Abstract
Cryptosporidiosis, caused by the protozoan parasite Cryptosporidium, causes self-limited diarrhea in immunocompetent hosts and severe life-threatening diarrhea in AIDS patients. Highly active antiretroviral therapy has been used to effectively treat cryptosporiosis in some but not all AIDS patients. Therefore, there is an urgent need for innovative drugs to treat this disease. Cryptosporidium infection results in intestinal pathophysiological changes such as glucose malabsorption, increased chloride ion (Cl−) secretion, and epithelial barrier disruption, leading to disease pathogenesis. In order to develop tools to combat this opportunistic pathogen, it is vital to understand mediators involved in disease pathogenesis. Substance P (SP), a neuropeptide and pain transmitter, is located in the gastrointestinal tract. SP can cause Cl− secretion in human gastrointestinal explants. However, its role in cryptosporidiosis has not been fully studied. Jejunal samples from macaques before and after Cryptosporidium parvum infection were assayed for SP and SP receptor mRNA and protein levels by reverse transcription-PCR and by immunohistochemistry and enzyme-linked immunosorbent assay, respectively. The role of SP in pathophysiological alterations, such as Cl− secretion and glucose malabsorption, was studied using tissues derived from macaques infected with C. parvum by the Ussing chamber technique. SP and SP receptor mRNA and protein expression levels were increased in jejunal samples following C. parvum infection and were accompanied by increased basal ion secretion and glucose malabsorption. In vitro treatment of samples obtained from infected macaques with the SP receptor antagonist aprepitant (Emend; Merck, Whitehouse Station, NJ) completely reversed the increase in basal ion secretion and corrected the glucose malabsorption. Our findings raise the possibility of using SP receptor antagonists for the treatment of symptoms associated with cryptosporidiosis.
Cryptosporidiosis is caused by the protozoan parasite Cryptosporidium. Individuals with congenital T- or B-lymphocyte deficiencies, severe combined immunodeficiency disease, chemotherapy-induced immunodeficiency, or AIDS are at risk for increased morbidity and potentially fatal infections following exposure to Cryptosporidium. The increasing population of immunodeficient persons together with recent outbreaks of cryptosporidiosis occurring as a result of the consumption of drinking water contaminated with Cryptosporidium oocysts have highlighted the importance of this pathogen. Unlike other intestinal pathogens, Cryptosporidium can survive in most environments for long periods of time due to its “hardy cyst” (20). Cryptosporidium also causes diarrheal disease in neonatal ruminants and farm animals, and these infections result in reservoirs of infectious oocysts that have economic, environmental, and zoonotic implications. In immunocompetent individuals, cryptosporidiosis is a self-limited form of diarrheal illness. Previous studies involving volunteers demonstrated that as few as 10 oocysts isolated from experimentally infected calves can cause infection in healthy adults (31). AIDS patients with cryptosporidiosis may experience severe debilitating diarrhea, resulting in the excretion of 0.6 to 20 liters of watery stools per day accompanied by chronic abdominal pain (19).
Little is known about the pathogenesis of cryptosporidiosis (16). Cryptosporidium infection results in intestinal structural and physiologic changes such as reduced glucose-NaCl absorption, increased chloride anion secretion (Cl−), and epithelial barrier disruption (10, 12, 17, 18). These alterations are known to lead to the pathogenesis of cryptosporidiosis, which includes dehydration, weight loss, diarrhea, and malabsorption.
Substance P (SP), a neuropeptide and pain transmitter, is located in areas of inflammation including the gastrointestinal tract. SP can cause chloride ion secretion in human gastrointestinal explants (34). In previous studies, we examined the expression of SP mRNA and protein in intestinal biopsies from healthy volunteers who were experimentally challenged with Cryptosporidium parvum (mild disease) and AIDS patients with naturally occurring uncontrolled cryptosporidiosis. We noted that SP correlated with the severity of illness; i.e., levels of SP mRNA and protein were increased in AIDS patients compared to those in healthy volunteers with self-limited disease (35). Although these human studies established the association of SP levels with the severity of diarrheal symptoms, they did not address the role of SP in the physiological changes, such as chloride ion secretion and glucose malabsorption, occurring in cryptosporidiosis. In the current studies, using a macaque model of cryptosporidiosis, we have evaluated the role of SP in cryptosporidiosis-associated physiological alterations. We determined the expression of SP and SP receptor (NK1) mRNA and protein expression and physiological alterations (by the Ussing chamber technique) in intestinal tissues derived from macaques before and after challenge with C. parvum. Furthermore, we studied physiological alterations in C. parvum-infected intestinal tissues with and without in vitro SP receptor antagonist treatment.
The studies outlined in the current paper demonstrated that levels of jejunal SP and SP receptor mRNA and protein are up-regulated during Cryptosporidium infection in macaques and that the treatment of jejunal samples from C. parvum-infected animals with SP receptor antagonist in vitro rectifies physiological alterations such as Cl− ion secretion and glucose malabsorption. These findings support a central role for SP in the pathogenesis of cryptosporidiosis and raise the possibility of using an SP receptor antagonist to combat the symptoms associated with this disease.
MATERIALS AND METHODS
Purification of Cryptosporidium oocysts.
C. parvum was purified from the feces of calves that were previously inoculated with C. parvum as described previously by Harp et al. (14). Briefly, oocyst suspensions were washed with sterile phosphate-buffered saline (PBS) (pH 7.2) and treated with a 1:3 bleach solution for 7 min for excystation of the oocysts. The excysted C. parvum oocyst was then washed several times with 1× PBS, followed by resuspension in 1 ml of Hanks balanced salt solution (and incubated at 37°C for 90 min). A hemocytometer was used to count the oocysts. The dose of C. parvum oocysts for inoculation in macaques was then adjusted to 2 × 107 oocysts in 5 ml.
Macaque model of Cryptosporidium infection.
Immunocompetent macaques were inoculated by per os gavage directly into the stomach with a 5-ml aliquot of Cryptosporidium oocyst suspension (2 × 107 C. parvum oocysts in PBS). Following infection, all the animals had diarrheal symptoms (watery stools). Biopsies of the jejunum (15 to 20 cm) were collected from healthy immunocompetent macaques before and 9 to 11 days after experimental cryptosporidiosis infection by laparoscopic surgery. Biopsies were (i) fixed in paraformaldehyde for immunohistochemistry for detection of substance P and NK1 receptor protein, (ii) snap-frozen for the detection of substance P protein and for reverse transcription (RT)-PCR studies for the detection of SP and NK1 receptor mRNA, or (iii) used immediately for the Ussing chamber studies. These studies were approved by the Animal Research Committee at the Tulane Primate Center.
Monitoring of physiological alterations by the Ussing chamber technique.
An electrophysiological technique was used to measure changes in short-circuit current via a dual voltage-current clamp in jejunal tissues mounted in Ussing chambers. Biopsies of the distal jejunum were obtained from three immunocompetent macaques before and after C. parvum challenge. The jejunum was then opened along the mesenteric border and rinsed with Krebs-Ringer bicarbonate solution (140 mM Na, 119.8 mM Cl, 25 mM HCO3, 1.2 mM Mg, 1.2 mM Ca, 4.8 mM K, 2.4 mM HPO4, and 0.4 mM H2PO4 [pH 7.4]). Three contiguous jejunal segments, 2 cm in length, from each animal were obtained and mounted as flat sheets in Ussing chambers with an aperture of 0.725 cm2. Each segment was bathed on its mucosal and serosal surfaces with 10 ml Krebs-Ringer bicarbonate solution. All bathing solutions were bubbled with 95% O2-5% CO2 and maintained at 37°C. The intestinal segments were voltage clamped at zero transmural potential difference using a VCC-600 voltage-current clamp (Physiologic Instruments, San Diego, CA). In order to keep the tissues alive, 100 μl of a 10 mM solution of glucose was added to the serosal side and 100 μl of a 10 mM solution of mannitol was added to the mucosal side at the beginning of the experiment.
Intestinal short-circuit current (Isc) was recorded continuously using a Kipp & Zonen (Delft, Holland) BD-41 recorder. Isc was expressed as μA/0.725 cm2. The measurement of the short-circuit current was performed in the jejunal tissues of macaques with and without cryptosporidiosis. Measurements of the short-circuit current in the jejunal tissues of macaques with cryptosporidiosis with and without substance P receptor antagonists (400 μg aprepitant [Emend; Merck, Whitehouse station, NJ] in 10 ml of Krebs solution) were also performed. Following substance P receptor antagonist treatment of the jejunal segments, the Isc was then recorded for 30 min to 1 h. The intestinal ion secretion of tissues with substance P receptor antagonist treatment was compared to that without substance P receptor antagonist treatment. We also studied changes in Isc (ΔIsc) induced by glucose as a marker to study absorption. Responsivenesses of the normal and Cryptosporidium-infected intestine to glucose were studied. We also studied the responsiveness of intestinal tissues derived from Cryptosporidium-infected tissues with and without substance P receptor antagonist pretreatment.
Immunohistochemistry.
Jejunal tissues obtained from three immunocompetent macaques before and after C. parvum challenge were used to examine SP and NK1 receptor protein expression by immunohistochemistry as described previously (35). Briefly, immunoperoxidase stains were done on 5-μm-thick frozen or paraformaldehyde-fixed intestinal tissues using polyclonal rabbit antibody to SP or SP receptor (Chemicon, CA) using the avidin-biotin method. Slides were considered to be positive if brown staining within the cytoplasm of cells above the level of nonspecific signal in tissue cells was noted. Controls included slides made of the same section stained using polyclonal isotype control primary rabbit antibody. Positive slides were graded as 1+ to 3+ by examining 25 fields by light microscopy in a blinded fashion (1+, <10% of cells were stained; 2+, 10 to 20% of cells were stained; 3+, over 20% of cells were stained).
Immunolocalization of substance P and CD3 by fluorescence deconvolution restoration microscopy.
Immunolocalization of substance P and CD3 by fluorescence deconvolution restoration microscopy was performed as described previously (1). Briefly, immunofluorescence stains were done on 5-μm-thick paraformaldehyde-fixed intestinal tissues. An incubation with 3% bovine serum albumin in 1× PBS was performed for 30 min to block nonspecific binding, followed by incubation with primary rabbit polyclonal antibody against substance P (catalog no. AB1566; Chemicon, Temecula, CA) or rat polyclonal antibody against CD3 (catalog no. MCA 1477; AbD Serotec, Raleigh, NC) according to the manufacturer's instructions. After three washes with 1× PBS, sections were incubated for 30 min with secondary goat anti-rabbit immunoglobulin G (IgG) antibody conjugated with Alexa Fluor 488 (catalog no. A11008; Invitrogen, Carlsbad, CA) or goat anti-rat IgG antibody conjugated with Texas red (catalog no. T6392; Invitrogen). Controls included slides made of the same section stained using nonimmune rabbit or rat IgG antibody. The sections were then washed three times with 1× PBS, which was followed by mounting a coverslip with Airvol containing 4′,6′-diamino-2-phenylindole (DAPI) for the blue staining of nuclei. Cells that stained positive for substance P were green, whereas cells expressing the lymphocyte marker CD3 stained red. Immunofluorescence images were acquired using a DeltaVision Spectris instrument (Applied Precision, Issaquah, WA), which consists of an Olympus IX-70 inverted microscope with a high-precision computer-controlled XYZ Stage Cool Snap Camera kit and Linux computers installed with softWoRx software (Applied Precision) for data acquisition and deconvolution. Maximum-intensity projected images were made from deconvolved image stacks.
SP and NK1 receptor mRNA quantitation by RT-PCR.
Total RNA was extracted from the jejunal tissues derived from macaques before and after C. parvum challenge using an RNeasy Mini kit (QIAGEN). Reverse transcription of mRNA was performed using TaqMan reverse transcription reagents (catalog no. N808-0234; Applied Biosystems) exactly as described in the manufacturer's package insert. Real-time PCR analysis was performed using 1 μg of RNA by using the ABI Prism 7700 sequence detection system (Perkin-Elmer/Applied Biosystems, Foster City, CA) as described previously (25). Optimized PCR primers and TaqMan probes for SP (catalog no. Hs00243225-m1), NK1 (catalog no. Hs00185530-m1), and 18S rRNA (catalog no. s99999901_s1) were purchased from Perkin-Elmer/Applied Biosystems and were proprietary. The PCR amplification consisted of a reaction mixture of 20 μl of the above-mentioned RT reaction mixture and 30 μl containing 0.25 mM deoxynucleoside triphosphates, AmpliTaq Gold (1.5 U), 5 mM MgCl2, and specific primers and probes for SP, NK1, or 18S (20 pmol each). Each PCR amplification run consisted of heat activation of AmpliTaq Gold for 5 min at 95°C, followed by 40 cycles at 95°C for 15 s and 40 cycles at 60°C for 1 min. All controls and samples were run in duplicate in the same plate. The measurement of 18S rRNA levels of the samples by real-time RT-PCR performed on the same plate was used as a control for loading and to normalize the mRNA contents among the samples tested. The cycle threshold of each duplicate determination was normalized by the subtraction of the cycle threshold for its corresponding 18S rRNA cycle threshold (ΔCT). Each ΔCT was then calibrated by subtracting the mean normalized cycle threshold, ΔCT, to the ΔCT values for a healthy control macaque that was not included as one of the three animals used for the generation of the data in the paper(ΔΔCT). The change (n-fold) relative to the normal SP or SP receptor mRNA levels in the postchallenge jejunal biopsies from Cryptosporidium-infected macaques compared to prechallenge biopsies before C. parvum infection expressed as relative units was calculated as 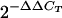 , as described elsewhere previously (32).
, as described elsewhere previously (32).
Extraction and quantitation of substance P protein.
Jejunal biopsies derived from macaques before and after C. parvum challenge were homogenized in 1% trifluoroacetic acid (1 ml/gram of tissue) and centrifuged at 17,000 × g for 15 min at 2 to 8°C. A Sep-Pak C18 cartridge (Waters Associates, Milford, MA) was prewetted with 100% acetonitrile followed by 1% trifluoroacetic acid in water. One milliliter of the supernatant containing 1 to 2 mg of total protein was then passed through the cartridge, followed by a wash with 10 to 20 ml of 1% trifluoroacetic acid. Protein was then eluted with 3 ml of a 60:40 solution of acetonitrile-1% trifluoroacetic acid and dried using a centrifugal concentrator under a vacuum. The dried samples were then reconstituted in assay buffer containing a protease inhibitor, Aprotinin (500 Kallikrein inhibitor units/ml; Sigma), and quantitated using an enzyme-linked immunosorbent assay (ELISA) kit from R&D Biosystems as outlined below.
Substance P protein quantitation.
A DE1400 competitive enzyme immunoassay ELISA kit from R&D systems was used to quantitate the SP protein present in the eluate derived from the prechallenge and postchallenge jejunal samples. The kit for the quantitative measurement of SP uses a polyclonal antibody to SP to bind in a competitive manner, the SP in the standard or sample, or an alkaline phosphatase molecule that has SP covalently attached to it. Microplates from the DE1400 ELISA kit that are coated with goat anti-rabbit antibody are used for the SP ELISA. Briefly, 50 μl of a diluted unknown sample or a control sample (containing 25 to 50 μg of total protein) was added to the wells of the above-mentioned microtiter plates, which was followed by the immediate consequent addition of 50 μl of alkaline phosphatase-conjugated SP and 50 μl of rabbit anti-SP. Also included were standards consisting of SP protein in concentrations ranging from 9.8 to 10,000 pg/ml. After the addition of the SP conjugate and the rabbit anti-SP to the unknown samples or the standards, the plates were incubated on a shaker (500 rpm) for 2 h. During the incubation, the rabbit polyclonal antibody bound to the goat anti-rabbit antibody coated into a microplate. Following three washes with Tris-buffered saline containing detergents (provided in the DE1400 kit; R&D systems) to remove excess conjugate and unbound sample, 50 μl of a p-nitrophenyl phosphate substrate solution (provided in the DE1400 kit; R&D systems) was added to the well and incubated for 1 h to determine the bound-enzyme activity. Immediately following color development, the absorbance at 405 nm was read. The intensity of bound yellow color is inversely proportional to the concentration of substance P in the sample. The measured optical density was used to calculate the concentration of SP. Results are expressed as picograms of SP in 1 mg of total protein.
Statistical analysis.
Statistical analysis was done using Primer software (1992; Stanton A. Glantz, McGraw-Hill). Statistical differences were determined using Student's or paired t tests based on the assumption of a normal distribution based upon identical measurements performed in larger numbers of mice.
RESULTS
Jejunal SP and NK1 receptor mRNA and protein expression levels are increased after Cryptosporidium infection in immunocompetent macaques.
To determine the effects of C. parvum infections on jejunal SP and NK1 mRNA levels in macaques, we isolated mRNA from the jejuna of macaques before and after C. parvum infection and measured mRNA levels by quantitative RT-PCR (Table 1). SP mRNA levels increased 33-fold following infection, from 2.35 ± 0.47 relative units before infection to 79.08 ± 25.05 relative units (P = 0.045, paired t test). Similarly, SP receptor or NK1 mRNA expression increased 57-fold, from 1.47 ± 0.29 relative units in uninfected macaques to 83.50 ± 28 relative units in infected macaques (P = 0.043, paired t test).
TABLE 1.
SP and SP receptor mRNA expression is up-regulated in immunocompetent macaques after Cryptosporidium parvum challenge
| Macaque | mRNA level (relative units)
|
|||
|---|---|---|---|---|
| SP
|
SP receptor
|
|||
| Prechallenge | Postchallenge | Prechallenge | Postchallenge | |
| 1 | 2.82 | 64 | 2 | 32 |
| 2 | 2.82 | 128 | 1 | 128 |
| 3 | 1.41 | 45 | 1.41 | 90.5 |
| Mean ± SEM | 2.35 ± 0.47a | 79.08 ± 25a | 1.47 ± 0.29b | 83.50 ± 28b |
P = 0.045, Student's paired t test.
P = 0.043, Student's paired t test.
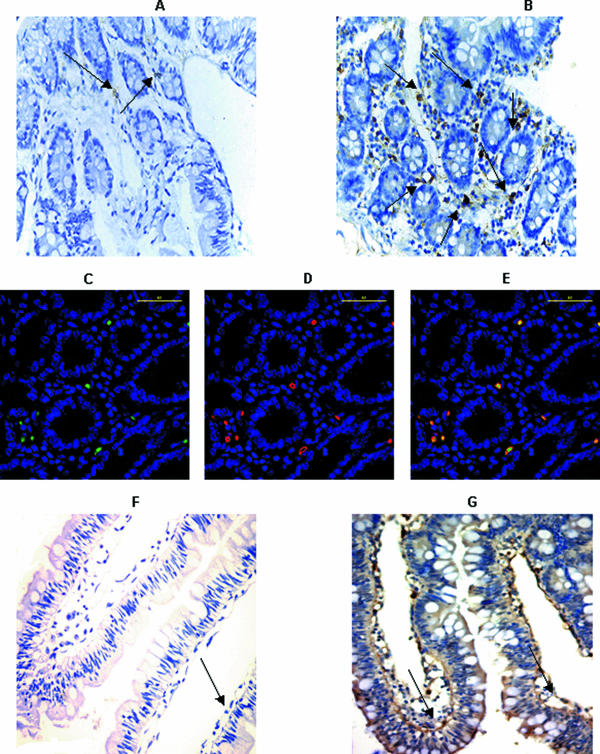
To determine if the increase in SP and NK1 mRNA expression was accompanied by increased SP and NK1 protein expression, we performed immunohistochemistry of jejunal tissues as described previously (35). Increased staining of these cells for SP protein was detected in the postchallenge biopsies compared to the prechallenge biopsies (Fig. 1A and B). SP protein staining was detected predominantly within cells located within the lamina propria. Using a semiquantitative grading scale, SP protein expression in postchallenge biopsies was assessed as 2+ (10 to 20% of the cells stained positive for SP), compared to 1+ in prechallenge biopsies (<10% of cells stained positive). To determine the type of cells that stained positive for SP, we performed double immunofluorescence staining. We noted that the cells that stained positive for SP were lymphocytes (Fig. 1C, D, and E) and not monocytes (data not shown).
FIG. 1.
Jejunal SP and NK1 are up-regulated after Cryptosporidium infection in macaques. SP protein was studied by immunohistochemistry using a polyclonal antibody to SP in paraformaldehyde-fixed paraffin-embedded jejunal biopsies derived from a representative macaque (A) before C. parvum challenge and (B) after C. parvum challenge (arrows in A and B represent cells that are stained positively for SP protein) (magnification, ×200). (C) Immunofluorescence deconvolution restoration microscopy using intestinal tissues derived from a representative macaque following C. parvum challenge showing substance P protein staining as detected by anti-SP polyclonal antibody (green and blue indicate substance P and nuclei, respectively). (D) CD3 protein staining as detected by anti-CD3 polyclonal antibody (red and blue indicate CD3 and nuclei, respectively). (E) Merged images showing orange/yellow CD3+ lymphocytes expressing substance P. The yellow scale bar in the upper right corner of each panel is 40 μm (magnification, ×400). SP receptor protein NK1 was studied by immunohistochemistry using a polyclonal antibody to NK1 in paraformaldehyde-fixed paraffin-embedded jejunal biopsies derived from a representative macaque (F) before C. parvum challenge and (G) after C. parvum challenge (an arrow in F represents a cell that is faintly positive for NK1 protein, and arrows in G represent strong positive staining for NK1 protein) (magnification, ×200).
Similar to substance P, increased staining of cells for NK1 protein was detected in the postchallenge biopsies compared to the prechallenge biopsies (Fig. 1F and G). SP receptor and NK1 protein staining was located predominantly within the epithelium of the villi and was also detected in a few lamina propria cells. Using a semiquantitative grading scale, NK1 protein expression in postchallenge biopsies was assessed as 2+ (10 to 20% of the cells stained positive for NK1), compared to 1+ in prechallenge biopsies (<10% of cells stained positive).
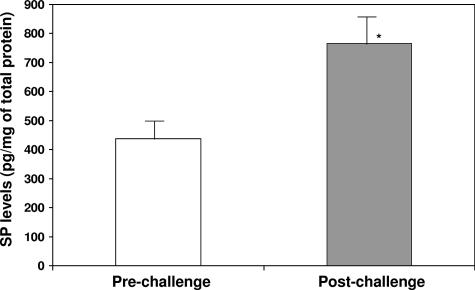
To confirm the results of immunohistochemistry, we performed a competitive ELISA for SP protein using protein extracts of prechallenge and postchallenge jejunal samples (Fig. 2). SP protein levels in the postchallenge jejunal samples (765 ± 89 pg/mg total protein) were increased 74% compared to those in the prechallenge samples (438 ± 72 pg/mg total protein) (P = 0.011, paired t test).
FIG. 2.
Quantitation of jejunal SP levels in macaques before and after Cryptosporidium infection. SP protein measurements were studied by competitive ELISA of jejunal biopsies derived from macaques (n = 3) before C. parvum challenge and after C. parvum challenge. Results are expressed as picograms of SP in 1 mg of total protein extract. *, P = 0.011 (Student's paired t test for SP protein level in prechallenge biopsies versus postchallenge biopsies derived from macaques) (error bars represent standard errors of the means).
Increased basal ion secretion and glucose malabsorption was observed in the jejunum of macaques in response to Cryptosporidium infection.
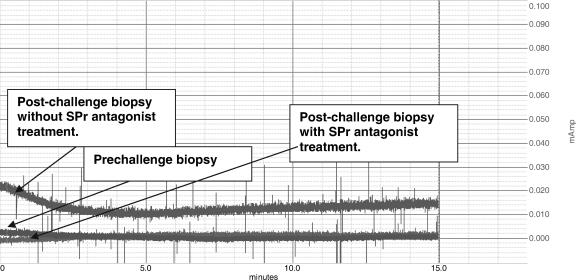
To assess whether or not the increase in SP and NK1 mRNA and protein levels following C. parvum challenge is accompanied by alterations in jejunal function, we measured basal jejunal ion secretion in samples derived from macaques before and after C. parvum challenge using the Ussing chamber technique. The jejunal samples were mounted as flat sheets in Ussing chambers with an aperture of 0.725 cm2. Basal short-circuit current (Isc), which reflects ion secretion, was recorded continuously on a Kipp & Zonen BD-41 recorder for 30 min to 1 h (Fig. 3). Results are expressed as changes in Isc (expressed as μA/0.725 cm2). The average ΔIsc increased over threefold, from 8.99 ± 1.0 μA in prechallenge samples to 30.66 ± 11.6 μA following challenge (P = 0.047, paired t test), indicating that basal ion secretion was increased following C. parvum challenge.
FIG. 3.
Basal chloride ion secretion is elevated in jejunal tissues derived from macaques infected with C. parvum, and in vitro SP receptor antagonist treatment rectifies this defect. SP induced chloride ion secretion as studied by the Ussing chamber technique using prechallenge jejunal biopsies, postchallenge biopsies without SP receptor antagonist treatment, and postchallenge biopsies with SP receptor antagonist treatment from a representative macaque. Changes in basal Isc (ΔIsc) are expressed as μA/0.725 cm2.
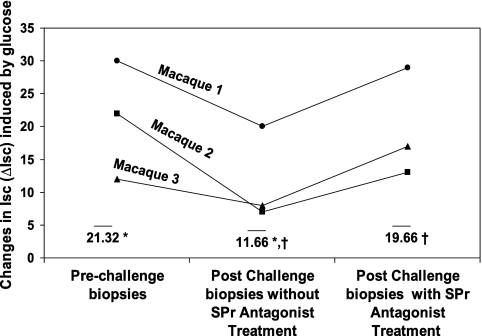
Other groups previously noted that malabsorption of solutes occurs in intestinal tissues derived from Cryptosporidium-infected mice and pigs (18, 30). The ΔIsc induced by glucose was used as a marker to study glucose absorption; there is a direct correlation between ΔIsc induced by glucose and the amount of glucose absorption. To establish whether or not C. parvum infection contributes to increased glucose malabsorption, glucose-induced Isc on jejunal tissues derived from macaques before and after challenge with C. parvum was recorded (Fig. 4). We noted that glucose-induced changes in jejunal short-circuit current responses decreased following C. parvum infection (Fig. 4). The average ΔIsc induced by glucose in prechallenge samples was 21.32 ± 9.02 μA; after challenge with C. parvum, the values decreased to 11.6 ± 7.23 μA (P = 0.046, Student's t test).
FIG. 4.
Glucose absorption is decreased in response to C. parvum infection in macaques and is rectified after in vitro SP receptor (SPr) antagonist pretreatment. Glucose absorption was studied by the Ussing chamber technique using prechallenge jejunal biopsies and postchallenge biopsies from macaques, respectively, before and after C. parvum challenge and in postchallenge biopsies with and without SP receptor antagonist treatment. Changes in Isc (ΔIsc) induced by glucose are presented as maximal elevations in Isc and are expressed as μA/0.725 cm2. Bars represent mean values derived from data for three macaques. *, P < 0.05 (Student's t test for glucose-induced ΔIsc in postchallenge biopsies versus prechallenge biopsies); ‡, P < 0.05 (Student's t test for glucose-induced ΔIsc in postchallenge biopsies with and without SP receptor antagonist treatment).
SP receptor antagonist treatment in vitro leads to inhibition of C. parvum-induced chloride ion secretion and rectifies C. parvum-induced glucose malabsorption.
SP is a known stimulator of chloride ion secretion (34); in order to establish whether or not the increase in SP and NK1 expression observed following C. parvum infections contributes to increased Cl− secretion, we performed Ussing chamber analyses on postchallenge jejunal samples with and without in vitro treatment with the SP receptor antagonist aprepitant (Merck, Whitehouse Station, NJ). The basal Isc was recorded continuously (Fig. 3). Results presented in the Fig. 4 are the basal Isc, expressed as μA/0.725 cm2. Treatment with aprepitant reduced the ΔIsc induced in postchallenge samples by 86%, from 30.66 ± 11.6 μA in untreated samples to 4.35 ± 4.35 μA in treated samples (P = 0.015, paired t test), a value that is indistinguishable from that obtained with prechallenge samples.
To establish whether or not the increase in SP and NK1 expression observed following C. parvum infections contributes to increased glucose malabsorption, glucose-induced Isc on postchallenge jejunal samples with and without treatment with aprepitant was recorded (Fig. 4). The average ΔIsc induced by glucose in postchallenge jejunum increased from 11.6 ± 7.23 μA before aprepitant treatment to 19.57 ± 8.33 μA following treatment (P = 0.015, Student's t test). These results indicate that SP receptor antagonist treatment normalizes both increased intestinal Cl− secretion and glucose malabsorption induced by C. parvum infection.
DISCUSSION
The current study was aimed at elucidating if substance P is a key mediator responsible for physiological alterations associated with cryptosporidiosis in immunocompetent macaques. Our previous studies demonstrated that SP mRNA and protein levels are increased in human cryptosporidiosis (35). However, due to the difficulty in obtaining larger sample volumes, we could not conduct physiological studies. Our findings reported here demonstrated that jejunal substance P and SP receptor (NK1) mRNA and protein levels were up-regulated following Cryptosporidium infection in macaques and were accompanied by increased basal intestinal ion secretion and glucose malabsorption. SP is a known stimulator of chloride ion secretion (34). In these studies, we noted that in vitro treatment of jejunal samples from postchallenge macaques with an SP receptor antagonist normalizes intestinal basal chloride ion secretion and glucose absorption. Thus, increased SP and SP receptor expression is a major contributor to these physiological alterations.
We do not know the exact mechanism by which SP is elevated in cryptosporidiosis and how the elevated levels of SP mediate alterations in cryptosporidiosis. Other studies have also shown that SP is located in the gastrointestinal tract and is produced by many types of inflammatory cells including monocytes, macrophages, lymphocytes, dendritic cells, eosinophils, and neutrophils (5, 11, 21, 22, 28, 29). All of these cells, including mast cells and epithelial cells, respond to substance P. Substance P is known to stimulate proinflammatory cytokines including gamma interferon, interleukin-1β, and tumor necrosis factor alpha (4, 7, 13, 33, 37). Proinflammatory cytokines are known to contribute to the pathogenesis of cryptosporidiosis. Villous atrophy and crypt hyperplasia occurring as a result of the inflammatory process are known to contribute to the impairment of absorption (9). Proinflammatory cytokines, specifically gamma interferon and tumor necrosis factor alpha, are known to disrupt the epithelial barrier, thus leading to a leaky and dysfunctional epithelium (25, 38). Furthermore, enterocytes in the gut are known to be responsible for chloride ion secretion. Enterocytes have SP receptors and hence can respond to SP with a resultant chloride ion secretion, thus contributing to disease pathogenesis. Therefore, most of the pathogenesis associated with cryptosporidiosis could be due to physiological changes that are induced by the elevated SP-induced responses.
In the current studies, we noted that the addition of SP receptor antagonist in vitro rectified the physiological alterations that occurred as a result of C. parvum infection. An explanation for this finding could be that elevated levels of endogenous SP present in macaque intestinal tissues may be responsible for the physiological alterations associated with cryptosporidiosis and that the inhibition of this endogenous SP in the tissues by treatment in vitro with aprepitant results in the inhibition of the physiological alterations.
Since the SP and SP receptor sequences are highly conserved among mammals, the SP receptor antagonist aprepitant can be used to antagonize SP receptors in humans, mice, and macaques. Also, in our preliminary unpublished data, we have successfully shown the inhibition of SP-mediated responses using SP receptor antagonist in mice. Also, pilot studies with aprepitant are successfully being conducted at the Tulane Primate Center in order to establish the pharmacokinetics of aprepitant in Rhesus macaques (S. D. Douglas and A. A. Lackner, unpublished observations).
We note that there is a discrepancy between the degree of the increase in SP mRNA and protein levels following C. parvum infection. Substance P is synthesized in ribosomes as a larger protein (preprotachykin A), and only part of it is enzymatically converted to an 11-amino-acid active peptide of SP. In our RT-PCR studies, we have probed for preprotachykinin mRNA, whereas in the ELISA used to measure protein levels, we measured SP. This could be the reason for the severalfold increase in mRNA compared to that of SP protein.
It could be argued that the recent laparoscopic surgery by itself may be responsible for inducing these alterations. However, as part of the optimization procedure, we had obtained tissue pieces by laprotomy from five animals with infection from 7 to 11 days after challenge with C. parvum. We noted that, as with all other species, physiological alterations began to occur only after certain days postchallenge. In macaques, we noted that only animals that were infected with C. parvum for at least 9 days demonstrated physiological alterations (similarly, our other unpublished observations show that physiological alterations occur in mice only between 3 and 7 days postchallenge). We therefore have data from two other animals that were infected with C. parvum for 7 and 8 days, respectively, that did not demonstrate any physiological alterations (data not shown). These two animals had prior laparoscopic surgery at 28 and 29 days, respectively; therefore, these animals could serve as controls to prove that recent laparoscopic surgery does not by itself induce these changes.
The basal Isc, which reflects ion secretion, was not significantly different between the pre- and postchallenge biopsies derived from these two macaques (28.5 μA in the prechallenge biopsies versus 22.5 μA in the postchallenge biopsies). Similarly, the change in jejunal short-circuit current induced by glucose was not significantly different between the pre- and postchallenge biopsies derived from the above-mentioned two macaques (28 μA in the prechallenge biopsies versus 25 μA in the postchallenge biopsies).
Recent developments with substance P antagonists have demonstrated the importance of substance P in several models of disease including asthma, chronic bronchitis, cystitis, inflammatory bowel disease, migraine, emesis, depression, pain, and seizures (2, 3, 6, 8, 15, 23, 24, 26, 27, 36, 39). Using an in vitro model, the studies outlined in this paper demonstrate that some of the effects of cryptosporidiosis can be decreased with an SP receptor antagonist. These studies raise the possibility of using SP receptor antagonists for the treatment of symptoms associated with cryptosporidiosis.
Acknowledgments
This work was supported by National Institutes of Health grant 1 R21 AI054205-01 to P. Robinson and grants DK 50550 and RR00164 to A. Lackner.
We gratefully acknowledge John Thornby and Edward Graviss for assistance with statistical analysis and Alan Burns for assistance with fluorescence deconvolution restoration microscopy.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Abe, Y., N. A. Bui-Thanh, C. M. Ballantyne, and A. R. Burns. 2005. Extra domain A and type III connecting segment of fibronectin in assembly and cleavage. Biochem. Biophys. Res. Commun. 338:1640-1647. [DOI] [PubMed] [Google Scholar]
- 2.Anton, P. A., and F. Shanahan. 1998. Neuroimmunomodulation in inflammatory bowel disease. How far from “bench” to “bedside”? Ann. N. Y. Acad. Sci. 840:723-734. [DOI] [PubMed] [Google Scholar]
- 3.Argyropoulos, S. V., and D. J. Nutt. 2000. Substance P antagonists: novel agents in the treatment of depression. Expert Opin. Investig. Drugs 9:1871-1875. [DOI] [PubMed] [Google Scholar]
- 4.Berczi, I., I. M. Chalmers, E. Nagy, and R. J. Warrington. 1996. The immune effects of neuropeptides. Baillieres Clin. Rheumatol. 10(2):227-257. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein, C. N., M. E. Robert, and V. E. Eysselein. 1993. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn's disease. Am. J. Gastroenterol. 88:908-913. [PubMed] [Google Scholar]
- 6.Bleiberg, H. 2000. A new class of antiemetics: the NK-1 receptor antagonists. Curr. Opin. Oncol. 12:284-288. [DOI] [PubMed] [Google Scholar]
- 7.Blum, A. M., M. A. G. Cook, R. C. Mathew, D. Elliott, and J. V. Weinstock. 1993. Substance P modulates antigen-induced, IFN-gamma production in murine Schistosomiasis mansoni. J. Immunol. 151:225-233. [PubMed] [Google Scholar]
- 8.Chu, H. W., M. Kraft, J. E. Krause, M. D. Rex, and R. J. Martin. 2000. Substance P and its receptor neurokinin 1 expression in asthmatic airways. J Allergy Clin. Immunol. 106:713-722. [DOI] [PubMed] [Google Scholar]
- 9.Farthing, M. 2000. Clinical aspects of human cryptosporidiosis. Contrib. Microbiol. 6:50-74. [DOI] [PubMed] [Google Scholar]
- 10.Genta, R. M., C. L. Chappell, A. C. White, Jr., K. T. Kimball, and R. W. Goodgame. 1993. Duodenal morphology and intensity of infection in AIDS-related intestinal cryptosporidiosis. Gastroenterology 105:1769-1775. [DOI] [PubMed] [Google Scholar]
- 11.Goldin, E., F. Karmeli, Z. Selinger, and D. Rachmilewitz. 1989. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig. Dis. Sci. 34:754-757. [DOI] [PubMed] [Google Scholar]
- 12.Goodgame, R. W., K. Kimball, C. N. Ou, A. C. White, Jr., R. M. Genta, C. H. Lifschitz, and C. L. Chappell. 1995. Intestinal function and injury in acquired immunodeficiency syndrome-related cryptosporidiosis. Gastroenterology 108:1075-1082. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, D., L. S. Ostlere, and C. A. Holden. 1997. Neuropeptide modulation of Th1 and Th2 cytokines in peripheral blood mononuclear leucocytes in atopic dermatitis and non-atopic controls. Br. J. Dermatol. 137:921-927. [PubMed] [Google Scholar]
- 14.Harp, J. A., W. Chen, and A. G. Harmsen. 1992. Resistance of severe combined immunodeficient mice to infection with Cryptosporidium parvum: the importance of intestinal microflora. Infect. Immun. 60:3509-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joos, G. F., and R. A. Pauwels. 2000. Pro-inflammatory effects of substance P: new perspectives for the treatment of airway diseases? Trends Pharmacol. Sci. 21:131-133. [DOI] [PubMed] [Google Scholar]
- 16.Juranek, D. D. 1995. Cryptosporidiosis: sources of infection and guidelines for prevention. Clin. Infect. Dis. 21(Suppl. 1):S57-S61. [DOI] [PubMed] [Google Scholar]
- 17.Kandil, H. M., H. M. Berschneider, and R. A. Argenzio. 1994. Tumour necrosis factor alpha changes porcine intestinal ion transport through a paracrine mechanism involving prostaglandins. Gut 35:934-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapel, N., J. F. Huneau, D. Magne, D. Tome, and J. G. Gobert. 1997. Cryptosporidiosis-induced impairment of ion transport and Na+-glucose absorption in adult immunocompromised mice. J. Infect. Dis. 176:834-837. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, P., A. V. Thillainayagam, J. Smithson, J. B. Hunt, A. Forbes, B. G. Gazzard, and M. J. Farthing. 1996. Jejunal water and electrolyte transport in human cryptosporidiosis. Dig. Dis. Sci. 41:2095-2099. [DOI] [PubMed] [Google Scholar]
- 20.Keusch, G. T., D. Hamer, A. Joe, M. Kelley, J. Griffiths, and H. Ward. 1995. Cryptosporidia—who is at risk? Schweiz. Med. Wochenschr. 125:899-908. [PubMed] [Google Scholar]
- 21.Lai, J. P., S. D. Douglas, E. Rappaport, J. M. Wu, and W. Z. Ho. 1998. Identification of a delta isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J. Neuroimmunol. 91:121-128. [DOI] [PubMed] [Google Scholar]
- 22.Lambrecht, B. N., P. R. Germonpre, E. G. Everaert, I. Carro-Muino, M. De Veerman, C. de Felipe, S. P. Hunt, K. Thielemans, G. F. Joos, and R. A. Pauwels. 1999. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur. J. Immunol. 29:3815-3825. [DOI] [PubMed] [Google Scholar]
- 23.Lieb, K., Y. Treffurth, M. Berger, and B. L. Fiebich. 2002. Substance P and affective disorders: new treatment opportunities by neurokinin 1 receptor antagonists? Neuropsychobiology 45:2-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H., Y. Cao, A. I. Basbaum, A. M. Mazarati, R. Sankar, and C. G. Wasterlain. 1999. Resistance to excitotoxin-induced seizures and neuronal death in mice lacking the preprotachykinin A gene. Proc. Natl. Acad. Sci. USA 96:12096-12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madara, J. L., and J. Stafford. 1989. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Investig. 83:724-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maunder, R. 2000. Mediators of stress effects in inflammatory bowel disease: not the usual suspects. J. Psychosom. Res. 48:569-577. [DOI] [PubMed] [Google Scholar]
- 27.May, A., and P. J. Goadsby. 2001. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 10:673-678. [DOI] [PubMed] [Google Scholar]
- 28.Mazumdar, S., and K. M. Das. 1992. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am. J. Gastroenterol. 87:176-181. [PubMed] [Google Scholar]
- 29.Metwali, A., A. M. Blum, L. Ferraris, J. S. Klein, C. Fiocchi, and J. V. Weinstock. 1994. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J. Neuroimmunol. 52:69-78. [DOI] [PubMed] [Google Scholar]
- 30.Moore, R., S. Tzipori, J. K. Griffiths, K. Johnson, L. De Montigny, and I. Lomakina. 1995. Temporal changes in permeability and structure of piglet ileum after site-specific infection by Cryptosporidium parvum. Gastroenterology 108:1030-1039. [DOI] [PubMed] [Google Scholar]
- 31.Okhuysen, P., C. L. Chappell, J. H. Crabb, C. R. Sterling, and H. L. DuPont. 1999. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 180:1275-1281. [DOI] [PubMed] [Google Scholar]
- 32.Ono, M., B. Yu, E. G. Hardison, M. A. Mastrangelo, and D. J. Tweardy. 2004. Increased susceptibility to liver injury after hemorrhagic shock in rats chronically fed ethanol: role of nuclear factor-kappa B, interleukin-6, and granulocyte colony-stimulating factor. Shock 21:519-525. [DOI] [PubMed] [Google Scholar]
- 33.Rameshwar, P., and P. Gascon. 1995. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86:482-490. [PubMed] [Google Scholar]
- 34.Riegler, M., I. Castagliuolo, P. T. So, M. Lotz, C. Wang, M. Wlk, T. Sogukoglu, E. Cosentini, G. Bischof, G. Hamilton, B. Teleky, E. Wenzl, J. B. Matthews, and C. Pothoulakis. 1999. Effects of substance P on human colonic mucosa in vitro. Am. J. Physiol. 276:G1473-1483. [DOI] [PubMed] [Google Scholar]
- 35.Robinson, P., P. C. Okhuysen, C. L. Chappell, J. V. Weinstock, D. E. Lewis, J. K. Actor, and A. C. White, Jr. 2003. Substance P expression correlates with severity of diarrhea in cryptosporidiosis. J. Infect. Dis. 188:290-296. [DOI] [PubMed] [Google Scholar]
- 36.Rupniak, N. M., and M. S. Kramer. 1999. Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol. Sci. 20:485-490. [DOI] [PubMed] [Google Scholar]
- 37.Ryu, S. Y., K. S. Jeong, W. K. Yoon, S. J. Park, B. N. Kang, S. H. Kim, B. K. Park, and S. W. Cho. 2000. Somatostatin and substance P induced in vivo by lipopolysaccharide and in peritoneal macrophages stimulated with lipopolysaccharide or interferon-gamma have differential effects on murine cytokine production. Neuroimmunomodulation 8:25-30. [DOI] [PubMed] [Google Scholar]
- 38.Stockmann, M., H. Schmitz, M. Fromm, W. Schmidt, K. Rokos, G. Pauli, P. Scholz, E. O. Riecken, and J. D. Schulzke. 1998. The mechanism of diarrhea in HIV is based on an impaired epithelial barrier function that could be induced by a specific cytokine pattern. Ann. N. Y. Acad. Sci. 859:267-270. [DOI] [PubMed] [Google Scholar]
- 39.Zachrisson, O., N. Lindefors, and S. Brene. 1998. A tachykinin NK1 receptor antagonist, CP-122,721-1, attenuates kainic acid-induced seizure activity. Brain Res. Mol. Brain Res. 60:291-295. [DOI] [PubMed] [Google Scholar]