Abstract
Molecular mimicry between lipooligosaccharides (LOS) of Campylobacter jejuni and gangliosides in peripheral nerves plays a crucial role in the pathogenesis of C. jejuni-related Guillain-Barré syndrome (GBS). We have analyzed the LOS outer core structures of 26 C. jejuni strains associated with GBS and its variant, Miller Fisher syndrome (MFS), by capillary electrophoresis coupled with electrospray ionization mass spectrometry. Sixteen out of 22 (73%) GBS-associated and all 4 (100%) MFS-associated strains expressed LOS with ganglioside mimics. GM1a was the most prevalent ganglioside mimic in GBS-associated strains (10/22, 45%), and in eight of these strains, GM1a was found in combination with GD1a mimics. All seven strains isolated from patients with ophthalmoplegia (GBS or MFS) expressed disialylated (GD3 or GD1c) mimics. Three out of 22 GBS-associated strains (14%) did not express sialylated ganglioside mimics because their LOS locus lacked the genes necessary for sialylation. Three other strains (14%) did not express ganglioside mimics because of frameshift mutations in either the cstII sialyltransferase gene or the cgtB galactosyltransferase gene. It is not possible to determine if these mutations were already present during C. jejuni infection. This is the first report in which mass spectrometry combined with DNA sequence data were used to infer the LOS outer core structures of a large number of neuropathy-associated C. jejuni strains. We conclude that molecular mimicry between gangliosides and C. jejuni LOS is the presumable pathogenic mechanism in most cases of C. jejuni-related GBS. However, our findings suggest that in some cases, other mechanisms may play a role. Further examination of the disease etiology in these patients is mandatory.
Gastroenteritis caused by Campylobacter jejuni is the most common infection preceding Guillain-Barré syndrome (GBS), an acute immune-mediated neuropathy (15, 29). Molecular mimicry between lipooligosaccharides (LOS) in the C. jejuni cell wall and gangliosides in peripheral nerves plays a crucial role in the pathogenesis of GBS (1). Gangliosides are membrane glycolipids that are highly enriched in the nervous system. They are composed of a highly variable oligosaccharide core containing one or more sialic acid molecules and a ceramide tail inserted in the cell membrane. Acute-phase sera of most patients with C. jejuni-associated GBS contain high titers of antibodies to various gangliosides that cross-react with C. jejuni LOS (2, 30). The specificities of these antiganglioside antibodies relate to specific antecedent infections and different clinical presentations of GBS. For example, anti-GM1 antibodies have been associated with a preceding Campylobacter infection and with a severe, pure motor form of GBS (16). Miller Fisher syndrome (MFS), a variant of GBS with oculomotor weakness and ataxia, is strongly associated with the presence of anti-GQ1b antibodies (8).
Since the first report in 1993, several studies have demonstrated ganglioside-like structures in the LOS outer core of C. jejuni strains isolated from GBS and MFS patients (31). Mass spectrometry (MS) and nuclear magnetic resonance analyses of individual strains have revealed the presence of GM1a, GD3, GD1a, and GT1a mimics in GBS-associated strains and of GD3 mimics in MFS-associated strains (4, 6, 24, 25, 32). Serological studies of larger collections of isolates have confirmed and extended these findings (2, 22). However, serological assays are not suitable to determine the exact chemical structure of the LOS outer core.
Detailed knowledge of the biosynthesis and structures of LOS outer cores in neuropathy-associated C. jejuni isolates may help to further elucidate the role of microbial factors in the pathogenesis of GBS, especially since C. jejuni displays considerable structural variation in its LOS outer core. Several genetic mechanisms responsible for this variation have been described (12). First, there is extensive variation in the gene content of the LOS biosynthesis gene locus (“LOS locus”). In addition, variation in homopolymeric tracts, single-base deletions, insertions, and mutations can lead to gene inactivations or glycosyltransferases with different acceptor specificities, resulting in the expression of different LOS structures. Previously, we analyzed the LOS locus of a collection of Dutch neuropathy-associated and control enteritis C. jejuni isolates (14). We found that the class A LOS locus was associated with GBS and the expression of GM1-like structures, whereas the class B LOS locus was associated with MFS and the expression of GQ1b-like structures. The presence of GM1-like and GQ1b-like structures was determined using serological assays, and the exact LOS structures were not known. The development of new MS methods combined with serotyping and preliminary genetic knowledge to predict LOS structures allows the quick screening of many strains (27). In the current study, we used this method to infer the LOS outer core structures of 26 GBS- and MFS-associated C. jejuni strains. Furthermore, we analyzed the genetic mechanisms responsible for the observed variation in these structures, and we related the different LOS structures to clinical symptoms in the corresponding patients.
MATERIALS AND METHODS
C. jejuni strains.
Twenty-two GBS-associated and four MFS-associated C. jejuni isolates were isolated from patients from The Netherlands, Belgium, and The Netherlands Antilles between 1991 and 2000 (Table 1). GB13 and GB14, and GB26 and GB27, were cultured from the diarrheal stools of family members of two GBS patients (3). In both families, there was an outbreak of C. jejuni enteritis, whereas only one family member developed GBS. We were unable to culture C. jejuni from either GBS patient, despite the serological evidence that the GBS patients had also been infected with C. jejuni. These paired isolates were found to be highly related by various genotyping methods (9, 10), suggesting that family members had been infected with the same C. jejuni strain. The degree of subculturing was kept to a minimum, but six to eight passages were necessary for isolation, storage, transport, and preparation of cells for mass spectrometry analysis.
TABLE 1.
C. jejuni strains and patient characteristics
| Strain | HS serotype(s)a | Origin | Patient diagnosis | GenBank accession no.b |
|---|---|---|---|---|
| GB1 | 1 | The Netherlands | GBS | EF066651 |
| GB2 | UT | The Netherlands | GBS | DQ813306 |
| GB3 | 19 | The Netherlands | GBS | DQ906040 |
| GB4 | 37 | The Netherlands | GBS | AY943308 |
| GB5 | 4, 64 | The Netherlands | GBS | AY854153 |
| MF6 | 4, 64 | The Netherlands | MFS | AY422196 |
| MF7 | 35 | The Netherlands | MFS | DQ140270 |
| MF8 | 23, 36 | The Netherlands | MFS | DQ102714 |
| GB11 | 2 | The Netherlands | GBS | AY422197 |
| GB13 | 2 | The Netherlands | Enteritis, family GBS | EF101695 |
| GB14 | 2 | The Netherlands | Enteritis, family GBS | EF101696 |
| GB15 | 5, 34 | The Netherlands | GBS | AY423554 |
| GB16 | 13, 66 | Belgium | GBS (with ophthalmoplegia) | EF076703 |
| GB17 | 4, 13, 64 | The Netherlands | GBS | EF094857 |
| GB18 | 19 | The Netherlands | GBS | DQ868320 |
| GB19 | 4, 50 | The Netherlands | GBS (with ophthalmoplegia) | DQ357237 |
| MF20 | 2 | The Netherlands | MFS | EF064287 |
| GB21 | 13, 65 | The Netherlands | GBS | EF076704 |
| GB22 | 13, 64 | The Netherlands Antilles | GBS | EF091821 |
| GB23 | 4, 13, 43 | The Netherlands | GBS | EF107518 |
| GB24 | 31 | The Netherlands | GBS | AY573819 |
| GB25 | 2 | The Netherlands | GBS (with ophthalmoplegia) | EF064288 |
| GB26 | 1, 44 | The Netherlands | Enteritis, family GBS | DQ351737 |
| GB27 | 1, 44 | The Netherlands | Enteritis, family GBS | EF095404 |
| GB28 | 19, 38 | The Netherlands Antilles | GBS | DQ906041 |
| GB31 | 13, 50 | The Netherlands Antilles | GBS | DQ518908 |
HS, heat stable (Penner serotyping system).
GenBank accession numbers for the partial DNA sequences within the LOS locus are given.
Determination of the LOS locus class by PCR.
The LOS locus class was determined as described previously (14). To distinguish between class D and class F, an additional primer set for the detection of orf17d (specific for class D) was included (23).
Mass spectrometry analysis.
Confluent growths from one agar plate (Mueller-Hinton medium) grown overnight were treated as described previously by Szymanski et al., except that we used proteinase K at 60 mg/ml, RNase A at 200 mg/ml, and DNase I at 100 mg/ml (27). The O-deacylated LOS samples were analyzed by capillary electrophoresis coupled with electrospray ionization MS (CE-ESI-MS) as described previously by St. Michael et al. (26). All CE-ESI-MS and CE-ESI-tandem MS (MS/MS) analyses were performed using a crystal model 310 capillary electrophoresis instrument (ATI Unicam, Boston, MA) coupled to an API 3000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Canada) via a microIon-spray interface.
DNA sequencing.
Genomic DNA was isolated using a DNeasy tissue kit (QIAGEN). Long PCR products were generated using an Advantage 2 PCR kit (Clontech Laboratories). The PCR products were sequenced using custom-made primers that were used previously to sequence the LOS locus in multiple strains (12). DNA sequencing was performed using an Applied Biosystems (Montreal, Canada) model 373 automated DNA sequencer and the manufacturer's cycle sequencing kit (see Table SXXVII in the supplemental material for additional details).
Cloning and expression of the cstII gene from C. jejuni GB26.
The GB26 cstII gene was amplified using Pwo polymerase (Roche Diagnostics, Laval, Canada) and the following primers: CJ-131 (5′-CTTAGGAGGTCATATGAAAAAAGTTATTATTGCTGGAAATG-3′ [41-mer] [the NdeI site is in italics]) and CJ-764 (5′-TTTAGGGTCGACTCAAAGATTAAAATTTTTTGAG-3′ [34-mer] [the SalI site is in italics]). These two primers amplified the region encoding amino acids 1 to 260 of cstII from C. jejuni GB26. The PCR product was digested with NdeI and SalI and cloned in pCWori+(-lacZ) (19), creating construct CST-125. Escherichia coli AD202, containing construct CST-125, was grown in 2× yeast extract-Tryptone medium containing 150 μg/ml ampicillin. The culture was incubated at 37°C until the A600 reached 0.35, induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and then incubated for 7 h at 37°C. The cells were broken using an Avestin C5 Emulsiflex cell disruptor (Avestin, Ottawa, Canada). α-2,3-Sialyltransferase and α-2,8-sialyltransferase activities were assayed as described previously (12).
Statistical analysis.
Differences in frequencies between groups were analyzed with the Fisher's exact test using InStat version 3.0 (Graphpad Software, San Diego, CA). Differences were considered significant at a P value of <0.05 after two-sided testing.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the newly determined nucleotide sequences of the LOS loci of the C. jejuni strains indicated in parentheses are EF066651 (GB1), DQ813306 (GB2), DQ906040 (GB3), AY943308 (GB4), DQ140270 (MF7), DQ102714 (MF8), EF101695 (GB13), EF101696 (GB14), AY423554 (GB15), EF076703 (GB16), EF094857 (GB17), DQ868320 (GB18), DQ357237 (GB19), EF064287 (MF20), EF076704 (GB21), EF091821 (GB22), EF107518 (GB23), AY573819 (GB24), EF064288 (GB25), DQ351737 (GB26), EF095404 (GB27), DQ906041 (GB28) and DQ518908 (GB31).
RESULTS
Determination of the LOS outer core structures.
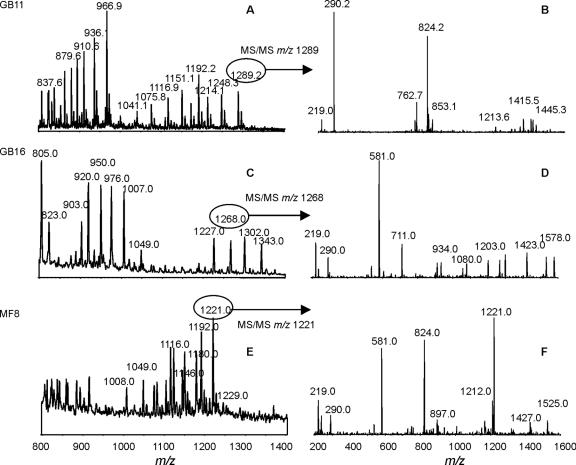
We used CE-ESI-MS on O-deacylated C. jejuni LOS to propose LOS outer core structures for the 26 GBS- and MFS-associated isolates (Fig. 1 and 2 and Table 2) (see Tables SI to SXXVI in the supplemental material). The CE-ESI-MS procedure did not provide linkage information but provided information about the sugar composition of the LOS outer core. The glycosyltransferase variants present in the LOS locus of each strain (Table 3) were used to help interpret the data obtained by CE-ESI-MS (see appendixes SA and SB in the supplemental material). For several strains, we could determine only the sugar composition of the LOS outer core and no structure. In class A and class B strains, the presence of a two-domain Cj1135 (glucosyltransferase) suggests that both heptoses are substituted with glucose, while the presence of a one-domain Cj1135 suggests that only HepI is substituted with glucose (11). The extension of the outer core from HepII is proposed for the strains that have an active Cj1136 variant, while the two strains (MF7 and MF8) that have an inactive Cj1136 variant due to frameshift mutations (GenBank accession numbers DQ140270 and DQ102714) are proposed to have an outer core extended from the glucose substituted to HepII. Based on our previous observations with strains whose LOS outer core structures were completely determined (11), we propose that the inner galactose is substituted with sialic acid in the strains that have no glucose on HepII and that have CgtA and CgtB variants that are specific for a sialylated acceptor. We propose that the inner galactose is not substituted with sialic acid in the case of class A and class B strains that have a glucose on HepII, an active Cj1136 variant, and CgtA/CgtB variants that are specific for nonsialylated acceptors.
FIG. 1.
Mass spectrometry analysis of O-deacylated LOS from representative C. jejuni strains with sialylated LOS outer cores. (A and B) C. jejuni GB11; (C and D) C. jejuni GB16; (E and F) C. jejuni MF8. Panels A, C, and E show extracted mass spectra from CE-MS. Panels B, D, and F show MS/MS of a representative peak from each CE-MS spectrum.
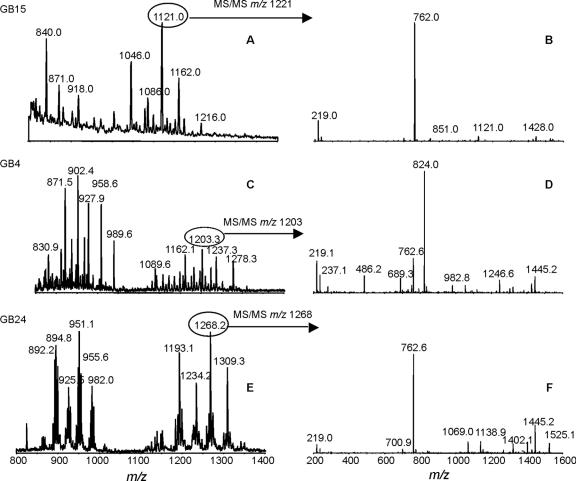
FIG. 2.
Mass spectrometry analysis of O-deacylated LOS from representative C. jejuni strains with nonsialylated LOS outer cores. (A and B) C. jejuni GB15; (C and D) C. jejuni GB4; (E and F) C. jejuni GB24. Panels A, C, and E show extracted mass spectra from CE-MS. Panels B, D, and F show MS/MS of a representative peak from each CE-MS spectrum.
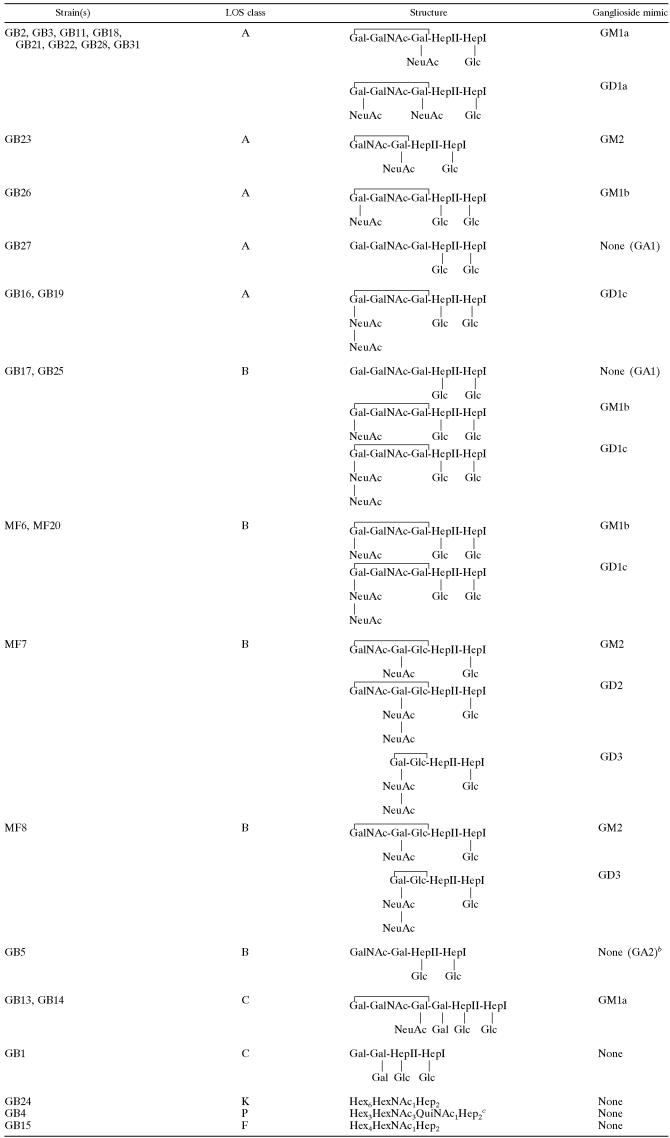
TABLE 2.
Proposed LOS outer core structures expressed by GBS- and MFS-associated strainsa
The brackets indicate the portions of the LOS outer cores that are mimicking gangliosides. The HepI of the inner core is linked to a Kdo residue that is linked to the lipid A portion of the LOS (4).
The exact structure could not be deduced from the observed masses, but the composition of the sugar residues was consistent with an “asialo-GM2”-like (GA2) structure.
The observed masses suggest that this LOS structure is related to the LOS outer core structure of strain ATCC 43431 (HS:3 type strain) (5).
TABLE 3.
Variants of the glycosyltransferases involved in the synthesis of the LOS outer core structures in C. jejuni strains with a class A or B LOS locusa
| Strain | LOS class | Variant
|
|||||
|---|---|---|---|---|---|---|---|
| Cj1135 | Cj1136 | CgtAI | CgtAII | CgtB | CstIIb | ||
| GB2 | A | One domain | On | Monosialylatedc | NAd | Monosialylated | Monofunctional |
| GB3 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB5 | B | Two domains | One | Nonsialylatedf | Monosialylated/disialylatedg | Offh | Off |
| MF6 | B | Two domains | On | Nonsialylated | Monosialylated/disialylated | Nonsialylated | Bifunctional |
| MF7 | B | Two domains | Off | Off | Monosialylated/disialylated | Off | Bifunctional |
| MF8 | B | Two domains | Off | Off | Monosialylated/disialylated | Monosialylated | Monofunctional |
| GB11 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB16 | A | Two domains | On | Nonsialylated | NA | Nonsialylated | Bifunctional |
| GB17 | B | Two domains | On | Nonsialylated | Monosialylated/disialylated | Nonsialylated | Bifunctional |
| GB18 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB19 | A | Two domains | On | Nonsialylated | NA | Nonsialylated | Bifunctional |
| MF20 | B | Two domains | On | Nonsialylated | Monosialylated/disialylated | Nonsialylated | Bifunctional |
| GB21 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB22 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB23 | A | One domain | On | Monosialylated | NA | Off | Monofunctional |
| GB25 | B | Two domains | On | Nonsialylated | Monosialylated/disialylated | Nonsialylated | Bifunctional |
| GB26 | A | Two domains | On | Nonsialylated | NA | Nonsialylated | Monofunctional |
| GB27 | A | Two domains | On | Nonsialylated | NA | Nonsialylated | Off |
| GB28 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
| GB31 | A | One domain | On | Monosialylated | NA | Monosialylated | Monofunctional |
Assignment of the glycosyltransferase variants is based on amino acid sequence comparisons with variants of known specificities (11, 12).
For CstII variants, monofunctional indicates that CstII has α-2,3-sialyltransferase activity, and bifunctional indicates that CstII has both α-2,3-sialyltransferase and α-2,8-sialyltransferase activity.
Monosialylated indicates that the glycosyltransferase is specific for the monosialylated acceptor.
NA, not applicable.
On indicates that a gene has no frameshift mutation.
Nonsialylated indicates that the glycosyltransferase is specific for a nonsialylated acceptor.
Monosialylated/disialylated indicates that the glycosyltransferase can use a monosialylated or a disialylated acceptor.
Off indicates that a gene is inactive because of a frameshift mutation.
Fifteen different outer core structures were identified among the 26 strains that were analyzed, and 14 strains expressed a mixture of at least two different outer core structures. It was not possible to quantify the proportions of the different outer core structures because their different sialic acid contents resulted in different ionization efficiencies, which then had an impact on the observed peak intensities. Several strains harboring the same LOS locus expressed different LOS structures. Within the class A strains, five different (mixtures of) LOS structures were detected. Clearly, knowledge of the LOS locus class is not sufficient to predict the LOS structure. It is also necessary to sequence the key glycosyltransferases to determine the variants involved and whether they encode complete or truncated products.
Expression of ganglioside mimics in the LOS.
Sixteen of 22 (73%) GBS-associated isolates and all four (100%) MFS-associated isolates expressed LOS with ganglioside mimics including GM1a, GM1b, GM2, GD1a, GD1c, GD2, and GD3. Ganglioside mimics were detected only in strains with a class A, B, or C LOS locus (the presence of ganglioside mimics in class A/B/C versus other classes was 20/23 versus 0/3 [P < 0.01]). In GBS-associated strains, GM1a was the most prevalent ganglioside mimic, present in 10 out of 22 strains (45%). Interestingly, in all eight GBS strains with a class A LOS locus and GM1a mimicry (36% of all GBS strains), the GM1a mimic was present as part of a GM1a/GD1a mixture (the presence of GM1a/GD1a in class A versus non-class A was 8/13 versus 0/13 [P < 0.01]). All seven strains isolated from patients with MFS or GBS with ophthalmoplegia expressed structures with a terminal di-NeuAc-Gal (GD3 and GD1c), versus only 1/19 other GBS-associated strains (5%; P < 0.01). These mimics were found predominantly in strains with a class B LOS locus (the presence of GD1c or GD3 in class B versus non-class B was 6/7 versus 2/19 [P < 0.01]). Both class A strains with a GD1c mimic were isolated from GBS patients with ophthalmoplegia.
LOS outer core structures without ganglioside mimics.
Ganglioside mimics could not be detected in the LOS of 6 out of 22 GBS-associated isolates (27%): GB1, GB4, GB5, GB15, GB24, and GB27 (Table 2). These strains were further analyzed to explain the absence of ganglioside mimics. The class C LOS locus of strain GB1 contains all genes necessary to synthesize sialylated LOS. However, we found a five-base deletion in the cstIII gene of GB1 (GenBank accession number EF066651), resulting in a truncated CstIII (219 amino acids instead of 294 amino acids). This will prevent the transfer of sialic acid and the subsequent addition of the terminal GalNAc to the LOS backbone. Strain GB5 also contains an LOS locus (class B) that is essentially capable of directing the synthesis of sialylated LOS, but single-base deletions in the cgtB and cstII genes result in the expression of a truncated LOS outer core without sialic acid (13). Although GB26 and GB27, isolated from two family members of a GBS patient, were indistinguishable by various phenotyping and genotyping methods (9, 14), mass spectrometry revealed that only GB26 expresses sialylated LOS. We detected a poly(G) tract in the cstII gene that leads to a frameshift and premature translation stop in GB27 (10-G tract) and mostly a complete translation product in GB26 (nine-G tract).
We sequenced the entire LOS biosynthesis locus of GB4, GB15, and GB24 and found that all three strains lack the genes necessary for sialylation of the LOS (GenBank accession numbers AY943308, AY423554, and AY573819, respectively). These three LOS loci do not contain either the sialyltransferase gene (cstII or cstIII) or the genes (neuA, neuB, and neuC) necessary for the synthesis of sialic acid and its activated donor, CMP-NeuAc. GB15 has a class F LOS locus (11), whereas both GB4 and GB24 contained novel LOS loci (classes P and K, respectively) (C. T. Parker et al., unpublished data). The exact LOS outer core structures of strains GB4 and GB24 could not be deduced from the mass spectrometry data, but the former structure is related to the LOS outer core of strain ATCC 43431 (HS:3 type strain), which does not contain a ganglioside mimic (5). The mass spectrometry profile suggests that the LOS outer core of GB15 is composed of four hexoses and one HexNAc. It is possible that the LOS outer core of GB15 mimics a human glycolipid of the globo or isoglobo series. However, none of the LOS outer cores of GB4, GB15, and GB24 contains sialic acid, as shown by the absence of the diagnostic ion (m/z 290) in CE-MS/MS spectra of O-deacylated LOS samples from these strains (Fig. 2). This was further confirmed by precursor ion scan experiments in which no glycoforms were detected with a precursor ion at m/z 290 (data not shown).
CstII variants and LOS structure.
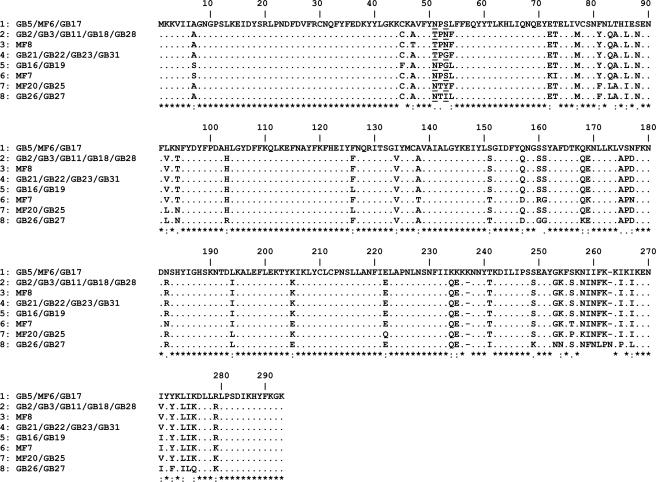
Polymorphisms in the cstII gene determine the extent of sialylation of the LOS (12). Therefore, we determined the correlation between CstII variants and LOS outer core structure. We have previously shown that amino acid residues 51 and 53 affect the level of activity and specificity of CstII (12). Most of the variants with Asn51 express disialylated LOS outer cores (bifunctional CstII; α-2,3- and α-2,8-sialyltransferase activity), while most of the variants with Thr51 express LOS outer cores with only α-2,3-linked sialic acids (monofunctional CstII; only α-2,3-sialyltransferase activity). CstIII, the CstII homologue present in class C strains, is always monofunctional and was therefore not analyzed in this study. We detected eight different variants among the 20 class A and class B neuropathy-associated strains (Fig. 3), 5 of which had Asn51. Seven out of 10 strains (70%) with an Asn51 variant expressed disialylated LOS, as opposed to 1 out of 10 strains (10%) with a Thr51 variant (P = 0.02). However, the correlation between CstII variants and LOS structure was not perfect. MF8 has the Thr51 variant and expresses a mixture of mono- and disialylated LOS outer cores, while GB26 has Asn51 and expresses only a monosialylated LOS outer core. The LOS loci of MF8 and HS:36 are identical except for a difference in the length of the G tract in cgtA (12). Although speculative, it is possible that a cgtA mostly in an “off status” enables CstII with very low α-2,8-sialyltransferase activity to add a second sialic acid, since the lack of a GalNAc addition preserves the acceptor for CstII. We cloned and expressed CstII from GB26 and found that it has only α-2,3-sialyltransferase activity (using in vitro assays) (see Fig. S1 in the supplemental material), although it has Asn51. CstII from GB26 has the sequence that diverges most from the other CstII sequences (Fig. 3), and it is possible that one (or several) amino acid substitution(s) has inactivated the α-2,8-sialyltransferase activity. We observed that the Asn51 variant was present in 6/7 class B strains (86%), whereas Thr51 was the most prevalent variant in class A strains (9/13; 69% [P = 0.06]). Likewise, the Asn51 variant was present in 6/7 (86%) of the MFS-associated strains and strains associated with GBS and ophthalmoplegia, whereas the Thr51 variant was found primarily in the other GBS-associated strains (9/13; 69% [P = 0.06]).
FIG. 3.
Alignment of the CstII sialyltransferase amino acid sequences from the GBS and MFS C. jejuni strains. Only variable residues are shown in addition to the consensus sequence. An “*” indicates conserved residues, a “:” indicates strongly similar residues, and a “.” indicates weakly similar residues. The amino acids that were shown to influence the activity and specificity of CstII are underlined (residues 51 to 53).
DISCUSSION
Ganglioside mimicry is considered to be a crucial factor in the pathogenesis of C. jejuni-associated GBS (1). Detailed knowledge of the bacterial components mimicking human structures, the genetic mechanisms responsible for the observed variation in these structures, and their relation to cross-reactive autoantibodies and clinical features may provide a better understanding of the role of molecular mimicry in postinfectious neuropathy. For the first time, MS combined with DNA sequence data were used to determine the LOS outer core structures of a large number of neuropathy-associated C. jejuni strains. Our data confirm that ganglioside mimicry is the most likely pathogenic mechanism underlying the majority of C. jejuni-associated GBS cases but that in some GBS patients, mimicry towards microbial structures other than ganglioside-like LOS or other mechanisms may lead to neurological damage.
Various ganglioside mimics were found in the LOS of neuropathy-associated strains. GM1a was the most prevalent ganglioside mimic in GBS strains, and it was predominantly present in combination with GD1a mimics (36% of all GBS strains). Although GM1a mimics were found in both class A and class C strains, the GM1a/GD1a mixture was present only in strains with a class A LOS locus, which has previously been associated with GBS (14, 20). Because the prevalence of a class A LOS locus in enteritis-associated strains is 14 to 17% (14, 20), it is expected that a maximum of 14 to 17% of these strains have the GM1a/GD1a mixture. The high prevalence of a GM1a/GD1a mixture in GBS-associated strains suggests that a cluster or complex of these two ganglioside mimics may be the target antigens in a subgroup of GBS rather than single ganglioside mimics. This finding is consistent with the results reported previously by Koga et al. but is in contrast with those reported previously by Nachamkin et al., who found that the expression of GD1a and not GM1 was associated with GBS (20, 22). Furthermore, our results are in agreement with recent observations that ganglioside complexes are important target antigens in GBS as well as in MFS (17, 18).
MFS and GBS with ophthalmoplegia (GBS/MFS overlap) have been associated with the presence of anti-GQ1b antibodies and with the presence of GQ1b-like LOS as determined by serological assays (2, 8). This association may be explained by the enrichment of GQ1b in the nerves that innervate the oculomotor muscles (7). Up to now, MS analysis has not demonstrated true GQ1b-like structures in C. jejuni LOS. The detection of structures with a terminal di-NeuAc-Gal in seven out of eight (87%) strains associated with ophthalmoplegia suggests that in these patients, pathogenic antibodies are raised against the disialylated GD3- or GD1c-like LOS and cross-react with GQ1b in human nerves.
There are several possible explanations for the observation that six GBS-associated strains did not express ganglioside mimics in their LOS. We previously demonstrated that a GBS patient was coinfected with two C. jejuni strains, while only one strain could be linked to GBS (13). In such cases, it is possible that a coinfecting strain, possibly a strain without ganglioside mimics, is isolated from the stool sample and wrongfully regarded as a “GBS-associated” strain. This may also have occurred in patients described here, related to strains that lacked ganglioside mimics. However, it is also possible that the expression of ganglioside mimics vanished during the infection or culture procedures due to mechanisms such as phase variation or single-base mutations or deletions. Strain GB1 did not express ganglioside mimics due to a frameshift mutation in the cstIII gene. It is possible that GBS was induced by a ganglioside-mimicking GB1 strain and that this mutation occurred later in the course of the infection or during laboratory processing. This hypothesis is concordant with the presence of antibodies against both GM1 and asialo-GM1 in the patient serum (2). The same scenario may also apply to strains GB26 and GB27, which were isolated from two family members of a GBS patient who did not develop neurological symptoms. Both isolates are genetically highly related, indicating that all family members had probably been infected with the same strain. Interestingly, we found that variation in the poly(G) tract of the cstII gene was responsible for the lack of ganglioside mimics in the LOS of GB27. In this case, GBS may have been triggered by the ganglioside-mimicking variant of the strain (GB26) and not by the variant without ganglioside mimics (GB27). Strain GB26 expresses an LOS outer core that mimics GM1b, which, unfortunately, is not commercially available. Consequently, we could not determine if the family member who developed GBS had any anti-GM1b antibodies.
On the other hand, our findings indicate that sometimes, molecular mimicry with nonsialylated LOS may be involved in the pathogenesis of GBS. We demonstrated previously that the GB5 patient serum contains anti-asialo-GM2 antibodies that are cross-reactive with GB5 LOS, which suggests that GBS was induced by molecular mimicry with C. jejuni LOS without ganglioside mimics (13). Other mechanisms, including mimicry with C. jejuni structures other than LOS, either sialylated or nonsialylated, or with other microorganisms, should also be considered in some cases. Strains GB4, GB15, and GB24 do not express ganglioside mimics because they do not have the genes that are required for sialylation of the LOS. The acute-phase patient sera with GB4, GB15, and GB24 did not contain antiganglioside antibodies (2; M. L. Kuijf, unpublished data), suggesting a pathogenic mechanism other than ganglioside mimicry. Further investigations are needed to elucidate the pathogenesis of GBS and the role of C. jejuni in these cases.
Genetic polymorphism of C. jejuni determines the LOS structure and thereby also the specificity of the antiganglioside antibody response and clinical features of GBS (2, 12, 14). The presence of and polymorphism within the cstII gene have been associated with the expression of ganglioside mimics and with clinical features of GBS (21, 28). We found that the CstII Asn51 variant was associated with the expression of disialylated LOS and seemed to occur more frequently in class B strains and strains related with clinical symptoms of MFS or GBS with ophthalmoplegia. The Thr51 variant was associated with monosialylated LOS and seemed to occur more frequently in class A strains and in GBS-related strains. These observations suggest that the previously described associations between a class A LOS locus and GBS and class B LOS locus and MFS may be based on the high prevalence of the Thr51 variant in the class A LOS locus and the Asn51 variant in the class B LOS locus (14). Our findings are concordant with recent data reported by Koga et al. (20, 21).
We conclude that the majority of C. jejuni strains isolated from GBS or MFS patients express single or multiple ganglioside mimics in their LOS. However, a substantial portion of the strains is apparently lacking the antigen that is supposed to give rise to the potentially pathogenic antiganglioside antibodies. Further examination of the disease etiology in these patients is mandatory.
Supplementary Material
Acknowledgments
This study was supported by grants from The Netherlands Organization for Scientific Research (920-03-225) to P.C.R.G. and from the Human Frontier Science Program (RGP 38/2003).
We thank Anna Cunningham for technical help, Warren Wakarchuk for helpful discussion, Monica Dzieciatkowska for help with mass spectrometry analysis, and Lawrence Price, Lai King Ng, and Helen Tabor for serotyping of our strains.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 29 January 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Ang, C. W., B. C. Jacobs, and J. D. Laman. 2004. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 25:61-66. [DOI] [PubMed] [Google Scholar]
- 2.Ang, C. W., J. D. Laman, H. J. Willison, E. R. Wagner, H. P. Endtz, M. A. de Klerk, A. P. Tio-Gillen, N. van den Braak, B. C. Jacobs, and P. A. van Doorn. 2002. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect. Immun. 70:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang, C. W., P. A. van Doorn, H. P. Endtz, I. S. J. Merkies, B. C. Jacobs, M. A. de Klerk, R. van Koningsveld, and F. G. A. van der Meché. 2000. A case of Guillain-Barré syndrome following a family outbreak of Campylobacter jejuni enteritis. J. Neuroimmunol. 111:229-233. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall, G. O., S. Fujimoto, A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect. Immun. 62:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinall, G. O., C. M. Lynch, H. Pang, R. T. Shaver, and A. P. Moran. 1995. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231:570-578. [PubMed] [Google Scholar]
- 6.Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry 33:241-249. [DOI] [PubMed] [Google Scholar]
- 7.Chiba, A., S. Kusunoki, H. Obata, R. Machinami, and I. Kanazawa. 1997. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res. 745:32-36. [DOI] [PubMed] [Google Scholar]
- 8.Chiba, A., S. Kusunoki, H. Obata, R. Machinami, and I. Kanazawa. 1993. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology 43:1911-1917. [DOI] [PubMed] [Google Scholar]
- 9.Dingle, K. E., N. van den Braak, F. M. Colles, L. J. Price, D. L. Woodward, F. G. Rodgers, H. P. Endtz, A. van Belkum, and M. C. J. Maiden. 2001. Sequence typing confirms that Campylobacter jejuni strains associated with Guillain-Barré and Miller Fisher syndromes are of diverse genetic lineage, serotype and flagella type. J. Clin. Microbiol. 39:3346-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endtz, H. P., C. W. Ang, N. van den Braak, B. Duim, A. Rigter, L. J. Price, D. L. Woodward, F. G. Rodgers, W. M. Johnson, J. A. Wagenaar, B. C. Jacobs, H. A. Verbrugh, and A. van Belkum. 2000. Molecular characterization of Campylobacter jejuni from patients with Guillain-Barré and Miller Fisher syndromes. J. Clin. Microbiol. 38:2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert, M., P. C. R. Godschalk, C. T. Parker, H. P. Endtz, and W. W. Wakarchuk. 2005. Genetic bases for the variation in the lipooligosaccharide outer core of Campylobacter jejuni and possible association of glycosyltransferase genes with post-infectious neuropathies, p. 219-248. In J. M. Ketley and M. E. Konkel (ed.), Campylobacter: molecular and cellular biology, 1st ed., Horizon Bioscience, Pullman, WA.
- 12.Gilbert, M., M.-F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A.-M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327-337. [DOI] [PubMed] [Google Scholar]
- 13.Godschalk, P. C., M. Gilbert, B. C. Jacobs, T. Kramers, A. P. Tio-Gillen, C. W. Ang, N. Van den Braak, J. Li, H. A. Verbrugh, A. van Belkum, and H. P. Endtz. 2006. Co-infection with two different Campylobacter jejuni strains in a patient with the Guillain-Barré syndrome. Microbes Infect. 8:248-253. [DOI] [PubMed] [Google Scholar]
- 14.Godschalk, P. C. R., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, B. C., P. H. Rothbarth, F. G. A. van der Meché, P. Herbrink, P. I. M. Schmitz, M. A. De Klerk, and P. A. van Doorn. 1998. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology 51:1110-1115. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, B. C., P. A. van Doorn, P. I. M. Schmitz, A. P. Tio-Gillen, P. Herbrink, L. H. Visser, H. Hooijkaas, and F. G. A. van der Meché. 1996. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann. Neurol. 40:181-187. [DOI] [PubMed] [Google Scholar]
- 17.Kaida, K., D. Morita, M. Kanzaki, K. Kamakura, K. Motoyoshi, M. Hirakawa, and S. Kusunoki. 2004. Ganglioside complexes as new target antigens in Guillain-Barré syndrome. Ann. Neurol. 56:567-571. [DOI] [PubMed] [Google Scholar]
- 18.Kaida, K. I., M. Kanzaki, D. Morita, K. Kamakura, K. Motoyoshi, M. Hirakawa, and S. Kusunoki. 13. April 2006, posting date. Anti-ganglioside complex antibodies in Miller Fisher syndrome. J. Neurol. Neurosurg. Psych. doi: 10.1136/jnnp.2006.087940. [DOI] [PMC free article] [PubMed]
- 19.Karwaski, M. F., W. W. Wakarchuk, and M. Gilbert. 2002. High-level expression of recombinant Neisseria CMP-sialic acid synthetase in Escherichia coli. Protein Expr. Purif. 25:237-240. [DOI] [PubMed] [Google Scholar]
- 20.Koga, M., M. Gilbert, M. Takahashi, J. Li, S. Koike, K. Hirata, and N. Yuki. 2006. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J. Infect. Dis. 193:547-555. [DOI] [PubMed] [Google Scholar]
- 21.Koga, M., M. Takahashi, M. Masuda, K. Hirata, and N. Yuki. 2005. Campylobacter gene polymorphism as a determinant of clinical features of Guillain-Barré syndrome. Neurology 65:1376-1381. [DOI] [PubMed] [Google Scholar]
- 22.Nachamkin, I., J. Liu, M. Li, H. Ung, A. P. Moran, M. M. Prendergast, and K. Sheikh. 2002. Campylobacter jejuni from patients with Guillain-Barré syndrome preferentially expresses a GD1a-like epitope. Infect. Immun. 70:5299-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker, C. T., S. T. Horn, M. Gilbert, W. G. Miller, D. L. Woodward, and R. E. Mandrell. 2005. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 43:2771-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prendergast, M. M., A. J. Lastovica, and A. P. Moran. 1998. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect. Immun. 66:3649-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salloway, S., L. A. Mermel, M. Seamans, G. O. Aspinall, J. E. N. Shin, L. A. Kurjanczyk, and J. L. Penner. 1996. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect. Immun. 64:2945-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St. Michael, F., C. M. Szymanski, J. Li, K. H. Chan, N. H. Khieu, S. Larocque, W. W. Wakarchuk, J.-R. Brisson, and M. A. Monteiro. 2002. The structures of the lipooligosaccharide and capsule polysaccharide of Campylobacter jejuni genome sequenced strain NCTC 11168. Eur. J. Biochem. 269:5119-5136. [DOI] [PubMed] [Google Scholar]
- 27.Szymanski, C. M., F. S. Michael, H. C. Jarrell, J. Li, M. Gilbert, S. Larocque, E. Vinogradov, and J.-R. Brisson. 2003. Detection of conserved N-linked glycans and phase-variable lipooligosaccharides and capsules from Campylobacter cells by mass spectrometry and high resolution magic angle spinning NMR spectroscopy. J. Biol. Chem. 278:24509-24520. [DOI] [PubMed] [Google Scholar]
- 28.van Belkum, A., N. van den Braak, P. Godschalk, W. Ang, B. Jacobs, M. Gilbert, W. Wakarchuk, H. Verbrugh, and H. Endtz. 2001. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 7:752-753. [DOI] [PubMed] [Google Scholar]
- 29.Winer, J. B., R. A. C. Hughes, M. J. Anderson, D. M. Jones, H. Kangro, and R. P. Watkins. 1988. A prospective study of acute idiopathic neuropathy. II. Antecedent events. J. Neurol. Neurosurg. Psych. 51:613-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuki, N. 2001. Infectious origins of, and molecular mimicry in, Guillain-Barré and Fisher syndromes. Lancet Infect. Dis. 1:29-37. [DOI] [PubMed] [Google Scholar]
- 31.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and T. Miyatake. 1993. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuki, N., T. Taki, M. Takahashi, K. Saito, T. Tai, T. Miyatake, and S. Handa. 1994. Penner's serotype 4 of Campylobacter jejuni has a lipopolysaccharide that bears a GM1 ganglioside epitope as well as one that bears a GD1 a epitope. Infect. Immun. 62:2101-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.