Abstract
The ability of exogenous interleukin-12 (IL-12) to elicit protective innate immune responses against the extracellular pathogen Streptococcus pneumoniae was tested by infecting BALB/c mice intranasally (i.n.) with S. pneumoniae after i.n. administration of IL-12. It was found that administration of IL-12 resulted in lower bacterial burdens in the infected mice and significantly improved survival rates. All IL-12-treated mice contained higher levels of pulmonary gamma interferon (IFN-γ) after infection and significantly more neutrophils than infected mice not treated with IL-12. IFN-γ was found to be essential for IL-12-induced resistance and for neutrophil influx into the lungs, and the observed changes correlated with increased levels of the IL-8 homologue keratinocyte-derived chemokine (KC). In addition, in vitro tumor necrosis factor alpha (TNF-α) production by alveolar macrophages stimulated with heat-killed pneumococci was enhanced by IFN-γ, and TNF-α in turn could enhance production of KC by lung cells. Finally, IL-12-induced protection was dependent upon the presence of neutrophils and the KC receptor CXCR2. Taken together, the results indicate that exogenous IL-12 can improve innate defense in the lung against S. pneumoniae by inducing IFN-γ production, which in turn enhances chemokine expression, and promotes pulmonary neutrophil recruitment into the infected lung. The findings show that IL-12 and IFN-γ can mediate a protective effect against respiratory infection caused by extracellular bacterial pathogens.
Streptococcus pneumoniae is a human pathogen that infects the host mainly through the respiratory tract. Once it crosses natural barriers, it can cause life-threatening diseases, such as pneumonia, bacteremia, and meningitis (10, 11). This gram-positive bacterial pathogen remains a leading cause of serious illness among infants, immunocompromised patients, and individuals over 60 years of age (11). The large number of pneumococcal serogroups limits the utility of vaccines, suggesting that alternative approaches that target innate host defense mechanisms may be necessary for optimal protection against this pathogen (10). Elimination of extracellular bacteria from the lung of a naive host is typically thought to be mediated primarily through phagocytosis and intracellular killing by alveolar macrophages and neutrophils recruited to the site of infection (2).
The importance of gamma interferon (IFN-γ) and the IFN-γ-inducing cytokine interleukin-12 (IL-12) in phagocyte activation and protection against intracellular pathogens is well accepted, but little is understood about the significance of these cytokines in protection against extracellular microbes (5). Indeed, it has been reported that IL-12 p40−/− BALB/c mice (mice genetically deficient in IL-12 p40 production) and IFN-γ−/− BALB/c mice (mice genetically deficient in IFN-γ production) are more resistant to pneumococcal infection than wild-type animals are (12, 16). We have now addressed the roles of IL-12 and IFN-γ in local protection against pulmonary S. pneumoniae infection by directly administering IL-12 intranasally (i.n.) to mice. The results show that exogenous IL-12 can induce increased protection against this extracellular pathogen, protection that appears to be due to enhanced IFN-γ-mediated neutrophil accumulation in the lung.
MATERIALS AND METHODS
Mice.
Six- to 10-week-old BALB/c mice were purchased from Taconic Laboratories (Germantown, NY). BALB/c IFN-γ−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at Albany Medical College. CXCR2+/− mice on a BALB/c background [C.129S2(B6)-Il8rbtm1Mwm/J] were purchased from Jackson Laboratories, homozygotes were obtained by breeding, and the genotypes of the offspring were confirmed by analysis of tail DNA according to the protocol provided by Jackson Laboratories. All mice were maintained at the Albany Medical College Animal Facility following Institutional Animal Care and Use Committee guidelines.
Treatment with IL-12.
Mice under light isoflurane anesthesia were inoculated i.n. 24 h before infection with 0.5 μg of recombinant murine IL-12 (provided by Wyeth Vaccines, Pearl River, NY) in 25 μl of phosphate-buffered saline (PBS) containing 1% normal mouse serum as the vehicle. Control mice received only the vehicle (PBS containing 1% normal mouse serum) before infection.
Bacterial infection.
The A66.1 S. pneumoniae strain, which expresses pneumococcal polysaccharide serotype 3 (14) (provided by David E. Briles, University of Alabama at Birmingham, Birmingham) was cultured at 37°C in Todd-Hewitt broth until mid-log phase, washed repeatedly, resuspended in fresh broth containing 15% glycerol, and stored at −70°C until use. To induce pneumonia, mice were anesthetized with ketamine HCl (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Phoenix Scientific, St. Joseph, MO) in PBS and inoculated i.n. with S. pneumoniae in 50 μl of Ringer's solution. Bacterial burden in the lungs and blood was measured by sacrificing infected mice at various time points as indicated, and plating serial 10-fold dilutions of whole-blood samples and lung homogenates onto blood agar plates. The plates were incubated at 37°C overnight, and CFU were enumerated 24 h later.
Expression of alveolar and lung inflammatory cells.
Bronchoalveolar lavage fluid (BALF) samples were collected by making an incision in the trachea and washing the lungs twice with 0.8 ml PBS, pH 7.2. Total leukocyte counts were determined using a hemacytometer. BALF cell populations were further evaluated using Diff-Quick-stained cytospin preparations. Neutrophil levels in lung tissue samples were quantified by measuring the activity of myeloperoxidase (MPO) (an intracellular enzyme specific for neutrophils) in lung homogenates as previously described (1). Briefly, 150 μl of lung homogenate was centrifuged at 2,000 × g for 10 min. The cell pellet was suspended in 300 μl of 0.2 mM potassium phosphate buffer, pH 7.4, containing 0.5% hexadecyltrimethylammonium bromide. The mixture was sonicated for 20 seconds and was centrifuged at 3,000 × g for 30 min. MPO activity was measured by adding 5 μl of supernatant fluid to 250 μl of reaction solution (0.01 mg/ml o-dianisidine and 0.004% H2O2 in potassium phosphate buffer). The reaction was stopped by adding 25 μl of 1% sodium azide, and optical density was read at 450 nm. All reagents were purchased from Sigma (St. Louis, MO).
ELISA.
BALF samples were harvested at the indicated time points and assayed for IL-1β, IFN-γ, IL-10, and keratinocyte-derived chemokine (KC) by an enzyme-linked immunosorbent assay (ELISA) using commercially available kits from R&D Systems (Minneapolis, MN). Tumor necrosis factor alpha (TNF-α) levels were determined using a sandwich ELISA developed in the laboratory. Briefly, microtiter plates were coated with MP-XT22 rat anti-mouse TNF-α monoclonal antibody (MAb) (isolated from hybridoma culture supernatants using anti-rat immunoglobulin G [IgG] agarose columns). After the plates were blocked with 1% bovine serum albumin in PBS, BALF samples were added to the wells and incubated for 2 h at room temperature. Biotin-labeled anti-TNF-α polyclonal antibody (BD Biosciences, San Diego, CA) was then added for 1 h at room temperature. Finally, an avidin-biotin-horseradish peroxidase complex (BD Biosciences) was added, and OptEIA substrate solution (BD Biosciences) was used for signal development.
Neutrophil depletion.
Anti-Ly6G MAb was purified from culture supernatants of the RB6-8C5 hybridoma using anti-rat IgG agarose columns (Sigma). Wild-type BALB/c mice were injected intraperitoneally with 0.5 mg of RB6-8C5 anti-Ly6G MAb or rat IgG as a control on days −1 and 0 of infection to deplete neutrophils. The efficiency of neutrophil depletion in BALF samples of pneumococcus-infected mice was confirmed by using Diff-Quick-stained cytospin preparations.
Intracellular cytokine staining.
Single-cell suspensions were obtained from lungs by digestion with 2.5 mg/ml collagenase D, 0.25 mg/ml DNase I (Roche Diagnostics, Mannheim, Germany), and 1 mM MgCl2 for 1 h at 37°C under constant agitation, followed by passage through a nylon mesh and density gradient centrifugation on Lympholyte M (Cedarlane Laboratories Ltd., Ontario, Canada). The lung lymphocytes from BALB/c mice were cultured with different stimuli in Dulbecco modified Eagle medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% fetal calf serum, 10 μg/ml penicillin, 10 μg/ml streptomycin, 4 mM l-glutamine, and 50 μM 2-mercaptoethanol for 4 h at 37°C and then for another 4 h in the presence of 10 μg/ml brefeldin A (Epicenter Technology, Madison, WI). The cells were fixed with 2% paraformaldehyde and stained with Alexa Fluor-conjugated anti-IFN-γ, fluorescein isothiocyanate-conjugated anti-CD3, and phycoerythrin-conjugated anti-DX5 MAbs by our standard procedure (13). The fluorescent MAbs were all obtained from BD Biosciences. The stained cells were stored in the dark at 4°C and analyzed within 24 h on a FACSCanto using FACSDiva software.
In vitro stimulation.
Airway cells from BALB/c mice were obtained by repeated lung lavage with PBS and added to a 96-well microplate (Nalge Nunc, Rochester, NY) at a concentration of 6 × 104 cells/well. The plates were incubated at 37°C for 2 h to allow adherence of macrophages, and the nonadherent cells were removed by gentle washing. IL-12 (5 ng/ml) or IFN-γ (1 ng/ml) was added to the cultures with or without heat-killed pneumococci equivalent to 2.5 × 105 CFU/well. Culture supernatants were then collected to determine TNF-α production by an ELISA. Similarly, to determine KC production, lung cell suspensions were incubated in 96-well plates (105 cells/well) at 37°C for 5 days. Nonadherent cells were removed, and the adherent cells were incubated overnight with IL-12 (5 ng/ml), IFN-γ (1 ng/ml), or TNF-α (10 ng/ml) with or without 2.5 × 104 CFU of heat-killed pneumococci/well.
Statistical analysis.
Mann-Whitney U test was used for analysis of bacterial loads in lung tissue and blood. Correlation between bacterial burden in the lungs and blood samples of individual mice was determined by the Spearman rank correlation test. Survival analyses were performed using the Kaplan-Meier log rank test. Student's t test was used to compare cytokine and neutrophil levels in IL-12- and PBS-treated groups. A P value of <0.05 was considered to be significant.
RESULTS
Exogenous IL-12 treatment protects BALB/c mice from lethal respiratory infection with S. pneumoniae.
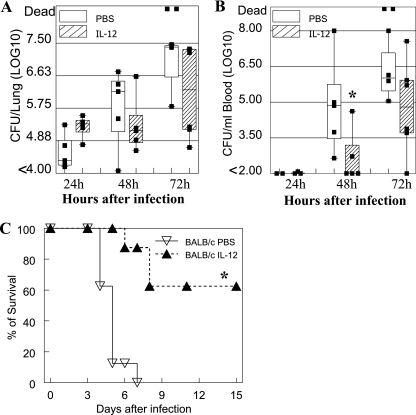
To determine the possible effect of exogenous IL-12 treatment on respiratory protection against S. pneumoniae infection, IL-12 was inoculated i.n. into BALB/c mice 24 h before i.n. bacterial challenge (1 × 105 CFU/mouse). Lower numbers of bacteria were found in the lungs of IL-12- versus PBS-treated mice 48 and 72 h after infection (Fig. 1A), although the differences were not significant due to two mice dying at 72 h from infection in the PBS-treated group (P = 0.055). Bacterial burdens in the blood of the IL-12-treated mice were also lower at 48 h after infection than those in the PBS-treated infected group, and this difference was statistically significant (P = 0.045) (Fig. 1B). By day 9 after infection, most of the surviving mice had completely cleared the infection from the blood (data not shown). In each individual mouse, the bacterial burdens in the lung and blood were significantly correlated as determined by the Spearman rank correlation test (P < 0.001). Most importantly, administration of IL-12 significantly improved the survival rates of lethally infected mice (1.5 × 105 CFU/mouse) (Fig. 1C). On the other hand, the same i.n. treatment with IL-12 did not improve survival of intraperitoneally infected mice (data not shown).
FIG. 1.
IL-12 improves protection of BALB/c mice from respiratory infection with S. pneumoniae. BALB/c mice were treated i.n. with 0.5 μg of IL-12 or with PBS vehicle 24 h before infection. Bacterial burdens were measured in the lungs (A) and blood (B) of mice infected i.n. with 1 × 105 CFU of S. pneumoniae. The box-and-whisker plots indicate the high and low values, medians, and interquartile ranges (at least five mice per group). The lower limit of detection was 102 CFU/ml. (C) Survival rates of BALB/c mice (eight mice/group) after i.n. infection with 1.5 × 105 CFU of S. pneumoniae. Values that were significantly different (P < 0.05) from those of the PBS-treated group are indicated (*). The data shown are representative of at least two independent experiments.
Importance of IFN-γ in IL-12-induced protection against pneumococcal infection.
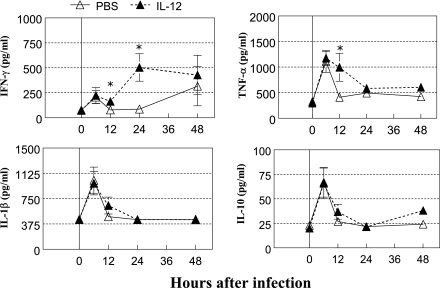
To elucidate the mechanism of IL-12-induced protection against pneumococcal infection, cytokine levels in the respiratory tract after pulmonary bacterial infection (7 × 104 CFU/mouse) were determined. The levels of TNF-α, IL-1β, and IL-10 in BALF samples increased after infection, reaching peak levels at 6 h and then declining thereafter (Fig. 2). IFN-γ expression showed a similar pattern, although in IL-12-treated mice, levels further increased 12 h after infection compared to the PBS-treated group. IL-12 given i.n. did not promote IFN-γ expression in blood after bacterial infection (data not shown), suggesting a local pulmonary effect of the IL-12 treatment. Expression of TNF-α was also significantly higher in IL-12-treated mice than in PBS-treated mice 12 h postinfection. The levels of IL-1β and IL-10 did not differ significantly between IL-12- and PBS-treated mice on any day of infection.
FIG. 2.
Cytokine levels in BALF after pneumococcal infection. BALB/c mice were treated i.n. with 0.5 μg of IL-12 or with PBS vehicle 24 h before infection. BALF samples were harvested 0, 6, 12, 24, and 48 h after infection with 7 × 104 CFU of S. pneumoniae and assayed for IFN-γ, TNF-α, IL-1β, and IL-10 levels by an ELISA. The results represent the mean levels ± standard errors of the means (error bars) of each cytokine (five mice/group at each time point). Values that were significantly different (P < 0.05) from those of the PBS-treated group are indicated (*). The data shown are representative of three independent experiments.
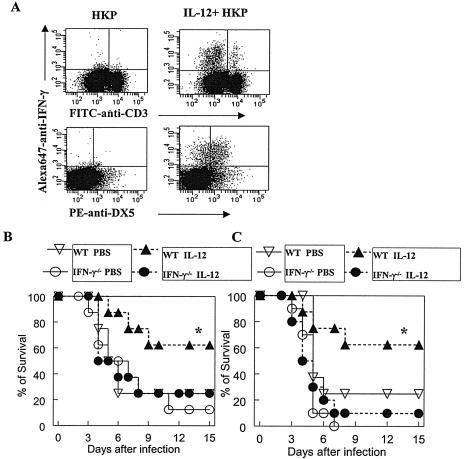
To determine the lung cell population(s) responsible for IFN-γ production after IL-12 stimulation, pulmonary lymphocytes from BALB/c mice were isolated and incubated for 8 h with heat-killed pneumococci in the presence or absence of IL-12. As determined by flow cytometric cell staining, incubation with heat-killed pneumococci induced IFN-γ-producing (IFN-γ+) cells only in the presence of IL-12, and nearly all of the cells producing IFN-γ were DX5+ CD3− NK cells (Fig. 3A).
FIG. 3.
Involvement of IFN-γ in IL-12-induced protection against pneumococcal infection. (A) Lung cells isolated from BALB/c mice were stimulated with heat-killed pneumococci equivalent to 5 × 106 CFU/ml alone or in the presence of 5 ng/ml IL-12 for 8 h, and intracellular IFN-γ expression was determined by flow cytometry, using fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (BD Biosciences) to identify T cells, phycoerythrin (PE)-conjugated anti-DX5 (BD Biosciences) to identify NK cells, and Alexa Fluor 647 (Alexa647)-conjugated anti-IFN-γ (BD Biosciences) to identify IFN-γ+ cells. Results from the lung lymphocyte gate are shown. The survival rates of wild-type (WT) BALB/c and IFN-γ−/− mice (eight mice/group) were measured after i.n. infection with 5 × 104 CFU/mouse (B) or 1 × 105 CFU/mouse (C). IL-12 did not improve protection against pneumococcal infection in IFN-γ−/− mice. Values that were significantly different (P < 0.05) from those of the PBS-treated group are indicated (*).
The requirement for IFN-γ in IL-12-mediated protection against pneumococcal infection was investigated using mice genetically deficient in IFN-γ production. There were no significant differences in susceptibility to pneumococcal infection between PBS-treated wild-type BALB/c and IFN-γ−/− mice (5 × 104 CFU/mouse and 1 × 105 CFU/mouse) (Fig. 3B and C). However, the protection observed in wild-type mice after IL-12 administration was not observed in IFN-γ−/− mice. These results suggest that IFN-γ plays an essential role in mediating IL-12-induced resistance against pulmonary pneumococcal infection.
Involvement of IFN-γ in neutrophil recruitment after pneumococcal infection.
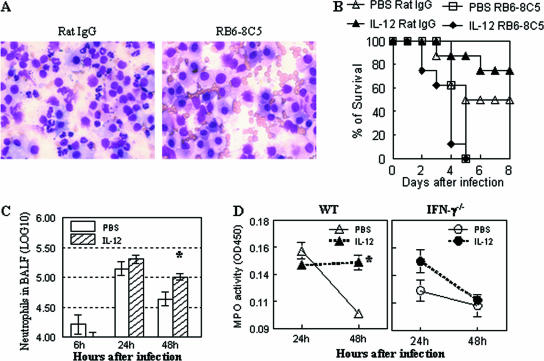
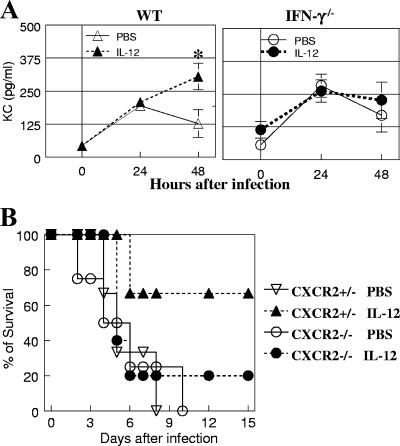
Neutrophils are known to play a critical role in protection from pneumococcal infection (6). Similarly, in our lung infection model, neutrophil depletion by anti-Ly6G antibody treatment resulted in increased susceptibility to pneumococcal infection regardless of IL-12 treatment (5 × 104 CFU/mouse) (Fig. 4A and B). The potential effect of IL-12 treatment on pulmonary neutrophil expression was thus investigated next. The numbers of neutrophils increased within 1 day after infection in both PBS- and IL-12-treated mice. However, while lung neutrophil numbers in the PBS-treated group were diminished dramatically by 48 h, there were still high levels of pulmonary neutrophils in IL-12-treated mice 48 h after infection, as indicated both by significantly elevated levels of cells in the BALF samples and increased MPO activity in lung tissue samples (Fig. 4C and D). In contrast to wild-type mice, MPO activity in lung homogenates of IL-12-treated IFN-γ−/− mice decreased by 48 h after infection, similar to that seen in PBS-treated wild-type mice (Fig. 4D). There were no significant differences in the levels of macrophages recovered from the BALF samples of IL-12- and PBS-treated infected mice, and incubation with IFN-γ did not enhance in vitro pneumococcal killing activity of alveolar macrophages (data not shown). These results suggest that IL-12 treatment primarily promotes neutrophil influx and/or retention in the lungs of infected mice, which correlates with increased IFN-γ expression and enhanced resistance to infection. In agreement with this, it was found that IL-12 treatment of wild-type mice, but not IFN-γ−/− mice, caused sustained expression of the neutrophil chemoattractant KC in lung tissue (Fig. 5A). The protective role of KC was further investigated in CXCR2−/− mice that have a targeted mutation in the receptor for KC. In the absence of IL-12 treatment, the susceptibility of CXCR2+/− mice to pneumococcal infection was similar to that of CXCR2−/− mice (3 × 104 CFU/mouse). However, CXCR2−/− mice were found to be highly susceptible to pneumococcal infection regardless of exogenous IL-12 treatment (Fig. 5B).
FIG. 4.
Involvement of IFN-γ in lung neutrophil expression after pneumococcal infection. (A) Diff-Quick-stained BALF cells from RB6-8C5 rat MAb-treated (neutrophil-depleted) and rat IgG-treated (control) mice 24 h after infection. (B) BALB/c mice were treated i.n. with 0.5 μg of IL-12 or with vehicle (PBS) 24 h before infection. Survival rates of RB6-8C5 rat MAb-treated (neutrophil-depleted) and rat IgG-treated (control) mice were measured after i.n. infection with 5 × 104 CFU/mouse. IL-12 did not improve protection against pneumococcal infection in neutrophil-depleted mice. (C) Numbers of neutrophils in BALF after infection with 7.5 × 104 CFU/mouse (five mice/group). (D) Neutrophil infiltration (MPO activity) in lung tissue of wild-type (WT) and IFN-γ−/− mice (five mice/group). OD450, optical density at 450 nm.
FIG. 5.
Role of KC in protection against pneumococcal infection. (A) KC levels in BALF of wild-type (WT) BALB/c and IFN-γ−/− mice (five mice/group). Results represent the means ± standard errors of the means (error bars). Values that were significantly different (P < 0.05) from those of the PBS-treated mice are indicated (*). (B) Survival rates of BALB/c CXCR2+/− (three mice/group) and CXCR2−/− (four or five mice/group) mice infected with 3 × 104 CFU/mouse in the presence or absence of IL-12.
Ability of IFN-γ to enhance TNF-α expression.
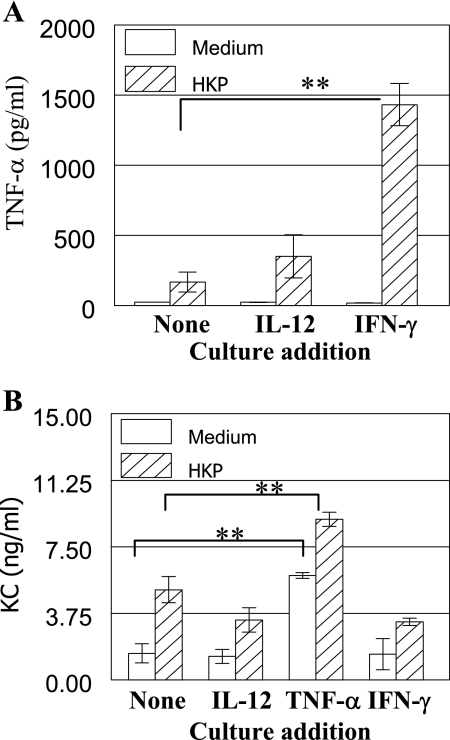
A possible mechanism by which IL-12-induced IFN-γ could improve protection is through enhancing macrophage production of TNF-α after infection, which in turn would induce KC expression and influx of neutrophils. Experiments were conducted to examine the in vitro effect of IFN-γ on the production of TNF-α by alveolar macrophages. TNF-α production was greatly increased when isolated macrophages were incubated with both heat-killed pneumococci (2.5 × 106 CFU/ml) and IFN-γ compared to either stimulant alone or after incubation with IL-12 plus heat-killed pneumococci (Fig. 6A). Furthermore, incubation of adherent lung cells with TNF-α increased KC production (Fig. 6B). Production of KC was further enhanced by coculture with TNF-α and heat-killed pneumococci, but there was minimal increase after stimulation with IL-12 or IFN-γ. These results indicate that IFN-γ in the lung increases macrophage TNF-α expression, which in turn enhances KC production and neutrophil recruitment.
FIG. 6.
In vitro stimulation of alveolar macrophages and cells adhering to the lung. (A) Purified alveolar macrophages from BALB/c mice were incubated in vitro with medium alone or with heat-killed pneumococci equivalent to 2.5 × 106 CFU/ml in the presence of the indicated cytokine for 24 h. Culture supernatants were assayed for TNF-α production by an ELISA. (B) Cells from BALB/c mice adhering to the wells were incubated in vitro with medium alone or heat-killed pneumococci (HKP) equivalent to 2.5 × 105 CFU/ml in the presence of the indicated cytokine for 24 h. Culture supernatants were assayed for KC production by an ELISA. The results are expressed as the means of four replicate cultures ± standard deviations (error bars) and are representative of at least two experiments. Values that were significantly different (P < 0.01) from those of the PBS-treated group are indicated (**).
DISCUSSION
The results presented in this study demonstrate that exogenous IL-12 given i.n. to BALB/c mice can induce protection against respiratory pneumococcal infection. Expression of IFN-γ was required for protection, which correlated with increased numbers of neutrophils and enhanced KC levels in the lung. NK cells were the primary cells responsible for IFN-γ production, and IFN-γ in turn was found to increase TNF-α production by bacterium-stimulated alveolar macrophages as shown by in vitro studies. The results suggest that i.n. inoculation of BALB/c mice with IL-12 can induce a cascade of events beginning with NK cell activation and IFN-γ production, followed by induction of TNF-α secretion by macrophages. TNF-α then mediates neutrophil recruitment through enhanced KC expression. Increased neutrophil levels in the lung are likely to be ultimately responsible for the observed IL-12-mediated protection.
We found that i.n. inoculation of exogenous IL-12 provided protection against subsequent respiratory infection with S. pneumoniae. Protection was observed in relation to both increased survival rates and decreased bacterial burdens in the lung and blood and was dependent upon expression of IFN-γ. The observed protective capacity of IL-12 was somewhat surprising, since it has been reported (12) that mice deficient in IL-12 expression (both IL-12 p35−/− and IL-12 p40−/− mice) are more resistant to pneumococcal infection than are wild-type mice. In addition, IFN-γ−/− mice have been found to have fewer bacteria in lungs after pneumococcal infection than IFN-γ+/+ mice do (16). These results, obtained using BALB/c mouse models, suggest a detrimental role for IL-12 and IFN-γ in protection against S. pneumoniae. However, IL-12 p40−/− and IFN-γ−/− mice on a C57BL/6 background have been reported to be more susceptible to pneumococcal infection than are wild-type mice (18, 21). The reason for the observed differences between our results using wild-type mice treated with exogenous IL-12 and the results of others using BALB/c mice with a deficient gene may relate to the fact that IL-12 in our study was delivered locally and directly into the lung. Local expression of IL-12 and IFN-γ appears to be beneficial, while systemic overexpression is likely to be detrimental and promote septic shock, a process that would be prevented in knockout mice. Similarly, differences among different mouse strains could relate to variability in the extent and kinetics of systemic IL-12 and/or IFN-γ production. We have found that local delivery of IL-12 to both wild-type BALB/c and C57BL/6 mice as well as SCID mice (data not shown) can be equally protective. Of note, we did not observe a significant increase in IL-12 expression in the lungs of mice 24 h after pneumococcal infection.
Pneumococcal infection was found to induce increased levels of IFN-γ in the BALF samples of IL-12-treated mice. The importance of this IFN-γ was demonstrated by the findings that IL-12 treatment did not protect IFN-γ−/− mice and that treatment of BALB/c mice with a neutralizing anti-IFN-γ antibody prevented IL-12-mediated bacterial lung clearance (data not shown). It is likely that lung NK cells are the major source of IFN-γ during infection, since culture of pulmonary lymphocytes with IL-12-treated and heat-killed pneumococci was shown to induce large numbers of IFN-γ-secreting NK cells as determined by intracellular cytokine analysis. We previously reported the presence of large numbers of IFN-γ-secreting NK cells in the lungs of mice after i.n. infection with Francisella tularensis (13), and others (3) have similarly found an involvement of NK cells in mice infected with Bordetella pertussis. Thus, it appears that NK cells play a major role in protection against various respiratory microbes, including the extracellular gram-positive pathogen S. pneumoniae.
Further experiments showed that IL-12 treatment led to increased levels of pulmonary neutrophils, as determined by both direct BALF cell counting and measurement of MPO activity in lung homogenates. The increase in neutrophil numbers correlated with increased levels of TNF-α as well as the IL-8 homologue KC, which is a neutrophil chemoattractant. TNF-α production was induced by culturing alveolar macrophages with IFN-γ plus heat-killed pneumococci, and KC expression was induced by TNF-α, but not IFN-γ (although increased expression of KC after exposure to IL-12 was not observed in IFN-γ−/− mice). We interpret these results to indicate that IL-12 enhances IFN-γ production by NK cells, which then acts on alveolar macrophages to induce production of TNF-α. TNF-α upregulates expression of KC, and this in turn causes increased recruitment and/or retention of lung neutrophils, cells that are ultimately responsible for mediating the enhanced bacterial clearance. Neutrophil influx into the lung is known to occur in response to many bacterial infections, including pneumococcal infections (6, 9). The CXC chemokines macrophage inflammatory protein 1 and KC, which are murine homologues of human IL-8, are the main chemokines responsible for attracting neutrophils to sites of inflammation in mice (20). It is possible that IFN-γ could act directly on neutrophils, since IFN-γ is known to increase expression of the gp91-phox subunit of NADPH oxidase, while downregulating expression of the p47-phox subunit (5). However, impairment of host defense has been found only in p47−/− mice, not in gp91−/− mice, during pneumococcal meningitis (19). It is also possible that IL-12 treatment directly causes lung macrophages to become more phagocytic. Nevertheless, we could not detect increased killing of pneumococci by the MH-S alveolar macrophage cell line after exposure to either IL-12 or IFN-γ, despite an increased production of nitric oxide (data not shown). Furthermore, exogenous IFN-γ treatment before infection did not lead to increased bacterial clearance in the lung (data not shown). Depletion of neutrophils abrogated the ability of IL-12 to enhance survival after infection but did not affect the number of lung macrophages.
IL-12 and IFN-γ are generally believed to play important roles in host defense against intracellular pathogens (17). We have now shown that IL-12 improves pulmonary clearance of extracellular gram-positive bacteria by promoting neutrophil accumulation through IFN-γ-dependent mechanisms. Of note, we did not observe pathological changes in mice given 1 μg of IL-12. i.n and IL-12 treatment alone did not induce significant levels of IFN-γ. There are only a limited number of reports of IL-12 inducing protection against extracellular organisms (4, 7, 15). Nevertheless, it is clear from the results presented in the current study that protection against extracellular bacterial pathogens in the respiratory tract represents an important function for this cytokine.
Acknowledgments
This research was supported by NIH grant RO1 AI 41715 and a grant from Philip Morris USA Inc./Philip Morris International.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Ayala, A., C. S. Chung, J. L. Lomas, G. Y. Song, L. A. Doughty, S. H. Gregory, W. G. Cioffi, B. W. LeBlanc, J. Reichner, H. H. Simms, and P. S. Grutkoski. 2002. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am. J. Pathol. 161:2283-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttery, J., and E. R. Moxon. 2002. Capsulate bacteria and the lung. Br. Med. Bull. 61:63-80. [DOI] [PubMed] [Google Scholar]
- 3.Byrne, P., P. McGuirk, S. Todryk, and K. H. Mills. 2004. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 34:2579-2588. [DOI] [PubMed] [Google Scholar]
- 4.Derrico, C. A., and K. J. Goodrum. 1996. Interleukin-12 and tumor necrosis factor alpha mediate innate production of gamma interferon by group B Streptococcus-treated splenocytes of severe combined immunodeficiency mice. Infect. Immun. 64:1314-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis, T. N., and B. L. Beaman. 2004. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 112:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvy, B. A., and A. G. Harmsen. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499-512. [DOI] [PubMed] [Google Scholar]
- 7.Hazlett, L. D., X. L. Rudner, S. A. McClellan, R. P. Barrett, and S. Lighvani. 2002. Role of IL-12 and IFN-gamma in Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Vis. Sci. 43:419-424. [PubMed] [Google Scholar]
- 8.Hunter, C. A., T. Slifer, and F. Araujo. 1996. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infect. Immun. 64:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, M. R., B. T. Simms, M. M. Lupa, M. S. Kogan, and J. P. Mizgerd. 2005. Lung NF-κB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 175:7530-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadioglu, A., and P. W. Andrew. 2004. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25:143-149. [DOI] [PubMed] [Google Scholar]
- 11.Klugman, K. P., and C. Feldman. 2001. Streptococcus pneumoniae respiratory tract infections. Curr. Opin. Infect. Dis. 14:173-179. [DOI] [PubMed] [Google Scholar]
- 12.Lauw, F. N., J. Branger, S. Florquin, P. Speelman, S. J. Van Deventer, S. Akira, and T. van der Poll. 2002. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J. Immunol. 168:372-378. [DOI] [PubMed] [Google Scholar]
- 13.Lopez, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 232:75-85. [DOI] [PubMed] [Google Scholar]
- 14.McDaniel, L. S., G. Scott, J. F. Kearney, and D. E. Briles. 1984. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160:386-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metzger, D. W., R. Raeder, V. H. Van Cleave, and M. D. Boyle. 1995. Protection of mice from group A streptococcal skin infection by interleukin-12. J. Infect. Dis. 171:1643-1645. [DOI] [PubMed] [Google Scholar]
- 16.Rijneveld, A. W., F. N. Lauw, M. J. Schultz, S. Florquin, A. A. Te Velde, P. Speelman, S. J. Van Deventer, and T. Van Der Poll. 2002. The role of interferon-gamma in murine pneumococcal pneumonia. J. Infect. Dis. 185:91-97. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig, S. D., and S. M. Holland. 2005. Defects in the interferon-gamma and interleukin-12 pathways. Immunol. Rev. 203:38-47. [DOI] [PubMed] [Google Scholar]
- 18.Rubins, J. B., and C. Pomeroy. 1997. Role of gamma interferon in the pathogenesis of bacteremic pneumococcal pneumonia. Infect. Immun. 65:2975-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaper, M., S. L. Leib, D. N. Meli, R. P. Brandes, M. G. Tauber, and S. Christen. 2003. Differential effect of p47phox and gp91phox deficiency on the course of pneumococcal meningitis. Infect. Immun. 71:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Damme, J., A. Wuyts, G. Froyen, E. Van Coillie, S. Struyf, A. Billiau, P. Proost, J. M. Wang, and G. Opdenakker. 1997. Granulocyte chemotactic protein-2 and related CXC chemokines: from gene regulation to receptor usage. J. Leukoc. Biol. 62:563-569. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto, N., K. Kawakami, Y. Kinjo, K. Miyagi, T. Kinjo, K. Uezu, C. Nakasone, M. Nakamatsu, and A. Saito. 2004. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect. 6:1241-1249. [DOI] [PubMed] [Google Scholar]