Abstract
Fasciola hepatica is a prevalent helminth parasite of livestock. Infection results in polarization of the host's immune response and generation of type 2 helper (Th2) immune responses, which are known to be inhibitory to Th1 responses. Bovine tuberculosis (BTB) is a bacterial disease of economic and zoonotic importance. Control polices for this disease rely on extensive annual testing and a test-and-slaughter policy. The correct diagnosis of BTB relies on cell-mediated immune responses. We established a model of coinfection of F. hepatica and Mycobacterium bovis BCG to examine the impact of helminth infection on correct diagnosis. We found the predictive capacity of tests to be compromised in coinfected animals and that F. hepatica infection altered macrophage function. Interleukin-4 and gamma interferon expression in whole-blood lymphocytes restimulated in vitro with M. bovis antigen was also altered in coinfected animals. These results raise the question of whether F. hepatica infection can affect the predictive capacity of tests for the diagnosis of BTB and possibly also influence susceptibility to BTB and other bacterial diseases. Further studies on the interplay between helminth infection and BTB are warranted.
The helminth parasite Fasciola hepatica is the causative agent of fasciolosis, a livestock disease prevalent in temperate regions. Fasciolosis causes losses to agribusiness estimated at around two billion U.S. dollars per year (32). The parasite has a complex life cycle utilizing the mud snail Galba truncatula as an intermediate host, while sheep and cattle, as well as various wildlife species, serve as the definitive hosts. Chemotherapy is the only treatment available (24); however, much effort is being focused on the production of a recombinant vaccine (12). The prevalence of fasciolosis is increasing, due in part perhaps to climatic changes (30), and a recent abattoir study indicates that 65% of the cull cows in Ireland are infected (26).
The immune response to F. hepatica is skewed toward type 2 helper (Th2) dominance, characterized by interleukin-4 (IL-4) production, eosinophilia, and a specific immunoglobulin G1 (IgG1) response with little or no specific IgG2 (5, 8, 25). Studies on the response in cattle suggest that animals experience a downregulation of Th1 responses, including gamma interferon (IFN-γ) production and lymphocyte responsiveness, by week 4 of infection (8, 9). These results are similar to those emerging from studies carried out with a murine model. Here, a complete downregulation of Th1 responses was seen to occur with an upregulation of the Th2 cytokines IL-4 and IL-5, with the magnitude of the effect dependent on the parasite burden (27). In murine models of coinfection, F. hepatica delays bacterial clearance and inhibits bacterium-specific IFN-γ production (4). These results suggest that F. hepatica, like many other helminths, modulates the host's immune system in order to evade detection, damage, or ultimately expulsion (22). A study of F. hepatica infection in the murine model has also revealed the presence of alternatively activated macrophages in infected animals (14). These macrophages were seen to produce increased levels of IL-10 and transforming growth factor β, both of which are regulatory cytokines, have anti-inflammatory properties, and could be responsible for reduced lymphocyte proliferation. Alternatively activated macrophages also have reduced microbicidal properties and could result in a poor innate defense against bacterial infection (19). These immunomodulatory effects may also be responsible for increased susceptibility to secondary bacterial diseases. Deworming of a human study group has been shown to lead to enhanced cellular responses to Mycobacterium tuberculosis antigens (17). In another study, Schistosoma mansoni and hepatitis C virus coinfection revealed that cellular immunity and cytokine production directed at hepatitis C virus were inhibited (18). Vaccination against experimental M. tuberculosis infection in mice with BCG was found to be less effective in cases where coinfection with S. mansoni occurred (15).
Bovine tuberculosis (BTB), caused by Mycobacterium bovis, is one of the most intractable diseases of livestock (13). Schemes for BTB eradication in the United Kingdom and Ireland rely on a test-and-slaughter policy that uses the single comparative intradermal tuberculin test (SCITT). This test detects animals that specifically react to intradermal inoculation of a specific antigen, purified protein derivative B (PPD-B), derived from M. bovis with a response greater than that directed against its equivalent derived from Mycobacterium avium, PPD-A. The development of a reaction to the SCITT relies on the animal forming a delayed-type hypersensitivity (DTH) response to PPD-B (23). Because of issues with low sensitivity in some situations, in Ireland, a second-line test, the whole-blood IFN-γ assay, was introduced for use in problem areas. This test relies on measuring the differences in IFN-γ production in vitro in response to PPD-B and PPD-A (20). The influence of other intercurrent infections on the performance characteristics of these tests is largely unknown. One previous study has demonstrated that acute bovine viral diarrhea virus infection could generate false-negative responses to both tests in BTB-infected calves (6). The possible influence of F. hepatica or other common helminths on the outcome of the SCITT or the whole-blood IFN-γ assay is relevant to BTB eradication and testing policies and also to the wider question of coinfection.
The objective of this study was to examine the influence of F. hepatica infection on the outcome of the two BTB diagnostic assays routinely used in control schemes. We also sought to determine if the timing of helminth infection has an influence on the response to tests and to outline possible mechanisms that could be responsible for altered responses in coinfected animals. We established a coinfection model with F. hepatica and M. bovis BCG, an avirulent strain of M. bovis that is commonly used for vaccination of humans. Vaccination with M. bovis BCG is known to induce a positive reaction on BTB diagnosis in cattle (34). Hence, it is a useful model for examining the effect of coinfection on the predictive capacity of tests used in the diagnosis of BTB. However, it is also important to bear in mind when interpreting these results that the model does not replicate in full the host-pathogen relationship developing in BTB, which involves a virulent, persistent organism that continues to multiply within the host.
MATERIALS AND METHODS
Experimental design.
Experimental animals were castrated male Friesian calves between 6 and 9 months of age purchased from herds free of BTB as determined by a negative result in the annual SCITT carried out as part of the Irish BTB Eradication Scheme. The most recent SCITT of this herd, which included these experimental calves, was carried out 3 months prior to the start of the experiment. The calves were free from exposure to F. hepatica infection as determined by history, serum antibody levels, and fecal examination. They were group housed in slatted pens with good ventilation and fed good-quality grass silage ad libitum. Animals were randomly assigned to one of four treatment groups as shown in Table 1. At week 0, animals in groups 1 and 2 were infected with 400 F. hepatica metacercariae (obtained from G. Coles, Department of Veterinary Clinical Sciences, University of Bristol) contained within a 5% gelatin bolus, as previously described (7). Animals in groups 3 and 4 were inoculated subcutaneously in the right shoulder with 106 to 107 CFU of M. bovis BCG Danish strain 1331 (Statens Serum Institute, Copenhagen, Denmark). Four weeks after the initial infection, animals in group 2 were inoculated with BCG as described above while animals in group 3 were infected with F. hepatica. Animals were necropsied 23 weeks following the initial infection or inoculation. All experiments were approved by the University Ethics Committee and were performed under license from the Irish Department of Health and Children.
TABLE 1.
Experimental designa
| Treatment group | Wk 0 | Wk 4 | Wk 13 | Wk 17 |
|---|---|---|---|---|
| 1 | F. hepatica | SCITT | ||
| 2 | F. hepatica | BCG | SCITT | |
| 3 | BCG | F. hepatica | SCITT | |
| 4 | BCG | SCITT |
Calves were allocated to one of four treatment groups, as shown. In the case of groups 2, 3, and 4, the SCITT was carried out 13 weeks post BCG immunization. In addition to the groups shown above, two additional calves, from the same farm as the other animals and within the same age range, were used as donors of blood monocytes for macrophage preparation and in vitro stimulation with PPD-B and F. hepatica ES products.
Antigens.
Excretory-secretory (ES) products were produced as previously described (14). To prepare endotoxin-free ES products, phase separation was used (1). Briefly, proteins adjusted to 1 mg/ml in sterile, endotoxin-free phosphate-buffered saline (PBS) were vortexed with 5% Triton X-114 and incubated on ice for 5 min and then at 37°C for 5 min. Following the last incubation, solutions were centrifuged at 5,000 × g for 7 s at 37°C. Following centrifugation, the upper phase of the solution, containing the endotoxin-free proteins, was collected. Protein concentration was quantified by the bicinchoninic acid assay (Pierce) with bovine serum albumin (BSA) as the standard. Proteins were determined to be endotoxin free by testing with the Cambrex QCL-1000 Chromogenic LAL Endpoint Assay in accordance with the manufacturer's instructions. Only ES products found to contain no endotoxin were used for macrophage stimulation.
ELISA for F. hepatica-specific antibodies.
Blood was collected by puncture of the coccygeal vein and allowed to clot. Serum was removed and stored at −20°C prior to analysis. Specific antibodies to F. hepatica were measured by enzyme-linked immunosorbent assay (ELISA) with recombinant F. hepatica cathepsin L1 as the antigen. This is a recombinant version of a molecule secreted by the parasite that has been shown to be useful in detecting animals exposed to F. hepatica infection (10). Briefly, 96-well plates (Sarstedt) were coated with 1 μg/ml antigen in carbonate-bicarbonate coating buffer and then incubated overnight at 36°C. Plates were washed in PBS containing 0.05% Tween 20 and blocked with 1% BSA-PBS. Samples diluted 1/200 in 1% BSA-PBS were loaded onto the plate (100 μl/well) with appropriate positive and negative controls, and doubling serial dilutions were performed. Following incubation (30 min at 37°C), plates were washed three times in PBS containing 0.05% Tween 20 and monoclonal mouse anti-bovine IgG1 (1/4,000; Cedi Diagnostics) was added. Following incubation and washing, polyclonal rabbit anti-mouse IgG-horseradish peroxidase (1/2,000; Dako) was added and the mixture was incubated as described above. Following a final wash, 100 μl of 3,3′5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich) was added and the reaction was stopped after 10 min by addition of 100 μl of H2SO4. Plates were read at a wavelength of 450 nm on a Dynatec 4500 plate reader, and results were expressed as log10 antibody titers.
BTB diagnostic tests.
Two tests used in the diagnosis of BTB were used in this experiment, the SCITT and the whole-blood IFN-γ assay. The SCITT was carried out and interpreted by standard procedures, as outlined in EU directive 80/219 EU, by a qualified veterinary surgeon who was unaware of the treatment groups to which the animals belonged. Hair was clipped from a site over the neck region. With McClintock syringes, avian tuberculin (0.5 mg/ml; Lelystad) was injected intradermally at one point on the neck and bovine tuberculin (1 mg/ml; Lelystad) was injected at a distinct site directly below. Skin thickness (millimeters) at both sites was measured before injection and again 72 h later. For the whole-blood IFN-γ assay, heparinized blood was collected and divided into 1.5-ml aliquots in 12-well tissue culture plates. These aliquots were stimulated in duplicate with PPD-A and PPD-B, both at 20 μg/ml, at 37°C and 5% CO2. Twenty-four hours later, plasma was collected and tested for the presence of IFN-γ with the Bovigam enzyme immunoassay (CSIRO) in accordance with the manufacturer's instructions. Results were interpreted as previously described (20). Further aliquots of the supernatant (plasma) from this assay were used for IL-4 measurement as described below.
Macrophage isolation and phenotyping.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood taken 14 weeks post BCG inoculation by centrifugation over Ficoll-Histopaque (Sigma). Monocytes were then further isolated from PBMC with anti-CD14 microbeads (Miltenyi Biotec) on a magnetic separation column (21). Adherent cells were incubated for 24 or 48 h in tissue culture flasks at 37°C and 5% CO2 in Dulbecco modified Eagle medium (Invitrogen) containing 10% fetal calf serum (Sigma), 200 U/ml penicillin (Sigma), and 200 μg/ml streptomycin (Sigma), after which they were tested for nitric oxide (NO) production and intracellular arginase levels. To generate macrophages from naive monocytes, blood was collected in heparinized tubes and PBMC and CD14+ cells were isolated as described above. Adherent cells were cultured for 10 days, with medium changes every 2 days until mature macrophages had formed. Cells were collected in trypsin-EDTA without Mg2+ or Ca2+ and plated at a density of 105/well for stimulation. Cell lysates and supernatants were then examined as detailed below.
NO measurement.
Cell culture supernatants were tested in duplicate for NO with the Griess reagent system (Promega). Briefly, 50 μl of supernatant was added to wells of a 96-well microtiter plate (Sarstedt). To this, 100 μl of sulfanilamide solution was added and the plate was incubated for 10 min in the dark at room temperature. Following this, 100 μl of N-1-napthylethylenediamine dihydrochloride (NED) solution was added and the plate was incubated as before. Readings were taken at 570 nm, and the NO concentration was determined by comparison with a standard curve made with serial dilutions of a 100 μM solution of nitrate.
Arginase activity.
Cell lysates were prepared by addition of 400 μl of 1% Triton X-100 (Sigma) and incubation on a rocking platform for 40 min. Lysate (50 μl) was added to 50 μl of Tris-HCl buffer, pH 7.5, and incubated at 55°C for 10 min to allow for enzyme activation. Following this, 25 μl of the activated lysate was added to 25 μl of arginine substrate at a concentration of 0.5 M (pH 9.7). This mixture was incubated at 37°C for 1 h. The reaction was stopped by addition of 400 μl of acid stop solution comprising H2SO4 (96%), H3PO4 (85%), and H2O in a ratio of 1:3:7. Color was developed by adding 25 μl of 9% isonitrosopriopherone (Sigma) and heating the mixture to 100°C for 45 min (11). A 1:20 dilution of beef liver homogenate was used as a positive control.
IL-4 detection.
IL-4 produced in response to PPD-B in plasma derived from the IFN-γ whole-blood assays was measured with a commercial ELISA (Endogen) in accordance with the manufacturer's instructions. Briefly, recombinant IL-4 capture antibody was used to coat 96-well plates that were then incubated overnight at 4°C. Samples were added in duplicate in 100-μl volumes, and the mixtures were incubated for 1.5 h. IL-4 detection antibody was added, and the mixture was incubated for a further hour. Streptavidin-horseradish peroxidase conjugate was added to the plate for 30 min of incubation, after which color was developed by substrate addition. The reaction was stopped, and the plate was read at 450 nm with reference wavelength of 550 nm. Results were quantified with a standard curve prepared from recombinant bovine IL-4 and reported as picograms per milliliter.
Statistical analysis.
SCITT and IFN-γ test results were expressed as the percentages of the animals in each group testing positive. Significant differences between groups were determined by the chi-square test, as appropriate for categorical data. Measurement of arginase activity, IL-4 levels, and antibody titers produced normal data, and differences between groups were accordingly tested with a one-tailed Student t test. All statistical analysis was carried out with MiniTab (Microsoft Inc.).
RESULTS
F. hepatica burdens and specific immune responses.
Analysis of the serum antibody responses of the three groups infected with F. hepatica showed that humoral immune responses specific for F. hepatica were unaffected by BCG immunization in the two groups of coinfected animals (Fig. 1). All infected groups seroconverted and continued to produce increasing levels of parasite-specific IgG1 throughout infection. There were no significant differences between mean IgG1 titers among groups. Parasite-specific serum IgG2 did not increase above the background during the course of the experiment (data not shown). Livers were collected postmortem and examined to detect parasites present. The average parasite burdens (±the standard error of the mean [SEM]) in groups 1, 2, and 3 were 26 ± 4, 15 ± 3, and 8 ± 5, respectively. These burdens were not statistically significantly different.
FIG. 1.
F. hepatica-specific IgG1 responses (±SEM) in groups 1, 2, 3, and 4. All animals in groups 1, 2, and 3 seroconverted, while those in group 4 did not. No significant difference in titers among groups 1, 2, and 3 were found. Parasite-specific IgG2 levels did not rise above the background during the course of the experiment.
BTB test results.
Thirteen weeks following immunization with BCG, all animals were tested with the SCITT, and on the same day blood was collected for use in the whole-blood IFN-γ assay. The results of both tests are shown in Table 2. Group 3 contained one animal that responded to the IFN-γ assay, while the same animal was also a reactor in the SCITT along with a second animal from this group. No animals from group 2 were classed as responders in either the IFN-γ assay or the SCITT. Group 2 animals had produced a patent infection at the time of BTB diagnosis (fecal egg positive; data not shown), while the animals in group 3 had a prepatent infection at the time of testing.
TABLE 2.
SCITT and IFN-γ assay resultsa
| Group | Treatment (no. of animals) | No. of animals
|
|||
|---|---|---|---|---|---|
| Whole-blood IFN-γ assay
|
SCITT
|
||||
| Positive | Negative | Positive | Negative | ||
| 1 | F. hepatica (4) | 4b | 4b | ||
| 2 | F. hepatica-BCG (4) | 4b | 4b | ||
| 3 | BCG-F. hepatica (5) | 1 | 4b | 2 | 3b |
| 4 | BCG (5) | 4 | 1 | 4 | 1 |
The whole-blood IFN-γ assay was carried out on the same day as injection of PPD antigens for the SCITT. The number of animals from each group that were classed as positive or reactors was expressed as a percentage for statistical analysis.
Result significantly different from that of the BCG-only group as determined by the χ2 test (P ≤ 0.05).
Macrophage phenotyping.
We sought to characterize the activation status (classically or alternatively activated) of blood monocyte-derived macrophages from animals in each treatment group. We isolated CD14-positive cells with a microbead system from peripheral blood mononuclear cells of animals in each treatment group, cultured adherent cells in vitro, and measured their production of NO and arginase (Fig. 2a). We found only trace amounts of NO (data not shown), while we found selective upregulation of arginase production in the macrophages from animals infected with F. hepatica. These cells all showed significantly greater levels of arginase than those taken from animals inoculated with BCG only or from naive control animals. We also examined the effect of in vitro stimulation with PPD-B or F. hepatica ES products on blood monocyte-derived macrophages from uninfected animals (two calves obtained from the same source as the infected groups and within the same age range). Mature macrophages were stimulated with PPD-B or F. hepatica ES products. Following incubation, arginase and NO levels were measured and the results confirmed findings that ES products induced alternatively activated macrophages while PPD-B stimulation favored NO generation indicative of classically activated macrophages (Fig. 2b and c).
FIG. 2.
(a) Levels of arginase production in blood monocyte-derived macrophages (± SEM) at 24 h postisolation from each group. Measurements were made 14 weeks post BCG inoculation and 18 weeks post F. hepatica infection, except in the case of group 3, when the sampling was 12 weeks post F. hepatica infection. Arginase production by cells from groups 1, 2, and 3 was significantly greater (P < 0.05) than that by cells from group 4. The latter did not produce levels significantly greater than those of cells from negative control animals. (b) NO production in blood monocyte-derived macrophages from naive (uninfected) calves stimulated with PPD-B and F. hepatica ES products at 10 μg/ml. The values are means (±SEM) from three experiments with cells from two animals. (c) Arginase production by macrophages under the conditions described for panel b.
Cytokine production in whole-blood assays.
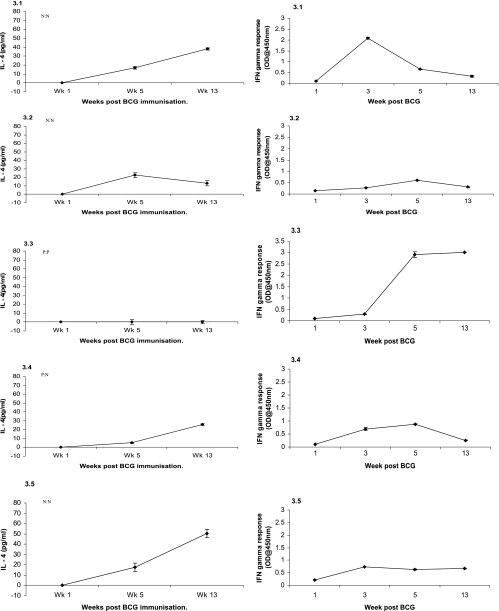
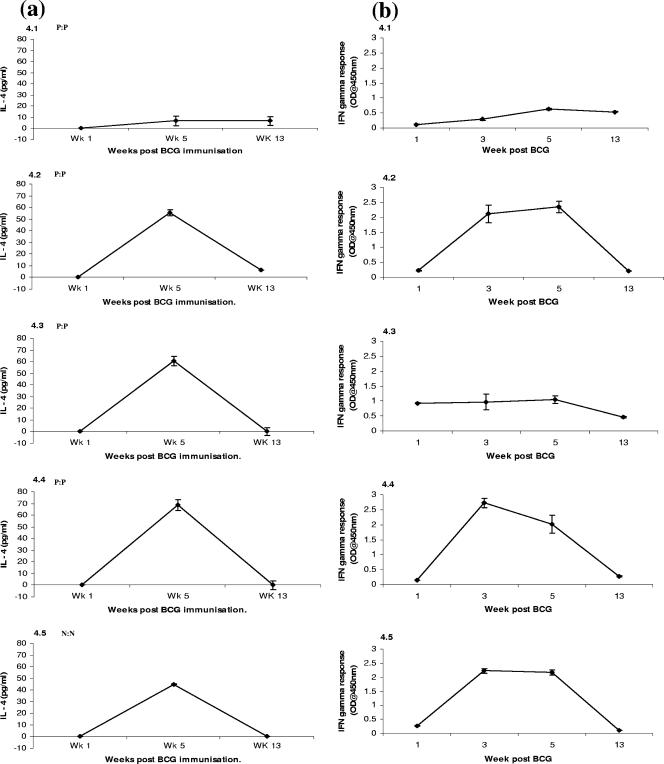
Levels of IL-4 and IFN-γ produced following incubation of whole blood with PPD-B are shown in Fig. 3, 4, and 5 for groups 2, 3, and 4, respectively. We found that group 4 animals produced a peak IL-4 response at week 5. However, group 3 animals displayed a different pattern of IL-4 expression. These animals produced a peak IL-4 response at week 13 while levels were depressed at week 5 (Fig. 3). Group 2 animals, in comparison with group 4 animals, had strong IL-4 responses directed at PPD-B 1 week following immunization. This group also showed elevated IL-4 responses at the time the SCITT was used; however, these levels were not significantly greater than those found in group 4.
FIG. 3.
IL-4 (a) and IFN-γ (b) production by individual animals (F. hepatica and BCG coinfected) in group 2. Cytokine levels were measured in plasma recovered from cultures of whole blood taken at 1 week and 13 weeks post BCG immunization and stimulated in vitro with PPD-B. Results are presented as the mean of triplicate wells ± the SEM. The response of each animal in the SCITT and its whole-blood IFN-γ BTB test results are also indicated in each panel as follows: N:N, negative in both assays; N:P, SCITT negative, whole-blood assay positive; P:N, SCITT positive, whole-blood assay negative; P:P, positive in both assays. OD, optical density.
FIG. 4.
IL-4 (a) and IFN-γ (b) production by individual animals (BCG and F. hepatica coinfected) in group 3. IL-4 was measured in animals at weeks 1, 5, and 13 post BCG immunization, and IFN-γ was measured at weeks 1, 3, 5, and 13 post BCG immunization, as described for Fig. 3. Responses to BTB diagnostic tests are also indicated as in Fig. 3. OD, optical density.
FIG. 5.
IL-4 (a) and IFN-γ (b) production by individual animals (BCG immunized) in group 4. Sampling times and other conditions were as described for Fig. 4. OD, optical density.
The IFN-γ response of animals to PPD-B in the whole-blood assay also showed that some coinfected animals differed with respect to the kinetics and magnitude of this response compared with BCG-infected animals (Fig. 4). Animals in group 4 that mounted an IFN-γ response produced levels that were maximal between weeks 3 and 5 postinfection; these animals were reactors on the SCITT. Two animals produced poor responses, but one of these was still a reactor in the SCITT. In group 3, one animal produced a strong and continuous IFN-γ response and was a reactor in the SCITT. The others produced weak IFN-γ responses and failed to become reactors in the SCITT. One animal produced an early IFN-γ response, similar to group 4 animals, but this response collapsed following F. hepatica infection and this animal subsequently failed to react in the SCITT. IL-4 is known to be inhibitory to IFN-γ production, and this relationship was evident in the coinfected groups. Coinfected animals had peak IL-4 responses when the SCITT was used, where IFN-γ is essential for the production of a positive response. Coinfected animals whose levels of IL-4 elevated above those of BCG-immunized animals were those whose IFN-γ levels were suppressed and consequently responded poorly in the SCITT.
DISCUSSION
The results presented here indicate that a secondary or concurrent helminth infection may influence the predictive capacity of tests used for diagnosis of BTB. The finding that, following experimental infection, SCITT detection rates fell from 80% in group 4 animals to 40% in group 3 animals to 0% in group 2 animals has grave implications for a test-and-slaughter control policy. There is also evidence for a potential effect of the parasite burden visible within coinfected groups. Group 3 animals had a mean parasite burden less than that of those in group 2; while this difference did not reach significant levels (P = 0.0662) because of the relatively high variance within groups (commonly seen following experimental F. hepatica infection), it could indicate that higher parasite levels in group 3 might have resulted in a further decrease in the number of animals that were reactors in the SCITT and the IFN-γ test. It also raises the possibility that BCG immunization had an “adjuvant” or nonspecific effect on the ability of the animals to contain an F. hepatica infection.
Group 2 animals were 17 weeks post F. hepatica infection while group 3 animals were 9 weeks post F. hepatica infection when the SCITT was used. This difference in the number of reactors between these two groups of coinfected animals may be due to the difference in the relative timing of exposure to F. hepatica and BCG. This may indicate that the duration of helminth infection or the presence of helminth infection prior to BCG immunization played some role in the influence on the response to BTB diagnostic tests.
Here we have shown that infection with a common helminth parasite can have a major effect on immune responses relevant to the diagnosis of BTB. Positive responses of BTB-infected animals in the SCITT and the whole-blood IFN-γ assay require sufficient cellular immune responses directed toward PPD-B (29). In general, dominance of humoral over cellular immunity in cattle infected with BTB is correlated with a more progressive form of the disease (35). Even if the effects of natural infection with F. hepatica on the response to diagnostic tests for BTB are much less than those seen here following experimental F. hepatica infection with BCG immunization, the potential implications for disease control programs are grave. Only one previous study (6) has examined the effect of an intercurrent infection on the performance of the SCITT and the IFN-γ test. Acute infection with bovine viral diarrhea virus resulted in temporary suppression of lymphocyte and IFN-γ responses.
The ability of helminths to modulate their host's immune system has been extensively reviewed (22). A number of studies detailing the effects of coinfection with helminths highlight the detrimental effect this modulation can have on immune responses directed at a secondary or concurrent pathogen. In a murine model of F. hepatica and Bordetella pertussis infection, cellular immunity directed toward B. pertussis was abrogated, resulting in delayed bacterial clearance of bacteria and poor B. pertussis-specific IFN-γ production (4). This effect could also be produced by injection of the F. hepatica protease cathepsin L1 (28). This effect was also seen to be dependent on IL-4, as IL-4 knockout mice did not suffer the same effects when cathepsin L1 was administered (28). In our study, we found IL-4 levels to be elevated in coinfected animals, corresponding to downregulated IFN-γ production and negative responses in the SCITT. This suggests that IL-4 plays a role in helminth immunomodulation in this model. S. mansoni infection is known to increase susceptibility to BCG infection in mice (16), with IFN-γ production in response to PPD-B downregulated in coinfected mice, while the Th2 cytokines IL-4 and -5 were found to be unchanged in coinfected animals in response to stimulation by S. mansoni antigen. Coinfected animals were also found to have higher numbers of CFU of BCG in their lungs, livers, and spleens at three different time points postinfection compared to singly infected animals. A study conducted with cattle infected with F. hepatica found that coinfection with F. hepatica and Salmonella dublin resulted in increased excretion of S. dublin and higher mortality levels (2), although no specific mechanisms were identified. In summary, suppression of Th1 responses is known to occur in a variety of helminth infections. This is likely to be one of the factors resulting in increased susceptibility to bacterial infections and in altered performance of diagnostic tests.
The macrophage plays a vital role in the outcome of mycobacterial infection, acting as a host cell and providing antimycobacterial defenses. Effective recruitment of macrophages is needed for the induction of a DTH response, which is vital in the SCITT. Macrophage activation can follow one of two pathways, classical or alternative, depending on the metabolism of l-arginine via inducible NO synthase or arginase, respectively (19). Phenotyping of macrophages can therefore be carried out by monitoring the production of NO and the levels of arginase activity. Alternatively activated macrophages are typically associated with helminth infections and have been highlighted as a possible cause of cellular anergy (33). In some cases, this has been linked to the direct upregulation of programmed death ligand 2 on the macrophage surface (33). Programmed death ligand 2 has been shown to be responsible for decreasing cellular responsiveness by direct contact with T cells. In this study, we examined the possible role of macrophages in coinfection and found that animals infected with F. hepatica and those coinfected with F. hepatica and BCG produced macrophages which were alternatively activated. It has been shown that helminth-induced alternatively activated macrophages have reduced IFN-γ and NO output (14). During the DTH response, Th1 cells are responsible for recruiting and activating macrophages via IFN-γ secretion. We have shown in this study that cellular immune responses relevant to BTB diagnosis are compromised in animals following experimental F. hepatica infection. This lack of Th1 effector mechanisms and presence of alternatively activated macrophages may be responsible for the poor SCITT responses elicited from coinfected animals. The cause-and-effect relationship between alternatively activated macrophages and failing Th1 effector mechanisms is still, to some extent, unclear.
Our data demonstrate the continuing presence of IL-4, a typical Th2 cytokine, at the time the SCITT was administered to coinfected animals. Previous studies have shown that peak IL-4 expression in BTB occurs between weeks 5 and 8 postinfection, with the response fading to background levels as infection proceeds (31). This is consistent with the results from group 4 animals in our study, with IL-4 production peaking at week 5 and levels declining to the week in which the SCITT was used. Levels of IL-4 in groups 2 and 3, however, indicate that cytokine expression patterns deviate from the norm in coinfected animals. In group 3, IL-4 levels were significantly reduced at week 5 while being significantly higher compared to those of group 4 when the SCITT was used. In group 2, levels were also higher when the SCITT was used but not significantly so. This suggests that a Th2 response was ongoing and specifically directed at PPD-B.
The differences in IFN-γ responses seen between BCG-only and coinfected groups may also have been responsible for the poor SCITT results. It appears that F. hepatica infection can cause the antigen-specific IFN-γ response to shut down. Notably, animal 3.3, the only one positive in both the SCITT and the IFN-γ test from either coinfected group, was also the only one not to have a detectable IL-4 response and whose IFN-γ response remained elevated 13 weeks post BTB immunization. The measurement of additional cytokines, such as IL-4, in helminth-infected animals in response to PPD-B restimulation of lymphocytes in vitro may offer additional diagnostic potential.
The experiments described here deal with the possible influence of diagnosis of BTB in animals with F. hepatica infection. This experimental model, using M. bovis BCG, while useful, fails to take into consideration the complex interplay that may take place in active BTB, where bacterial multiplication will have a greater influence due to prolonged exposure to mycobacterial antigen. In view of the continuing spread of BTB, uncertainty in relation to transmission dynamics, and the impact of proposed control measures on wildlife populations, we consider it important to carry out further experimental and epidemiological studies on the effect of helminth parasitism on the diagnosis and control of this disease, including a coinfection study involving an active BTB infection. Conclusions from this study may also have wider implications in the context of human health, where multiple coinfections are common and vaccination is seen as the most sensible route toward the eradication of such diseases (3).
Acknowledgments
R.F. was funded by IRCSET under the EMBARK Initiative. Funding for this study was also provided by the EU Commission under Framework 6, Project ref. FOOD-CT-2005-02305-DELIVER.
We thank E. Gormley in the School of Agriculture, Food Science and Veterinary Medicine, UCD, and E. Costello at the Department of Agriculture and Food for providing M. bovis antigens and J. McNair, AFBI, Belfast, for helpful discussions.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191-195. [DOI] [PubMed] [Google Scholar]
- 2.Aitken, M. M., P. W. Jones, G. A. Hall, D. L. Hughes, and K. A. Collis. 1978. Effects of experimental Salmonella dublin infection in cattle given Fasciola hepatica thirteen weeks previously. J. Comp. Pathol. 88:75-84. [DOI] [PubMed] [Google Scholar]
- 3.Borkow, G., and Z. Bentwich. 2000. Eradication of helminth infections may be essential for successful vaccination against HIV and tuberculosis. Bull. W. H. O. 78:1368-1369. [PMC free article] [PubMed] [Google Scholar]
- 4.Brady, M. T., S. M. O'Neill, J. P. Dalton, and K. H. Mills. 1999. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 67:5372-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, W. C., W. C. Davis, D. A Dobbelaere, and A. C. Rice-Ficht. 1994. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Infect. Immun. 62:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charleston, B., J. C. Hope, B. V. Carr, and C. J. Howard. 2001. Masking of two in vitro immunological assays for Mycobacterium bovis (BCG) in calves acutely infected with non-cytopathic bovine viral diarrhea virus. Vet. Rec. 149:481-484. [DOI] [PubMed] [Google Scholar]
- 7.Clery, D. G., P. O'Mahony, and G. Mulcahy. 1995. A novel method for the administration of metacercariae of Fasciola hepatica to adult cattle. Res. Vet. Sci. 58:290-291. [DOI] [PubMed] [Google Scholar]
- 8.Clery, D. G., and G. Mulcahy. 1998. Lymphocyte and cytokine responses of young cattle during primary infection with Fasciola hepatica. Res. Vet. Sci. 65:169-171. [DOI] [PubMed] [Google Scholar]
- 9.Clery, D., P. Torgerson, and G. Mulcahy. 1996. Immune responses of chronically infected adult cattle to Fasciola hepatica. Vet. Parasitol. 62:71-82. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen, J. B., C. P. Gassenbeek, W. Boersma, F. H. Borgsteede, and F. J. van Milligen. 1999. Use of a pre-selected epitope of cathepsin-L1 in a highly specific peptide-based immunoassay for the diagnosis of Fasciola hepatica infections in cattle. Int. J. Parasitol. 29:685-696. [DOI] [PubMed] [Google Scholar]
- 11.Corraliza, I. M., M. L. Campo, G. Soler, and M. Modolell. 1994. Determination of arginase activity in macrophages: a micromethod. J. Immunol. Methods 174:231-235. [DOI] [PubMed] [Google Scholar]
- 12.Dalton, J. P., S. O'Neill, C. Stack, P. Collins, A. Walshe, M. Sekiya, S. Doyle, G. Mulcahy, D. Hoyle, E. Khaznadji, N. Moire, G. Brennan, A. Mousley, N. Kreshchenko, A. G. Maule, and S. M. Donnelly. 2003. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int. J. Parasitol. 33:1173-1181. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly, C. A., R. Woodroffe, D. R. Cox, J. Bourne, G. Gettinby, A. M. Le Fevre, J. P. McInerney, and W. I. Morrison. 2003. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature 426:834-837. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly, S., S. M. O'Neill, M. Sekiya, G. Mulcahy, and J. P. Dalton. 2005. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias, D., H. Akuffo, A. Pawlowski, M. Haile, T. Schon, and S. Britton. 2005. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 23:1326-1334. [DOI] [PubMed] [Google Scholar]
- 16.Elias, D., H. Akuffo, C. Thors, A. Pawlowski, and S. Britton. 2005. Low dose chronic Schistosoma mansoni infection increases susceptibility to Mycobacterium bovis BCG infection in mice. Clin. Exp. Immunol. 139:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farid, A., M. Al-Sherbiny, A. Osman, N. Mohamed, A. Saad, M. T. Shata, D. H. Lee, A. M. Prince, and G. T. Strickland. 2005. Schistosoma infection inhibits cellular immune responses to core HCV peptides. Parasite Immunol. 27:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon, S. 2002. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 20.Gormley, E., M. B. Doyle, T. Fitzsimons, K. McGill, and J. D. Collins. 2006. Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam) assay. Vet. Microbiol. 112:171-179. [DOI] [PubMed] [Google Scholar]
- 21.Liebana, E., A. Aranaz, M. Welsh, S. D. Neill, and J. M. Pollock. 2000. In vitro T-cell activation of monocyte-derived macrophages by soluble messengers or cell-to-cell contact in bovine tuberculosis. Immunology 100:194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 23.Monaghan, M. L., M. L. Doherty, J. D. Collins, J. F. Kazda, and P. J. Quinn. 1994. The tuberculin test. Vet. Microbiol. 40:111-124. [DOI] [PubMed] [Google Scholar]
- 24.Mas-Coma, S., M. D. Bergues, and J. G. Esteban. 1999. Human fasciolosis, p. 411-434. In J. P. Dalton (ed.), Fasciolosis. CAB International, Wallingford, United Kingdom.
- 25.Mulcahy, G., P. Joyce, and J. P. Dalton. 1999. Immunology of Fasciola hepatica infection, p. 341-376. In J. P. Dalton (ed.), Fasciolosis. CAB International, Wallingford, United Kingdom.
- 26.Murphy, T. M., K. N. Fahy, A. McAuliffe, A. B. Forbes, T. A. Clegg, and D. J. O'Brien. 2006. A study of helminth parasites in culled cows from Ireland. Prev. Vet. Med. 76:1-10. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill, S. M., M. T. Brady, J. J. Callanan, G. Mulcahy, P. Joyce, K. H. Mills, and J. P. Dalton. 2000. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 22:147-155. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, S. M., K. H. Mills, and J. P. Dalton. 2001. Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferon-gamma production in vivo. Parasite Immunol. 23:541-547. [DOI] [PubMed] [Google Scholar]
- 29.Pollock, J. M., J. McNair, M. D. Welsh, R. M. Girvin, H. E. Kennedy, D. P. Mackie, and S. D. Neill. 2001. Immune responses in bovine tuberculosis. Tuberculosis 81:103-107. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard, G. C., A. B. Forbes, D. J. Williams, M. R. Salimi-Bejestani, and R. G. Daniel. 2005. Emergence of fasciolosis in cattle in East Anglia. Vet. Rec. 157:578-582. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spithill, T. W., P. M. Smooker, J. L. Sexton, E. Bozas, C. A. Morrison, J. Creany, and J. C. Parsons. 1999. Development of vaccines against Fasciola hepatica, p. 377-401. In J. P. Dalton (ed.), Fasciolosis. CAB International, Wallingford, United Kingdom.
- 33.Terrazas, L. I., D. Montero, C. A. Terrazas, J. L. Reyes, and M. Rodriguez-Sosa. 2005. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 35:1349-1358. [DOI] [PubMed] [Google Scholar]
- 34.Vordermeier, H. M., M. A. Chambers, B. M. Buddle, J. M. Pollock, and R. G. Hewinson. 2006. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet. J. 171:229-244. [DOI] [PubMed] [Google Scholar]
- 35.Welsh, M. D., R. T. Cunningham, D. M. Corbett, R. M. Girvin, J. McNair, R. A. Skuce, D. G. Bryson, and J. M. Pollock. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]