Abstract
Neospora caninum is an obligate intracellular protozoan parasite that causes abortion in cattle. It is normally found as a latent infection controlled by a T-helper-cell type 1 response involving CD4+ cytotoxic T cells and gamma interferon. Cattle may be infected by two different routes: transplacentally as a result of activation of the latent infection in the mother causing congenital infection or abortion and by ingestion of oocysts, which, if it occurs during gestation, can also result in abortion. Here, for the first time, we establish proof that live vaccination protects against fetal death, whereas immunization using whole-tachyzoite lysate in different adjuvants fails to protect against fetal death. Strong antibody responses were induced in all the vaccinated groups, and the quality and magnitude of these responses were similar in the live- and the lysate-vaccinated groups. In contrast, only the group immunized with live tachyzoites had strong cellular and gamma interferon responses prior to challenge, and these responses correlated with protection against fetopathy. These results suggest that a cellular immune response may be important in the mechanisms involved in protection against N. caninum-associated abortions.
Neospora caninum is an obligate, intracellular, protozoan parasite that causes abortion in cattle. It is normally found as a latent infection controlled by a Th1 response involving CD4+ cytotoxic T cells and gamma interferon (IFN-γ) (2, 25, 32). Adult cattle show no clinical signs other than abortion. During pregnancy, possibly as a result of the altered balance of the immune system by fetally derived regulatory cytokines such as interleukin-10 (IL-10) and IL-4, bradyzoites contained within tissue cysts recrudesce and differentiate, resulting in a tachyzoite parasitemia (reviewed in reference 33). Parasites cross the placenta and infect the fetus, causing abortion or congenital infection; the time during gestation when the fetus becomes infected determines whether it is killed or survives to term (32). Infection appears to persist for the life of a congenitally infected cow, and she may abort or infect the fetus in every gestation. Cattle may also acquire infection postnatally by ingesting oocysts excreted by the canine definitive host (7, 9, 27). Epidemiological evidence suggests that abortion storms associated with N. caninum are linked to exogenous transplacental transmission as a result of the exposure of cattle to oocysts during pregnancy (17, 28).
Several studies have shown that immunization using live tachyzoites, tachyzoite lysate, or select specific antigens, notably SAG1 and SRS2, can protect against transplacental transmission resulting from an experimental challenge with tachyzoites during pregnancy in mice (11, 14, 18, 20, 21, 22). However, it has proven to be difficult to demonstrate protection against abortion because of the lack of an appropriate model system.
There is evidence to suggest that protective immunity to exogenous transplacental transmission exists in cattle since naturally, chronically infected animals have a response that protects the fetus from abortion following exogenous challenge (34). Furthermore, immunization with live tachyzoites prior to pregnancy protects against fetal infection if the mother is challenged during pregnancy (12). However, cattle harboring latent infections can infect their fetuses in sequential pregnancies, resulting in congenitally infected calves or reabortion, suggesting that the maternal immune response does not protect against endogenous transplacental transmission (3, 10).
There are no drugs available to prevent Neospora-associated abortions; a vaccine based on killed tachyzoites is available in the United States, but the data on the efficacy of this vaccine are ambiguous (23). In other parts of the world, control relies solely on effective management. Once an abortion storm begins, however, there is nothing that can be done to prevent it from running its full course, and this has considerable economic and welfare implications.
The aim of this study was to establish whether protective immunity could be induced against N. caninum-associated abortion in cattle, and we demonstrate that the method of immunization is critical to protection.
MATERIALS AND METHODS
Cattle.
Thirty-five Holstein Friesian heifers were purchased from herds that had no history of Neospora-associated abortions. Cows were negative for antibodies to Leptospira hardjo, infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, and N. caninum (Veterinary Laboratories Agency, Preston, United Kingdom). Cattle were housed in dog- and fox-proof accommodations and fed as described previously (32).
Immunization protocol.
Thirty cattle were randomly allocated into five groups of six cows. Each group was treated as described in Table 1.
TABLE 1.
Immunization protocol
| Group | Immunization | AI | Challenge strain |
|---|---|---|---|
| 1 | None | √ | Nc Liv |
| 2 | Live Nc Nowra | √ | Nc Liv |
| 3 | Lysatea in VSA3 | √ | Nc Liv |
| 4 | Lysatea in Quil A | √ | Nc Liv |
| 5 | Adjuvant control | √ | Nc Liv |
Lysate was derived from the Nc Nowra isolate of N. caninum.
Group 1 cows were sham inoculated with sterile phosphate-buffered saline (PBS) (pH 7.2), and group 2 cows were immunized intravenously with 107 tachyzoites of Nc Nowra in PBS 9 weeks prior to artificial insemination.
Groups 3 and 4 were immunized with two doses of Nc Nowra tachyzoite lysate in either VSA3 or Quil A adjuvant administered subcutaneously in the neck region 4 weeks apart. Group 5 included three cows given VSA3 adjuvant alone and three cows given Quil A alone following the same immunization regimen. Groups 3, 4, and 5 were artificially inseminated 5 weeks after the second immunization.
Cattle were progesterone synchronized and artificially inseminated as described previously (32). To confirm that cattle were pregnant, they were scanned by transrectal ultrasonography at 35 days postinsemination. Two animals, one from group 3 and one from group 5, did not become pregnant and were excluded from the experiment.
All five groups were challenged intravenously with 107 Nc Liv tachyzoites at 70 days of gestation. A nonpregnant sentinel control animal was housed with each group, and these animals remained negative for N. caninum in antibody and cell proliferation assays for the duration of the experiment.
Fetal viability was confirmed using ultrasonography to monitor fetal heartbeat and movement every 2 days following challenge for 5 weeks. Thereafter, cattle were scanned weekly until 120 days of gestation. Where there was an absence of fetal heartbeat on two consecutive scans within 24 h, 5 ml of prostaglandin (Lutalyse; Pfizer Animal Health Ltd., United Kingdom) was given by intramuscular injection to cause luteolysis and subsequent expulsion of the fetus.
Blood samples were collected by jugular venepuncture at weekly intervals following immunization and/or challenge until 4 weeks after abortion or calving. Pre- and postcolostral blood samples were taken from calves at birth.
All procedures were carried out in accordance with the United Kingdom Animals (Scientific Procedures) Act, 1986, and were approved through the University of Liverpool's ethical review process.
Parasites.
Two different isolates of N. caninum were used: Nc Nowra (19) was used for immunization, and Nc Liv (5) was used for the challenge dose. Both isolates were maintained under the same conditions in a continuous passage of Vero cells at 37°C and 5% CO2 in air and in RPMI 1640 medium supplemented with 2% horse serum and penicillin-streptomycin (100 IU/ml-100 μg/ml; Biowhittaker). Tachyzoites were harvested when the culture was at maximum growth as described previously (32).
Preparation of parasite lysate for immunization.
Purified Nc Nowra tachyzoites were resuspended in lysis buffer containing PBS with 1 mM phehylmethanesulfonyl fluoride (VWR International), 2 mM dithiothreitol (VWR International), and 1 mM benzimidine HCl (Fisher Scientific, United Kingdom); subjected to three freeze-thaw cycles; and then sonicated using 18 cycles of 60 s at 25 kHz with a probe sonicator (Molecular Probes), with 5 min of cooling on ice between cycles. After each sonication, an aliquot was checked for parasite disruption. The total lysate was used for immunization. The protein concentration was estimated using the Bio-Rad protein assay. To confirm that there were no viable parasites in the final lysate preparation, 10 μl of lysate was inoculated into five 25-cm3 tissue culture flasks of Vero cells. Cultures were checked daily for the appearance of parasites. No tachyzoites were seen in the Vero cell culture up to 4 weeks after inoculation.
The immunizing doses were prepared in Quil A or VSA3 to give 100 μg per dose in a final volume of 2 ml per cow. All formulations were tested for sterility after mixing at 48 h, 7 days, and 14 days postformation. No contamination was detected.
Detection and quantification of the anti-N. caninum-specific antibody response.
N. caninum-specific antibodies were measured in serum using the Mastazyme enzyme-linked immunosorbent assay (Mast Diagnostics, Bootle, Merseyside, United Kingdom) (31). Results were calculated as the percent positivity (PP), where the optical density of the sample was expressed as a percentage of the high-positive control. For each plate, a negative, a mid-positive, and a high-positive control were included. The plate was rejected if the control values fell outside the range recommended by the manufacturer. A PP value of ≥20 indicated a positive result. Antibody titers were calculated as described previously by Williams et al. (32). The avidity of the immunoglobulin G antibodies was analyzed according to a method described previously by Björkman et al. (6). An avidity index (AI) of less than 35 was considered to be low, an AI of 35 to 50 was considered to be medium, and an AI of >50 was considered to be high.
Peripheral blood mononuclear cell proliferation and gamma interferon assays.
Peripheral blood mononuclear cell proliferation assays and IFN-γ enzyme-linked immunosorbent assays were conducted using standard methods as described previously (32). In all assays, medium-alone controls were included. Proliferation data are expressed as a ratio of the medium-alone control, and IFN-γ was not detected in the medium-alone control wells for each animal at each time point.
The antigen used for these assays was prepared from Nc Nowra tachyzoites, and a soluble fraction of antigen was used as described previously (32).
Necropsy of fetuses, calves, and cows.
Fetuses expelled following prostaglandin injection were dissected, and brain and myocardium were removed for analysis by PCR and histological examination. Calves were euthanized within 7 days of birth by intravenous injection of concentrated pentobarbitone sodium (Euthatal; Merial Animal Health Ltd., United Kingdom), and brain, myocardium, and spinal cord were removed for analysis. Cows were killed 4 to 8 weeks postcalving at an over-30-month-registered establishment (under United Kingdom bovine spongiform encephalopathy regulations), and brain tissue was collected for examination. Tissues for histological examination were placed into 10% buffered formalin, embedded in paraffin wax, sectioned at 4 μm, and stained with hematoxylin and eosin using standard techniques or were used for immunocytochemistry. A known positive tissue sample and a known negative tissue sample were used as controls for each run (5).
Detection of N. caninum DNA by PCR.
Four or six 50-mg samples of brain and/or myocardium were pooled from fetal, calf, or adult tissues. N. caninum DNA was detected as described previously (10) by using a method described previously by Uggla et al. (30). For each run, the positive control consisted of purified tachyzoites and negative controls consisted of brain tissue from a cow known to be negative for N. caninum, and a water control was also included in each run.
Statistical analysis.
Group comparisons were analyzed using a Kruskall-Wallis nonparametric test. Where significant group differences were identified, they were followed by a Mann-Whitney test. Alternatively, a one-way analysis of variance was used to compare groups with a posttest Tukey's multiple comparison of means. The critical probability was set at a P value of 0.05, and all analyses were done using Minitab v14 for Windows.
RESULTS
Immunization with live tachyzoites prevents fetal death, while lysate fails to protect.
Following the challenge infection on day 70 of gestation, dead fetuses were detected in five of the six cows in group 1 (challenge control) 21 to 30 days postchallenge (Table 2). Four of the five fetuses were recovered and were positive for N. caninum by PCR and immunohistochemistry. The remaining cow gave birth to a live, clinically normal calf at term. The calf was uninfected as shown by a negative precolostral serum sample and by PCR.
TABLE 2.
Outcome of challenge infection with N. caninum at 70 days of gestation in cattle that had been immunized with live tachyzoites or tachyzoite lysate in different adjuvants 10 weeks before pregnancy
| Group | Treatment | No. of fetal deaths/no. of cattle infected | Time to fetal death (days after infection at 70 days of gestation ± SD) | Detection of N. caninum in fetal tissues by PCR (no. of positives/no. tested) |
|---|---|---|---|---|
| 1 | Challenge control | 5/6 | 27.0 ± 3.74 | 4/4b |
| 2 | Immunized with live tachyzoites | 0/6 | NAa | NA |
| 3 | Immunized with tachyzoite lysate in VSA3 | 5/5 | 24.2 ± 3.10 | 4/4b |
| 4 | Immunized with tachyzoite lysate in Quil A | 5/6 | 24.2 ± 0.41 | 5/5 |
| 5 | Adjuvant alone control | 4/5 | 22.4 ± 3.50 | 4/4 |
NA, not applicable.
One fetus was not recovered.
In group 2 (live immunization), all six fetuses survived to term and were born clinically normal (Table 2). All six were antibody negative, and no parasite DNA was detected in heart or brain tissue at postmortem examination.
In groups 3, 4, and 5 (lysate-immunized and adjuvant control groups), following challenge at 70 days gestation, dead fetuses were detected in five out of six cows immunized with lysate in Quil A, in all five animals immunized with lysate in VSA3, and in four out of five animals in the adjuvant-alone control group (Table 2). The nonaborting cows in groups 4 and 5 each produced a healthy, uninfected calf at full term. Evidence of N. caninum DNA was detected in brain tissues of all 17 aborted fetuses (Table 2), and parasites were detected in myocardia from 14 of the 17 fetuses by immunohistochemistry.
In groups 1, 3, 4, and 5, the time to fetal death ranged from 20 to 40 days after infection. There was no significant difference in the time to death among any of the four groups in which fetuses died (P > 0.05 by analysis of variance).
No N. caninum DNA was found in brain tissues from any of the adult cattle.
Protection is not associated with the quality of the antibody response.
The maternal N. caninum-specific antibody responses were investigated by analyzing the PP values, the titers, and the avidity indices 2 weeks after the second immunization (groups 3 and 4) or 6 weeks after the live immunization (group 2), on the day of challenge, and at 2 weeks postchallenge (Table 3).
TABLE 3.
Evaluation of the quality and quantity of antibody responses in cattle immunized using live N. caninum tachyzoites or tachyzoite lysate in two different adjuvants and challenged with a fetopathic infection at 70 days of gestationa
| Group (immunization) | Sera taken 2 wk after second immunization or 6 wk after live immunization (1 SD)
|
Sera taken immediately before the challenge infection (1 SD)
|
Sera taken two wk post challenge infection (1 SD)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PP | Titer | AI | PP | Titer | AI | PP | Titer | AI | |
| 2 (live) | 58.8b (15.6) | 10.3d (1.0) | 34.0e (15.4) | 37.6f (7.3) | 8.2h (0.4) | 61.5j (5.7) | 77.2k (19.7) | 10.4m (0.6) | 63.6j (7.7) |
| 3 (VSA) | 61.4b,c (29.9) | 8.8d (0.5) | 34.6e (18.4) | 20.2g (10.1) | 5.0i (1.0) | 48.0j (15.0) | 105.4l (5.7) | 13.0n (1.4) | 54.6j (16.5) |
| 4 (Quil A) | 96.8c (18.3) | 9.3d (1.4) | 26.0e (6.0) | 27.6f (6.9) | 5.5i (0.8) | 50.0j (18.0) | 117.7l (16.3) | 13.0n (1.6) | 58.3j (12.3) |
Values sharing the same superscript letter denote values that are not significantly different from each other. Values with different superscripts are significantly different.
Two weeks following the second immunization, there was no significant difference in the PP values, titers, or avidities between the lysate-immunized groups, groups 3 and 4. There was no significant difference between the PP values in the live-immunized group (group 2) compared to the lysate-plus-VSA3 group, group 3, but the PP values of group 2 were significantly lower than those of lysate-plus-Quil A group, group 4 (P = 0.013). However, when the two lysate groups were combined, there was no significant difference between group 2 and groups 3 and 4 (P = 0.79).
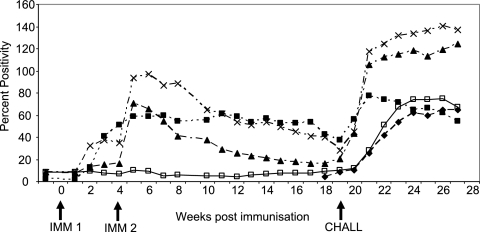
All three immunized groups had a positive antibody response on the day of challenge (Fig. 1); the PP values and antibody titers of the live-immunized group (group 2) were significantly higher than those of the VSA3-immunized group (group 3), but there was no significant difference between the PP values for the live-immunized group and those for the Quil A-plus-lysate group (Table 3). There was no significant difference among the three groups in the AI values, and the AIs were significantly higher in all three groups on the day of challenge than 6 weeks after immunization.
FIG. 1.
Antibody responses presented as mean percent positivity values in cattle following immunization (IMM) with live tachyzoites or whole-tachyzoite lysate 10 weeks prior to artificial insemination and challenged with a fetopathic infection of N. caninum Nc Liv tachyzoites 19 weeks after the first immunizing dose. ⧫, group 1 (challenge control); ▪, group 2 (live immunization); ▴, group 3 (lysate plus VSA3); ×, group 4 (lysate plus QuilA); □, group 5 (adjuvant control).
There was evidence of an anamnestic antibody response in all three immunized groups, since the antibody levels increased rapidly following challenge at 10 weeks of gestation (19 weeks following immunization) compared to the adjuvant and challenge control groups (Fig. 1).
Two weeks after challenge, the PP values and titers were significantly lower in the live-immunized group (group 2) than in the lysate-immunized groups (groups 3 and 4), but there was no significant difference in the avidity indices between groups 2, 3, and 4, and all were greater than 50, which is indicative of a mature antibody response (Table 3).
A persistent cell-mediated response is induced by live immunization but not by lysate.
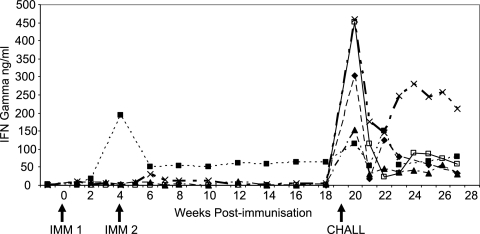
Antigen-specific T-cell responses were compared by measuring antigen-specific IFN-γ secretion and cell proliferation over the course of the experiment. The IFN-γ responses are shown in Fig. 2. A similar pattern was detected in the antigen-specific proliferation responses. No antigen-specific proliferation or IFN-γ secretion was detected in the control animals prior to challenge.
FIG. 2.
Mean gamma interferon responses (ng ml−1) in cattle following immunization with live tachyzoites or whole-tachyzoite lysate 10 weeks prior to artificial insemination and challenged with a fetopathic infection of N. caninum Nc Liv tachyzoites 19 weeks after the first immunizing dose. ⧫, group 1 (challenge control); ▪, group 2 (live immunization); ▴, group 3 (lysate plus VSA3); ×, group 4 (lysate plus QuilA; □, group 5 (adjuvant control).
Weak, transient proliferation responses and IFN-γ responses were detected in the lysate-immunized groups between 2 and 4 weeks after the second vaccination. Antigen-specific proliferation and IFN-γ responses were detected 1 week after live immunization in group 2, which peaked 4 weeks after immunization and remained detectable until the day of challenge 19 weeks after immunization.
The IFN-γ responses immediately prior to the challenge infection (day 0) and 1 week later are shown in Table 4; the cell proliferation results followed the same pattern but with greater animal-to-animal variation. Immediately prior to challenge, a significant, specific IFN-γ response was detected in group 2, which was immunized with live tachyzoites 19 weeks previously (P < 0.01). In contrast, no antigen-specific proliferation or IFN-γ secretion was detected in the challenge control groups (groups 1 and 5) or in the lysate-immunized groups (groups 3 and 4). One week after challenge infection, all five groups had detectable IFN-γ and antigen-specific proliferation responses, and there was no significant difference between the groups.
TABLE 4.
Gamma interferon secretion by peripheral blood mononuclear cells following antigen stimulation at the time of challenge infection and 1 week later
| Group (description) | IFN-γ response (1 SD) [ng/ml−1]
|
|
|---|---|---|
| Immediately prior to challenge | Week 1 postchallenge | |
| 1 (challenge control) | 0.82 (1.0) | 258.65 (183.8) |
| 2 (live immunization) | 47.47 (31.77) | 89.19 (87.84) |
| 3 (lysate + VSA3) | 1.96 (1.25) | 152.3 (206.1) |
| 4 (lysate + Quil A) | 1.79 (1.74) | 459.93 (653.0) |
| 5 (adjuvant control) | 0.47 (0.76) | 450.55 (383.99) |
DISCUSSION
This study demonstrates conclusively that immunization with live tachyzoites prior to pregnancy conferred 100% protection against Neospora-induced fetopathy in cattle, whereas immunization with tachyzoite lysate failed to confer any protection, irrespective of the adjuvant used. Protection was associated with a cellular response, and although strong, high-avidity antibody responses were elicited by both live and lysate immunization, there was no correlation with protection against a fetopathic challenge. We used two different isolates in this study: an Australian bovine isolate, Nc Nowra, was used to vaccinate the cattle, and they were challenged with a heterologous isolate from a British dog (Nc Liv). The results suggest that the protection induced was not isolate specific.
This is the first time that successful vaccination against Neospora-mediated fetal death in mice or cattle has been described, and this study extends previous observations that live immunization induces protection against vertical transmission in both cattle and mice (12, 15, 18). In contrast, vaccination using whole, killed tachyzoites failed to protect against vertical transmission in cattle (3, 4), and the use of single recombinant antigens or whole-tachyzoite lysate to induce protection in mice has yielded variable results. NcSRS2 administered in immunostimulating complexes or Freund's adjuvant or as a vaccinia virus construct elicits partial protection from vertical transmission in BALB/c mice (11, 21, 22), while whole crude lysate has been shown to induce complete protection or partial protection in two different mouse models (14, 18). Because of the effectiveness of live vaccination, we went to great lengths to prove that our lysate did not contain any live tachyzoites. Tachyzoites are very robust and can survive freeze-thawing and several rounds of sonication. It is therefore fundamental for any study using lysates that the immunizing preparation is tested by inoculation onto cell cultures or into susceptible mice to establish that it is free of contaminating live tachyzoites.
In order to evaluate the quality and quantity of the antibody response, we measured the titer and the avidity of the response following vaccination, at the time of challenge, and 2 weeks after challenge. Two weeks after the second immunization, the antibody response was higher in the group immunized with lysate in Quil A than in the group immunized with lysate in VSA3, although these differences were not statistically significant. This fits with what is known about the adjuvants. VSA3 is an oil-in-water emulsion and licensed for use in cattle. It acts as a depot, providing the gradual release of antigen over several weeks. Quil A is a mixture of saponins derived from the Quilaja saponaria tree and is considered to be an inducer of Th1 responses, since immunoglobulin G2a responses are elicited in vaccinated animals (24).
Two weeks after the final immunization and 2 weeks after challenge, the PP values and the antibody titers were higher in the lysate-immunized groups than in the live-immunized group, while the AIs were similar in all three groups. By the time the challenge infection was administered, the AIs were above 50, which is indicative of a mature response (6). These results suggest that the lysate immunization stimulated an antibody response comparable to that in the live-immunized group, that Quil A stimulated a stronger antibody response than VSA3, and that an anamnestic response was induced in all three groups. However, this did not correlate with protection, as the fetuses in 10 out of the 11 cows immunized with lysate were killed by the challenge infection, whereas all six fetuses survived in the live-immunized group, and suggests that the antibody response did not play a fundamental role in protection. This is in contrast to previous work that suggested that antibodies are important in protection against N. caninum, since B-cell-deficient μMT mice died earlier and had a higher parasite load than did intact mice (8). Similarly, there is evidence that in mice infected with the closely related parasite Toxoplasma gondii, B cells are also involved in resistance to infection (13). However, in the former study, the μMT mice, as well as having no B-cell response, also had lower IFN-γ and IL-10 responses than did wild-type mice, suggesting that factors other than antibody may have been involved in the increased susceptibility in this strain; furthermore, while a B-cell response was elicited following immunization of BALB/c mice with whole structural N. caninum antigens, the mice had an impaired IFN-γ response and were more susceptible to lethal challenge than nonimmunized mice, supporting the view that B cells alone were not instrumental in the control of infection (26).
Intracellular protozoan parasites normally induce and are controlled by cellular immune responses. Because N. caninum is a relatively newly described parasite, there is less known about the immune response that it induces compared to those of other related parasites. However, it is generally accepted that IFN-γ is involved in controlling parasite growth and that cytotoxic T cells, probably expressing the CD4+ phenotype, are involved in killing infected cells, with IL-12 and IL-18 involved in the induction of the response (25, 33). Our data support this hypothesis, since peripheral blood mononuclear cells from animals immunized with live tachyzoites 19 weeks previously contained cells that proliferated and secreted IFN-γ following restimulation with specific antigen. This suggests that a population of memory T cells was present in these animals. Others have previously shown that proliferative responses in N. caninum-infected cattle are associated with CD4+ T cells and that these are the principal secretors of IFN-γ (1, 16, 29). In contrast, although the two lysate-immunized groups developed IFN-γ and proliferation responses immediately following immunization, either no memory cells were induced or the precursor frequency of antigen-specific T cells was too low to produce enough IFN-γ to be detected in our assays in these animals on the day of challenge, 15 weeks after the second immunization.
While with a live immunization, we had, in effect, infected the cattle, there was no evidence that we had established a persistent infection since DNA was not detected in the predilection sites, i.e., the brains and hearts of the infected mothers, at postmortem 28 days after parturition. Moreover, in previous experiments, in cattle infected before pregnancy with an intravenous inoculation of tachyzoites, there was no subsequent transplacental transmission and infection of fetuses (32). To date, we have not found any evidence that experimental postnatal infection of cattle with either oocysts or tachyzoites results in the establishment of a persistent infection and subsequent transplacental transmission (32; C. McCann, M. M. McAllister, D. J. L. Williams, and A. J. Trees, unpublished observations).
In conclusion, our data suggest that a live-tachyzoite vaccine may be a safe and effective option, if given prior to pregnancy, for the control of N. caninum-associated abortions in cattle. In contrast, individual antigens or combinations of antigens will be effective only if they can be delivered in such a way as to induce an effective T-cell response.
Acknowledgments
We are grateful to the staff of the Animal Husbandry Farm for their care of the animals involved in this study. We thank Peter Cripps for statistical advice and Katarina Näslund for excellent technical assistance.
This study was funded by Novartis Animal Health Ltd.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Andrianarivo, A. G., M. L. Anderson, J. D. Rowe, I. A. Gardner, J. P. Reynolds, L. Choromanski, and P. A. Conrad. 2005. Immune responses during pregnancy in heifers naturally infected with Neospora caninum with and without immunization. Parasitol. Res. 96:24-31. [DOI] [PubMed] [Google Scholar]
- 2.Andrianarivo, A. G., B. C. Barr, M. L. Anderson, J. D. Rowe, A. E. Packham, K. W. Sverlow, and P. A. Conrad. 2001. Immune responses in pregnant cattle and bovine fetuses following experimental infection with Neospora caninum. Parasitol. Res. 87:817-825. [DOI] [PubMed] [Google Scholar]
- 3.Andrianarivo, A. G., L. Choromanski, S. P. McDonough, A. E. Packham, and P. A. Conrad. 1999. Immunogenicity of a killed whole Neospora caninum tachyzoite preparation formulated with different adjuvants. Int. J. Parasitol. 29:1613-1625. [DOI] [PubMed] [Google Scholar]
- 4.Andrianarivo, A. G., J. D. Rowe, B. C. Barr, M. L. Anderson, A. E. Packham, K. W. Sverlow, L. Choromanski, C. Loui, A. Grace, and P. A. Conrad. 2000. A POLYGEN-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int. J. Parasitol. 30:985-990. [DOI] [PubMed] [Google Scholar]
- 5.Barber, J. S., O. J. Holmdahl, M. R. Owen, F. Guy, A. Uggla, and A. J. Trees. 1995. Characterization of the first European isolate of Neospora caninum (Dubey, Carpenter, Speer, Topper and Uggla). Parasitology 111:563-568. [DOI] [PubMed] [Google Scholar]
- 6.Björkman, C., K. Näslund, S. Stenlund, S. W. Maley, D. Buxton, and A. Uggla. 1999. An IgG avidity ELISA to discriminate between recent and chronic Neospora infection. J. Vet. Diagn. Investig. 11:41-44. [DOI] [PubMed] [Google Scholar]
- 7.De Marez, T., S. Liddell, J. P. Dubey, M. C. Jenkins, and L. Gasbarre. 1999. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int. J. Parasitol. 29:1647-1657. [DOI] [PubMed] [Google Scholar]
- 8.Eperon, S., K. Bronnimann, A. Hemphill, and B. Gottstein. 1999. Susceptibility of B-cell deficient C57BL/6 (microMT) mice to Neospora caninum infection. Parasite Immunol. 21:225-236. [DOI] [PubMed] [Google Scholar]
- 9.Gondim, L. F., M. M. McAllister, W. C. Pitt, and D. E. Zemlicka. 2004. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 34:159-161. [DOI] [PubMed] [Google Scholar]
- 10.Guy, C. S., D. J. L. Williams, D. F. Kelly, J. M. McGarry, F. Guy, C. Björkman, R. F. Smith, and A. J. Trees. 2001. Neospora caninum in chronically infected pregnant cows: spontaneous transplacental infection is associated with an acute rise in maternal antibody. Vet. Rec. 149:443-449. [DOI] [PubMed] [Google Scholar]
- 11.Haldorson, G. J., B. A. Mathison, K. Wenberg, P. A. Conrad, J. P. Dubey, A. J. Trees, I. Yamane, and T. V. Baszler. 2005. Immunisation with native surface protein NcSRS2 induces a Th2 immune response and reduces congenital Neospora caninum transmission in mice. Int. J. Parasitol. 35:1407-1415. [DOI] [PubMed] [Google Scholar]
- 12.Innes, E. A., S. E. Wright, S. Maley, A. Rae, A. Schock, E. Kirvar, P. Bartley, C. Hamilton, I. M. Carey, and D. Buxton. 2001. Protection against vertical transmission in bovine neosporosis. Int. J. Parasitol. 31:1523-1534. [DOI] [PubMed] [Google Scholar]
- 13.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 14.Liddell, S., M. C. Jenkins, C. M. Collica, and J. P. Dubey. 1999. Prevention of vertical transfer of Neospora caninum in BALB/c mice by vaccination. J. Parasitol. 85:1072-1075. [PubMed] [Google Scholar]
- 15.Lindsay, D. S., S. D. Lenz, B. L. Blagburn, and D. A. Brake. 1999. Characterization of temperature-sensitive strains of Neospora caninum in mice. J. Parasitol. 85:64-67. [PubMed] [Google Scholar]
- 16.Lunden, A., J. Marks, S. W. Maley, and E. A. Innes. 1998. Cellular immune responses in cattle experimentally infected with Neospora caninum. Parasite Immunol. 20:519-526. [DOI] [PubMed] [Google Scholar]
- 17.McAllister, M. M., C. Bjorkman, R. Anderson-Sprecher, and D. G. Rogers. 2000. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J. Am. Vet. Med. Assoc. 217:881-887. [DOI] [PubMed] [Google Scholar]
- 18.Miller, C., H. Quinn, C. Ryce, M. P. Reichel, and J. T. Ellis. 2005. Reduction in transplacental transmission of Neospora caninum in outbred mice by vaccination. Int. J. Parasitol. 35:821-828. [DOI] [PubMed] [Google Scholar]
- 19.Miller, C. M. D., H. E. Quinn, P. A. Windsor, and J. T. Ellis. 2002. Characterisation of the first Australian isolate of Neospora caninum from cattle. Aust. Vet. J. 80:620-625. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa, Y., N. Inoue, X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Protective efficacy of vaccination by recombinant vaccinia virus against Neospora caninum infection. Vaccine 19:1381-1390. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2001. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 19:1710-1711. [DOI] [PubMed] [Google Scholar]
- 22.Pinitkiatisakul, S., J. G. Mattsson, M. Wikman, M. Friedman, K. L. Bengtsson, S. Stahl, and A. Lunden. 2005. Immunisation of mice against neosporosis with recombinant NcSRS2 iscoms. Vet. Parasitol. 129:25-34. [DOI] [PubMed] [Google Scholar]
- 23.Romero, J. J., E. Perez, and K. Frankena. 2004. Effect of a killed whole Neospora caninum tachyzoite vaccine on the crude abortion rate of Costa Rican dairy cows under field conditions. Vet. Parasitol. 123:149-159. [DOI] [PubMed] [Google Scholar]
- 24.Singh, M., and D. T. O'Hagan. 2003. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol. 33:469-478. [DOI] [PubMed] [Google Scholar]
- 25.Staska, L. M., T. C. McGuire, C. J. Davies, H. A. Lewin, and T. V. Baszler. 2003. Neospora caninum-infected cattle develop parasite-specific CD4+ cytotoxic T lymphocytes. Infect. Immun. 71:3272-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira, L., A. Marques, C. S. Meireles, A. R. Seabra, D. Rodrigues, P. Madureira, A. M. R. Faustino, C. Silva, A. Ribeiro, P. Ferreira, J. M. Correia da Costa, N. Canada, and M. Vilanova. 2005. Characterisation of the B-cell immune response elicited in BALB/c mice challenged with Neospora caninum tachyzoites. Immunology 116:38-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trees, A. J., M. M. McAllister, C. S. Guy, J. W. McGarry, R. F. Smith, and D. J. L. Williams. 2002. Neospora caninum: oocyst challenge of pregnant cows. Vet. Parasitol. 109:147-154. [DOI] [PubMed] [Google Scholar]
- 28.Trees, A. J., and D. J. L. Williams. 2005. Endogenous and exogenous transplacental transmission—a critical distinction. Trends Parasitol. 21:558-561. [DOI] [PubMed] [Google Scholar]
- 29.Tuo, W., W. C. Davis, R. Fetterer, M. Jenkins, P. C. Boyd, L. C. Gasbarre, and J. P. Dubey. 2004. Establishment of Neospora caninum antigen-specific T cell lines of primarily CD4+ T cells. Parasite Immunol. 26:243-246. [DOI] [PubMed] [Google Scholar]
- 30.Uggla. A., S. Stenlund, O. J. Holmdahl, E. B. Jakubek, P. Thebo, H. Kindahl, and C. Bjorkman. 1998. Oral Neospora caninum inoculation of neonatal calves. Int. J. Parasitol. 28:1467-1472. [DOI] [PubMed] [Google Scholar]
- 31.Williams, D. J. L., H. C. Davison, J. McGarry, B. Helmick, F. Guy, P. Douglas, D. Hogben, A. Otter, and A. J. Trees. 1999. Evaluation of the MASTAZYME ELISA for the detection of serum antibody to Neospora caninum in cattle. Vet. Rec. 145:571-575. [DOI] [PubMed] [Google Scholar]
- 32.Williams, D. J. L., C. S. Guy, J. W. McGarry, F. Guy, L. Tasker, R. Smith, K. MacEachern, P. J. Cripps, D. F. Kelly, and A. J. Trees. 2000. Neospora-associated abortion in cattle: the time of experimentally-induced parasitaemia during gestation determines foetal survival. Parasitology 121:347-358. [DOI] [PubMed] [Google Scholar]
- 33.Williams, D. J. L., and A. J. Trees. 2006. Protecting babies: vaccine strategies to prevent foetal infection in Neospora caninum infected cattle. Parasite Immunol. 28:61-67. [DOI] [PubMed] [Google Scholar]
- 34.Williams, D. J. L., C. S. Guy, R. F. Smith, J. McKay, F. Guy, J. W. McGarry, and A. J. Trees. 2003. First demonstration of protective immunity in cattle with latent Neospora caninum infections. Int. J. Parasitol. 33:1059-1065. [DOI] [PubMed] [Google Scholar]