precise mode of antibody-mediated immunity is not clear, opsonizing (46) and agglutinating (50) antibodies have been suggested as possible mechanisms of protection.
VMP 001 is comprised of 9 of the 20 repeats present in P. vivax CS protein. All repeat units present in CS molecule may not be necessary to induce effective immunity. In fact, RTS,S (1, 21) and several synthetic CS-based vaccines containing limited copies of repeats, as multiple antigenic peptides, mounted high levels of anti-repeat responses (23, 40, 49). Therefore, we designed a molecule with limited, but diverse, number of repeats. Although the total number of repeats was decreased, we maintained the order found in the natural isolates. Due to the almost complete identity at the N-, and C-terminal regions in P. vivax CS protein, we designed a vaccine based on the VK210 backbone with the addition of a single VK247 repeat. The structure of the final construct is depicted in Fig. 1. We anticipate that this CS-based vaccine will be able to target all of the field isolates with the help of adequate T and B-cell epitopes.
FIG. 1.
Structure of the chimeric CS molecule (not drawn to scale). The repeat region is flanked by region I at the N-terminal end and the 12-amino-acid insert at the C-terminal end. The hatched box in the C-terminal region represents region II. The central region represents nine VK210 repeats and one VK247 repeat.
Expression, purification, and characterization of VMP 001.
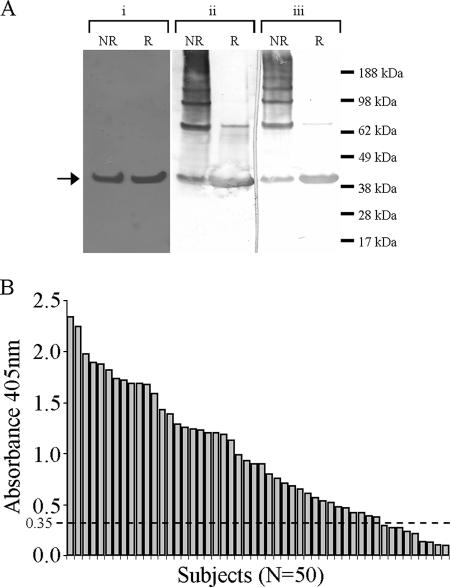
We optimized and matched the frequency of the P. vivax CS codons to E. coli codons. Of the 257 amino acids encoding for VMP 001, 167 (ca. 65%) of the codons were changed. This codon-optimized gene was cloned into an IPTG-inducible E. coli expression plasmid. The recombinant construct produced high levels of soluble protein in the BL21(DE3) strain of E. coli (Fig. 2A). After affinity and ion-exchange chromatography under nondenaturing conditions the recombinant protein was purified to >95% purity to yield 20 to 30 mg of protein/liter with low endotoxin levels suitable for human use.
FIG. 2.
(A) Coomassie blue-stained gel shows a reduced (R) and nonreduced (NR) VMP001 (i) that is recognized in a Western blot by MAb NVS-3 (ii) and MAb 247 (iii) specific for VK210 and VK247 sporozoites, respectively. (B) Sera from acutely infected Thai individuals react to VMP 001 in an ELISA.
Antigenic characterization of VMP 001. (i) Recognition by monoclonal antibodies.
VMP 001 was recognized in a Western blot analysis by monoclonal antibodies NVS-3 and Pv247 (Fig. 2A), mouse monoclonal antibodies that specifically recognize dominant repeat units on VK210 and VK247 sporozoites, respectively. Due to their strict strain specificity these antibodies are reliable markers used to identify and characterize P. vivax isolates (51). Strain-specific antibodies are also known to be induced in natural infections (3). Our results show that the introduction of a single copy of VK247 repeat is sufficient for recognition by the specific monoclonal antibody and may therefore, upon immunization, elicit antibodies that recognize the VK247 sporozoites. Recognition of the recombinant protein by the protective monoclonal NVS-3 suggests that the protective epitope is expressed and is accessible on the protein to the protective antibody.
(ii) Recognition of VMP 001 by human sera after acute P. vivax infection.
To determine the breadth of natural infection-induced immune responses, we tested IgG reactivity in serum samples from 50 individuals from Mae Sod Province in Thailand who were diagnosed with acute P. vivax infections by ELISA. A range of ELISA titers were observed, in which 82% of the individuals tested were positive (Fig. 2B). This demonstrates that recombinant VMP 001 is recognized after natural P. vivax infection and also confirmed that modifications introduced in the chimeric protein maintained the conformation of B-cell epitopes. In addition, four serum samples that were categorized as VK247 alone based on standard diagnostic ELISA and PCR were also found to be positive in our assay, further indicating that VMP 001 expresses detectable levels of the epitope representing a single VK 247 repeat sequence.
In previous reports, the anti-CS antibody responses varied as a result of both the geographic origin of P. vivax infections and the nature of the CS protein used to measure that response. When a yeast-expressed recombinant protein was used, the percentage of subjects positive for antibodies ranged from 15% in Peru (17), 63% in Thailand (48), and 20 to 37.5% in Papua New Guinea (7), and with synthetic peptides it was ca. 68 to 75% in different parts of Columbia (3). In general in these studies the titers against the recombinant antigen have been low; however, our results indicate that VMP 001 is highly antigenic and is recognized by sporozoite-induced antibodies. This could thus infer that in clinical trials VMP 001 might induce high levels of immune responses.
Biological characterization of VMP 001: heparan sulfate binding of recombinant CS.
Heparan sulfate present on hepatocytes serves as a receptor for the binding and invasion of sporozoites. This binding is mediated by two charged motifs: region I in the N-terminal of the molecule and region II-plus in the C-terminal region of the molecule (53). We tested VMP 001 binding to heparan sulfate and the hepatocyte cell line HCO-4 (44). Our results show that recombinant CS bound to both soluble heparan sulfate (Fig. 3) and HCO-4 (data not shown) in a dose-dependent manner.
FIG. 3.
VMP 001 (⋄), but not BSA (▪), binds to heparan sulfate in a dose-dependent manner.
The repeat region and region III of the CS protein are thought to be involved in maintaining the conformation of the molecule such that the charged motifs involved in hepatocyte binding are exposed (27). Our results demonstrate that recombinant CS is biologically functional and retains its key role of binding to hepatic cells.
Immunogenicity of CS protein in mice.
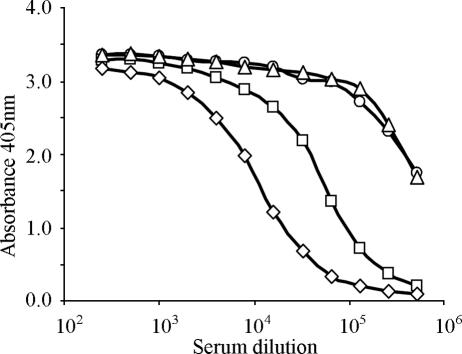
The immunogenicity of VMP 001 was first determined in outbred CD1 mice. Mice were immunized with either 10, 1, or 0.1 μg of protein emulsified in Freund's adjuvant. After the primary injection in complete Freund's adjuvant, mice generated antibodies, as determined by ELISA (data not shown). After a booster immunization in incomplete Freund's adjuvant, mice immunized with the highest dose of 10 μg had a titer of 106 (Fig. 4). Even the lowest immunization dose of 0.1 μg produced a titer of 2 × 105 at an OD of 1, demonstrating that the protein is highly immunogenic.
FIG. 4.
Immunogenicity of CS. Outbred CD1 mice immunized with 10 μg (○), 1 μg (▵), and 0.1 μg (□) of protein in Freund's adjuvant develop high antibodies 2 weeks after two immunizations. Preimmune serum (⧫) did not show any reactivity.
In previous studies, the immune response to CS has been shown to be determined by major histocompatibility antigens of the host. To determine whether recombinant CS is recognized in a diverse major histocompatibility complex background, we compared its immunogenicity in four different strains of mice. Outbred CD1 mice and three inbred strains—BALB/c, C57BL/6, and B10.BR mice—were immunized with 10 μg of VMP 001 in Montanide ISA 51. Outbred CD1 mice and C57BL/6 (H-2b) induced high ELISA IgG titers (Fig. 5; OD of 1 = 8.4 × 105 and 7.6 × 105, respectively). B10.BR (H-2k) mice were found to be intermediate responders with an OD of 1 at a serum dilution of ca. 105, whereas BALB/c (H-2d) induced the lowest level of antibodies (OD of 1 = ca. 2.2 × 104). Previous studies using a yeast-expressed recombinant P. vivax CS protein vaccine in Freund's adjuvant (7, 37) have reported poor antibody and cellular immune responses in H-2d mice. In our studies, after three doses, BALB/c (H-2d) mice had titers of 9 × 104 (data not shown), suggesting that this vaccine is highly immunogenic and, with repeated immunizations, can overcome partial genetic restriction in a mouse haplotype previously found to be a nonresponder (37) or poor responder (7).
FIG. 5.
Immunogenicity of CS. Two weeks after two immunizations, mice immunized with 1 μg of VMP 001 in Montanide ISA 51 adjuvant induced antibodies in CD1 (○), C57BL/6 (▵), B10.BR (□), and BALB/c (⋄) mice.
The higher immunogenicity of our construct could be explained by the fact that it is larger than the one used in the above studies and incorporates additional helper epitopes present in the N- and C-terminal regions of the CS protein (2, 48), which may provide additional B-cell help. While H-2d mice do not respond as well as the other strains tested, the presence of the additional epitopes is possibly the reason that these mice are able to overcome genetic restriction and, compared to the other strains, show the maximum amount of increase in antibody titers after booster immunizations (data not shown). As opposed to the previously tested recombinant vaccines, VMP 001 includes the entire sequence for p25, a promiscuous T-cell epitope at the C-terminal region of the P. vivax CS protein, the inclusion of which has been shown to improve the immunogenicity of MAPs based on the repeat sequence (23). Peptide mapping studies also reveal the presence of CD8 epitopes within the first 40 amino acids that are recognized by BALB/c mice (2). A combination of CD8 and CD4/T-helper epitopes could thus be the key to improving a vaccine's immunogenicity. Detailed analysis of cellular responses should provide further answers. Furthermore, it is highly possible that codon optimization of VMP 001 produced an immunologically superior immunogen through improved conformation.
Thus, the antibody analysis shows that recombinant CS is highly immunogenic with all three adjuvants tested and, in mice, the immune response transcends genetic restriction and induced a degree of antibody responses that were greater than that observed with earlier recombinant P. vivax CS protein vaccines.
A previous recombinant P. vivax CS protein vaccine that encodes for the repeat region fused to 81 amino acids from the N-terminal of the influenza virus nonstructural protein induced very poor immune response in human volunteers (19). In that study, booster immunizations failed to enhance the immune response, which could be due to a lack of adequate T-cell help. It is believed that in order to be protective high titers of antibodies must be generated (9). In this regard, the high level of immune response generated by VMP 001 raises the prospect that this vaccine, when tested in humans, might induce protective immune responses in HLA-diverse human populations.
Epitope analysis of immune response.
To delineate the specificity of anti-VMP 001 antibodies, we first tested the reactivity of pooled mouse serum against recombinantly produced N- and C-terminal subunits. High titers of antibodies were detected to the C-terminal region (Fig. 6) that includes region II and the 12-amino-acid insert. Antibody titers against the N-terminal portion of the protein, ending just before the repeats at region I, were detected but were lower than those for the C-terminal subunit and full-length VMP 001 (Fig. 6). Previously, immunization of Aotus monkeys with synthetic peptide-based vaccines based on either the N- or C-terminal peptides indicated that the C-terminal peptide had higher antibody titers than the N-terminal peptide (3).
FIG. 6.
Sera from mice immunized with VMP 001 recognize the C-terminal (▵) and N-terminal (□) proteins, in addition to VMP 001 (⋄).
We synthesized peptides representing various regions of the CS antigen to further delineate the fine specificity of the antibody response induced in mice by VMP 001 vaccine. The highest titers were obtained against two peptides, the 12-amino-acid insert and the type 1 repeat (Fig. 7), which is represented nine times in the VMP 001 construct. The practical implications for the role of these antibodies in protection can only be determined in a human challenge model. The remaining peptides spanning the entire recombinant molecule had relatively lower, but reasonable, titers ranging from about 4,000 to 5,000 at an OD of 1.0 (Fig. 7).
FIG. 7.
Immunogenicity of CS: fine specificity of anti-VMP 001 antibodies. Anti-VMP 001 sera were tested for their recognition of peptides representing VK210 repeat (○), VK247 repeat (⧫), region I (▴), AGDRx3 (▵), 12-amino-acid insert (□), and a C-terminal peptide (⋄). Prebleed (▪) and scrambled peptide (•) served as negative controls.
The role of antibodies to repeat regions in protection against sporozoite is not completely clear. Several studies using passive antibody transfers and immunizations with synthetic peptides suggest that immune response against repeats is sufficient to induce protection. A monoclonal antibody that has been mapped to a four-amino-acid motif (AGDR) within the VK210 sequence protected monkeys immunized passively with this antibody (11); however, immunization with a repeat-based vaccine (NS81V20) did not lead to high titers of antibodies to the AGDR motif (26). In another study using synthetic MAPs as vaccines, there was a positive correlation between protection and anti-AGDR titers (49). It is very likely that there are protective regions within the repeat region; however, these and other epitopes of the CS molecule may be masked due to a dominant response to nonprotective regions of the molecule which serves as a decoy to subvert the immune response, directing it away from more essential regions of the molecule.
Our results show that VMP 001 is able to induce antibodies recognizing both VK210 and VK247 sequences (Fig. 7). Thus, if anti-repeat antibodies play an important role in protection, then our recombinant protein generates antibodies that should be able to recognize, and neutralize, both types of sporozoites. In addition, immunizations with VMP 001 are able to generate antibodies against the AGDR motif (ELISA titer of 4,750), a part of the central repeat region that has been shown to correlate to protection.
Antibodies from VMP 001-immunized mice recognized peptides from both the N- and C-terminal regions of the CS protein, indicating that all regions of the molecules are recognized by the immune system. Our ability to detect antibodies to region I suggests that immunization with this vaccine may prevent the receptor-ligand interaction, an essential step in sporozoite invasion in liver cells. This region has been thought to be a part of an immunologically cryptic epitope that is not recognized in the context of the entire protein but is able to induce invasion-inhibitory antibodies when used as an immunogen (38). Sporozoite infection leads to low levels of antibodies to the C-terminal region of the molecule in very few of the exposed individuals (7, 48). This has been attributed to poor availability of the epitope (8), epitope competition (45), or sequence homology to thrombospondin. However, other studies have demonstrated that immunization with a peptide spanning this epitope does generate a humoral immune response against it (7, 12). VMP 001 has been optimally designed to expose various epitopes, and after vaccination could allow the generation of immune responses to the cryptic epitopes that are not presented during sporozoite exposure.
Assessment of biological activity of antibodies induced by VMP 001.
To assess whether antibodies generated against this chimeric molecule are able to recognize native protein, immunofluorescence assays were performed with live VK210 sporozoites. Sera from CD1 mice immunized with VMP 001 had strong immunofluorescence reactivity with sporozoites (Fig. 8); preimmune serum had no reactivity (data not shown).
FIG. 8.
In a wet immunofluorescence assay using live VK210 sporozoites anti-VMP 001 serum recognized the sporozoites and caused then to clump (A), and the serum reacted with fixed VK210 (B) and VK247 (C) sporozoites, demonstrating that the serum recognizes both strains of P. vivax.
Furthermore, incubation of live sporozoites showed agglutination in the presence of immune serum (Fig. 8A), which has been shown to inactivate the sporozoites and render them noninfectious (50). Using intravital microscopy (50) it was demonstrated that sporozoite-immunized mice with high titers of antibodies prevented the sporozoites from migrating from the skin into blood vessels, thus preventing the establishment of infection. Taken into context, our results suggest that vaccination with VMP 001 may induce antibodies that result in the inactivation of the sporozoites and thus prevent the onset of the hepatic stage of infection.
Based on the ELISA reactivity, it appears that immunization with the chimeric CS generated antibodies against both VK210 and VK247 peptides. We confirmed this reactivity to native protein using both VK210 and VK247 sporozoites in an immunofluorescence assay with anti-VMP 001 antibodies (Fig. 8B and C).
Preliminary experiments to investigate the induction of cellular immune responses in mice immunized with VMP 001 indicate the generation of gamma interferon, tumor necrosis factor alpha, and granulocyte-macrophage colony-stimulating factor. Further analysis is ongoing to confirm, and quantitate, the immune response, as well as analyze the cell populations responsible for the production of cytokines.
In summary, we have constructed, expressed, and purified a chimeric vaccine that incorporates the protein domains from the conserved and variant regions of the P. vivax CS protein. We believe that the level of expression and the degree of purity achieved is compatible with the requirements to produce the VMP 001 for clinical studies. The recombinant protein possesses the biological properties exhibited by native CS proteins and induces high titers of antibodies that recognize and cause agglutination of live P. vivax sporozoites, an indicator for the loss of sporozoite virulence. Recombinant VMP 001 appears to have induced an immunologically “balanced” response against the N-terminal, central repeats and the C-terminal region of the molecule from the two major field isolates of P. vivax. This VMP 001 vaccine construct warrants development and testing against P. vivax malaria in humans.
Acknowledgments
We thank Wil Nicoll for invaluable assistance with the figures; Clara Brando, Ted Hall, and Shannon McGrath for helpful discussions; D. Gray Heppner and W. Ripley Ballou for their support of the project; and members of the Department of Entomology, AFRIMS, and students from the Penn State Co-Op Program for technical assistance.
This study was supported by the Medical Infectious Diseases Research Program of the U.S. Army Medical Research and Materiel Command, Fort Detrick, Maryland.
The views and opinions expressed in this study are those of the authors and should not be construed as the official opinion of the U.S. Departments of Defense, the U.S. Army, or the U.S. Food and Drug Administration.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (NRC Publication, 1996 ed.).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo-Herrera, M., and S. Herrera. 2001. Plasmodium vivax malaria vaccine development. Mol. Immunol. 38:443-455. [DOI] [PubMed] [Google Scholar]
- 3.Arevalo-Herrera, M., M. A. Roggero, J. M. Gonzalez, J. Vergara, G. Corradin, J. A. Lopez, and S. Herrera. 1998. Mapping and comparison of the B-cell epitopes recognized on the Plasmodium vivax circumsporozoite protein by immune Colombians and immunized Aotus monkeys. Ann. Trop. Med. Parasitol. 92:539-551. [PubMed] [Google Scholar]
- 4.Arevalo-Herrera, M., A. Z. Valencia, J. Vergara, A. Bonelo, K. Fleischhauer, J. M. Gonzalez, J. C. Restrepo, J. A. Lopez, D. Valmori, G. Corradin, and S. Herrera. 2002. Identification of HLA-A2 restricted CD8+ T-lymphocyte responses to Plasmodium vivax circumsporozoite protein in individuals naturally exposed to malaria. Parasite Immunol. 24:161-169. [DOI] [PubMed] [Google Scholar]
- 5.Arnot, D. E., J. W. Barnwell, J. P. Tam, V. Nussenzweig, R. S. Nussenzweig, and V. Enea. 1985. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science 230:815-818. [DOI] [PubMed] [Google Scholar]
- 6.Barr, P. J., H. L. Gibson, V. Enea, D. E. Arnot, M. R. Hollingdale, and V. Nussenzweig. 1987. Expression in yeast of a Plasmodium vivax antigen of potential use in a human malaria vaccine. J. Exp. Med. 165:1160-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilsborough, J., K. Baumgart, I. Bathurst, P. Barr, and M. F. Good. 1997. Fine epitope specificity of antibodies to region II of the Plasmodium vivax circumsporozoite protein correlates with ability to bind recombinant protein and sporozoites. Acta Trop. 65:59-80. [DOI] [PubMed] [Google Scholar]
- 8.Cerami, C., U. Frevert, P. Sinnis, B. Takacs, P. Clavijo, M. J. Santos, and V. Nussenzweig. 1992. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70:1021-1033. [DOI] [PubMed] [Google Scholar]
- 9.Chappel, J. A., W. O. Rogers, S. L. Hoffman, and A. S. Kang. 2004. Molecular dissection of the human antibody response to the structural repeat epitope of Plasmodium falciparum sporozoite from a protected donor. Malaria J. 29:3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappel, J. A., M. R. Hollingdale, and A. S. Kang. 2004. IgG(4) Pf NPNA-1 a human anti-Plasmodium falciparum sporozoite monoclonal antibody cloned from a protected individual inhibits parasite invasion of hepatocytes. Hum. Antibodies 13:91-96. [PubMed] [Google Scholar]
- 11.Charoenvit, Y., W. E. Collins, T. R. Jones, P. Millet, L. Yuan, G. H. Campbell, R. L. Beaudoin, J. R. Broderson, and S. L. Hoffman. 1991. Inability of malaria vaccine to induce antibodies to a protective epitope within its sequence. Science 251:668-671. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee, S., M. Wery, P. Sharma, and V. S. Chauhan. 1995. A conserved peptide sequence of the Plasmodium falciparum Circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect. Immun. 63:4375-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clyde, D. F. 1975. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 24:397-401. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane, A. H., J. W. Barnwell, W. E. Collins, and R. S. Nussenzweig. 1985. Monoclonal antibodies produced against sporozoites of the human parasite Plasmodium malariae abolish infectivity of sporozoites of the simian parasite Plasmodium brasilianum. Infect. Immun. 50:58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins, W. E., R. S. Nussenzweig, W. R. Ballou, T. K. Ruebush II, E. H. Nardin, J. D. Chulay, W. R. Majarian, J. F. Young, G. F. Wasserman, I. Bathurst, H. L. Gibson, P. J. Barr, S. L. Hoffman, S. S. Wasserman, J. R. Broderson, J. C. Skinner, P. M. Procell, V. R. Filipski, and C. L. Wilson. 1989. Immunization of Saimiri sciureus boliviensis with recombinant vaccines based on the circumsporozoite protein of Plasmodium vivax. Am. J. Trop. Med. Hyg. 40:455-464. [DOI] [PubMed] [Google Scholar]
- 16.Doolan, D. L., and S. L. Hoffman. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453-1462. [DOI] [PubMed] [Google Scholar]
- 17.Franke, E. D., C. M. Lucas, E. San Roman, and R. A. Wirtz. 1992. Prevalence of antibody to the variant repeat of the circumsporozoite protein of Plasmodium vivax in Peru. Am. J. Trop. Med. Hyg. 46:708-710. [DOI] [PubMed] [Google Scholar]
- 18.Good, M. F. 2005. Genetically modified Plasmodium highlights the potential of whole parasite vaccine strategies. Trends Immunol. 26:295-297. [DOI] [PubMed] [Google Scholar]
- 19.Gordon, D. M., T. M. Cosgriff, I. Schneider, G. F. Wasserman, W. R. Majarian, M. R. Hollingdale, and J. D. Chulay. 1990. Safety and immunogenicity of a Plasmodium vivax sporozoite vaccine. Am. J. Trop. Med. Hyg. 42:527-531. [DOI] [PubMed] [Google Scholar]
- 20.Guerra, C. A., R. W. Snow, and S. I. Hay. 2006. Mapping the global extent of malaria in 2005. Trends Parasitol. 22:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heppner, D. G., Jr., K. E. Kester, C. F. Ockenhouse, N. Tornieporth, O. Ofori, J. A. Lyon, V. A. Stewart, P. Dubois, D. E. Lanar, U. Krzych, P. Moris, E. Angov, J. F. Cummings, A. Leach, B. T. Hall, S. Dutta, R. Schwenk, C. Hillier, A. Barbosa, L. A. Ware, L. Nair, C. A. Darko, M. R. Withers, B. Ogutu, M. E. Polhemus, M. Fukuda, S. Pichyangkul, M. Gettyacamin, C. Diggs, L. Soisson, J. Milman, M. C. Dubois, N. Garcon, K. Tucker, J. Wittes, C. V. Plowe, M. A. Thera, O. K. Duombo, M. G. Pau, J. Goudsmit, W. R. Ballou, and J. Cohen. 2005. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine 23:2243-2250. [DOI] [PubMed] [Google Scholar]
- 22.Herrera, S., A. Bonelo, B. L. Perlaza, O. L. Fernandez, L. Victoria, A. M. Lenis, L. Soto, H. Hurtado, L. M. Acuna, J. D. Velez, R. Palacios, M. Chen-Mok, G. Corradin, and M. Arevalo-Herrera. 2005. Safety and elicitation of humoral and cellular responses in Colombian malaria-naive volunteers by a Plasmodium vivax circumsporozoite protein-derived synthetic vaccine. Am. J. Trop. Med. Hyg. 73:3-9. [DOI] [PubMed] [Google Scholar]
- 23.Herrera, S., C. De Plata, M. Gonzalez, B. L. Perlaza, F. Bettens, G. Corradin, and M. Arevalo-Herrera. 1997. Antigenicity and immunogenicity of multiple antigen peptides (MAP) containing Plasmodium vivax CS epitopes in Aotus monkeys. Parasite Immunol. 19:161-170. [DOI] [PubMed] [Google Scholar]
- 24.Herrington, D. A., E. H. Nardin, G. Losonsky, I. C. Bathurst, P. J. Barr, M. R. Hollingdale, R. Edelman, and M. M. Levine. 1991. Safety and immunogenicity of a recombinant sporozoite malaria vaccine against Plasmodium vivax. Am. J. Trop. Med. Hyg. 45:695-701. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman, S. L., L. M. Goh, T. C. Luke, I. Schneider, T. P. Le, D. L. Doolan, J. Sacci, P. de la Vega, M. Dowler, C. Paul, D. M. Gordon, J. A. Stoute, L. W. Church, M. Sedegah, D. G. Heppner, W. R. Ballou, and T. L. Richie. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185:1155-1164. [DOI] [PubMed] [Google Scholar]
- 26.Jones, T. R., L. F. Yuan, H. A. Marwoto, D. M. Gordon, R. A. Wirtz, and S. L. Hoffman. 1992. Low immunogenicity of a Plasmodium vivax circumsporozoite protein epitope bound by a protective monoclonal antibody. Am. J. Trop. Med. Hyg. 47:837-843. [DOI] [PubMed] [Google Scholar]
- 27.Kappe, S. H., C. A. Buscaglia, and V. Nussenzweig. 2004. Plasmodium sporozoite molecular cell biology. Annu. Rev. Cell Dev. Biol. 20:29-59. [DOI] [PubMed] [Google Scholar]
- 28.Luke, T. C., and S. L. Hoffman. 2003. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J. Exp. Biol. 206:3803-3808. [DOI] [PubMed] [Google Scholar]
- 29.Mann, V. H., T. Huang, Q. Cheng, and A. Saul. 1994. Sequence variation in the circumsporozoite protein gene of Plasmodium vivax appears to be regionally biased. Mol. Biochem. Parasitol. 68:45-52. [DOI] [PubMed] [Google Scholar]
- 30.McCutchan, T. F., J. C. Kissinger, M. G. Touray, M. J. Rogers, J. Li, M. Sullivan, E. M. Braga, A. U. Krettli, and L. H. Miller. 1996. Comparison of circumsporozoite proteins from avian and mammalian malarias: biological and phylogenetic implications. Proc. Natl. Acad. Sci. USA 93:11889-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCutchan, T. F., A. A. Lal, V. F. de la Cruz, L. H. Miller, W. L. Maloy, Y. Charoenvit, R. L. Beaudoin, P. Guerry, R. Wistar, Jr., S. L. Hoffman, W. T. Hockmeyer, W. E. Collins, and D. Wirth. 1985. Sequence of the immunodominant epitope for the surface protein on sporozoites of Plasmodium vivax. Science 230:1381-1383. [DOI] [PubMed] [Google Scholar]
- 32.Meraldi, V., J. F. Romero, C. Kensil, and G. Corradin. 2005. A strong CD8+ T-cell response is elicited using the synthetic polypeptide from the C terminus of the circumsporozoite protein of Plasmodium berghei together with the adjuvant QS-21: quantitative and phenotypic comparison with the vaccine model of irradiated sporozoites. Vaccine 23:2801-2812. [DOI] [PubMed] [Google Scholar]
- 33.Millet, P., C. Chizzolini, R. A. Wirtz, I. Bathurst, J. R. Broderson, G. H. Campbell, and W. E. Collins. 1992. Inhibitory activity against sporozoites induced by antibodies directed against nonrepetitive regions of the circumsporozoite protein of Plasmodium vivax. Eur. J. Immunol. 22:519-524. [DOI] [PubMed] [Google Scholar]
- 34.Mota, M. M., and A. Rodriguez. 2004. Migration through host cells: the first steps of Plasmodium sporozoites in the mammalian host. Cell Microbiol. 6:1113-1118. [DOI] [PubMed] [Google Scholar]
- 35.Mueller, A. K., M. Labaied, S. H. Kappe, and K. Matuschewski. 2005. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433:164-167. [DOI] [PubMed] [Google Scholar]
- 36.Nardin, E., F. Zavala, V. Nussenzweig, and R. S. Nussenzweig. 1999. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parasitologia 41:397-402. [PubMed] [Google Scholar]
- 37.Nardin, E. H., P. J. Barr, E. Heimer, and H. M. Etlinger. 1988. Genetic restriction of the murine humoral response to a recombinant Plasmodium vivax circumsporozoite protein. Eur. J. Immunol. 18:1119-1122. [DOI] [PubMed] [Google Scholar]
- 38.Rathore, D., R. Nagarkatti, D. Jani, R. Chattopadhyay, P. de la Vega, S. Kumar, and T. F. McCutchan. 2005. An immunologically cryptic epitope of Plasmodium falciparum circumsporozoite protein facilitates liver cell recognition and induces protective antibodies that block liver cell invasion. J. Biol. Chem. 280:20524-20529. [DOI] [PubMed] [Google Scholar]
- 39.Rathore, D., J. B. Sacci, P. de la Vega, and T. F. McCutchan. 2002. Binding and invasion of liver cells by Plasmodium falciparum sporozoites: essential involvement of the amino terminus of circumsporozoite protein. J. Biol. Chem. 277:7092-7098. [DOI] [PubMed] [Google Scholar]
- 40.Reed, R. C., V. Louis-Wileman, E. V. Cosmai, S. Fang, D. L. Jue, R. M. Wohlhueter, R. L. Hunter, and A. A. Lal. 1997. Multiple antigen constructs (MACs): induction of sterile immunity against sporozoite stage of rodent malaria parasites, Plasmodium berghei and Plasmodium yoelii. Vaccine 15:482-488. [DOI] [PubMed] [Google Scholar]
- 41.Robert, L. L., P. D. Santos-Ciminera, R. G. Andre, G. W. Schultz, P. G. Lawyer, J. Nigro, P. Masuoka, R. A. Wirtz, J. Neely, D. Gaines, C. E. Cannon, D. Pettit, C. W. Garvey, D. Goodfriend, and D. R. Roberts. 2005. Plasmodium-infected Anopheles mosquitoes collected in Virginia and Maryland following local transmission of Plasmodium vivax malaria in Loudoun County, Virginia. J. Am. Mos. Qcontrol. Assoc. 21:187-193. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Morales, A. J., E. Sanchez, M. Vargas, C. Piccolo, R. Colina, and M. Arria. 2006. Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J. Trop. Pediatr. 52:49-51. [DOI] [PubMed] [Google Scholar]
- 43.Rogers, W. O., W. R. Weiss, A. Kumar, J. C. Aguiar, J. A. Tine, R. Gwadz, J. G. Harre, K. Gowda, D. Rathore, S. Kumar, and S. L. Hoffman. 2002. Protection of rhesus macaques against lethal Plasmodium knowlesi malaria by a heterologous DNA priming and poxvirus boosting immunization regimen. Infect. Immun. 70:4329-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattabongkot, J., N. Yimamnuaychoke, S. Leelaudomlipi, M. Rasameesoraj, R. Jenwithisuk, R. E. Coleman, R. Udomsangpetch, L. Cui, and T. G. Brewer. 2006. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop. Med. Hyg. 74:708-715. [PubMed] [Google Scholar]
- 45.Schofield, L., and P. Uadia. 1990. Lack of Ir gene control in the immune response to malaria. I. A thymus-independent antibody response to the repetitive surface protein of sporozoites. J. Immunol. 144:2781-2788. [PubMed] [Google Scholar]
- 46.Schwenk, R., L. V. Asher, I. Chalom, D. Lanar, P. Sun, K. White, D. Keil, K. E. Kester, J. Stoute, D. G. Heppner, and U. Krzych. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 25:17-25. [DOI] [PubMed] [Google Scholar]
- 47.Shi, Y. P., V. Udhayakumar, M. P. Alpers, M. M. Povoa, A. J. Oloo, T. K. Ruebush II, and A. A. Lal. 1993. Natural antibody responses against the non-repeat-sequence-based B-cell epitopes of the Plasmodium falciparum circumsporozoite protein. Infect. Immun. 61:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suphavilai, C., S. Looareesuwan, and M. F. Good. 2004. Analysis of circumsporozoite protein-specific immune responses following recent infection with Plasmodium vivax. Am. J. Trop. Med. Hyg. 71:29-39. [PubMed] [Google Scholar]
- 49.Udhayakumar, V., A. Saekhou, S. Fang, D. Jue, R. M. Wohlhueter, and A. A. Lal. 1998. Immunogenicity of Plasmodium falciparum and Plasmodium vivax circumsporozoite protein repeat multiple antigen constructs (MAC). Vaccine 16:982-988. [DOI] [PubMed] [Google Scholar]
- 50.Vanderberg, J. P., and U. Frevert. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991-996. [DOI] [PubMed] [Google Scholar]
- 51.Wirtz, R. A., Y. Charoenvit, T. R. Burkot, K. M. Esser, R. L. Beaudoin, W. E. Collins, and R. G. Andre. 1991. Evaluation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med. Vet. Entomol. 5:17-22. [DOI] [PubMed] [Google Scholar]
- 52.Yadava, A., and C. F. Ockenhouse. 2003. Effect of codon optimization on expression levels of a functionally folded malaria vaccine candidate in prokaryotic and eukaryotic expression systems. Infect. Immun. 71:4961-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying, P., M. Shakibaei, M. S. Patankar, P. Clavijo, R. C. Beavis, G. F. Clark, and U. Frevert. 1997. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp. Parasitol. 85:168-182. [DOI] [PubMed] [Google Scholar]
- 54.Zakeri, S., A. Abouie-Mehrizi, N. D. Djadid, and G. Snounou. 2006. Circumsporozoite protein gene diversity among temperate and tropical Plasmodium vivax isolates from Iran. Trop. Med. Int. Health 11:729-737. [DOI] [PubMed] [Google Scholar]