Abstract
An acute and persistent eosinophil infiltration is observed during Mycobacterium bovis BCG pleural infection in mice. Eosinophil accumulation, lipid body formation, and eotaxin production were significantly reduced in BCG-infected Toll-like receptor-2 (TLR2)-deficient mice compared to wild-type mice. Neutralization of eotaxin or CCR3 drastically inhibited BCG-induced eosinophil accumulation and lipid body formation, indicating that BCG-induced eosinophil recruitment and activation is largely dependent of TLR2-mediated eotaxin generation.
Eosinophils are fundamentally found in tissues that have an interface with the external environment and its bacterial flora, such as the gastrointestinal and respiratory tracts. Accumulating evidence has established eosinophils as multifunctional leukocytes with varied effector and immunomodulatory functions not only in allergic or helminthic disease but also in the initiation and amplification of numerous inflammatory and infectious responses and as modulators of innate and adaptive immunity (1, 22).
Mycobacterial infections frequently show eosinophil recruitment in both naturally occurring infections in human patients (11, 26) and in animal experimental infections (6, 14, 17, 20, 27). Enhanced eosinophil recruitment to sites of infection has been associated with unrestricted growth of mycobacteria observed in mouse susceptible strains in contrast to resistant ones (27) and in mice deficient in gamma interferon (IFN-γ−/−) that are highly susceptible to infection with tuberculosis-causing organisms (13). Moreover, eosinophilia has been associated with higher rates of active tuberculosis in human immunodeficiency virus type 1-infected adults (9), suggesting that eosinophils may have roles in pathogenesis and susceptibility to mycobacterial infection. Although different studies have demonstrated that eosinophils accumulate at sites of mycobacterial infection, including celomatic cavities, skin, lungs, and airways, the mechanisms involved in eosinophil trafficking and activation are not well characterized. In the present study, we used a murine model of pleural tuberculosis to investigate the mechanism involved in eosinophil recruitment and activation.
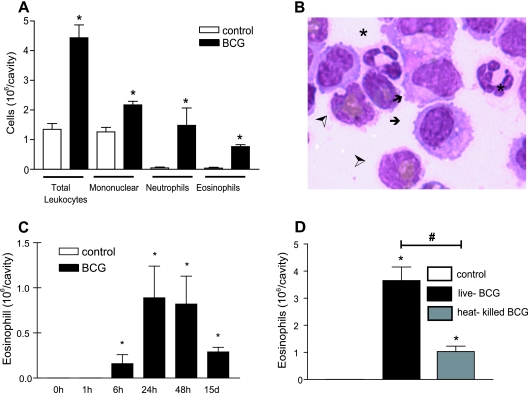
Pleurisy in mice was induced in susceptible C57BL/6 mice by intrapleural injection of live or heat-killed Mycobacterium bovis BCG (Moreau strain, Fundação Ataulpho de Paiva, Brazil) at 5 × 106 bacilli/cavity as previously described (7), with protocols approved by the Fundação Oswaldo Cruz animal welfare committee. The first phase of pleural leukocyte accumulation induced by BCG infection was characterized by a marked increased in neutrophil numbers (mean ± the standard error of the mean [SEM]: from undetected in control animals to 0.12 ± 0.05 × 106/cavity at 1 h and 8.5 ± 0.67 × 106/cavity at 6 h). After 6 h of BCG infection a significant influx of eosinophils, macrophages, and lymphocytes was observed with a peak at 24 h (Fig. 1A to C), and the numbers of these cells remained increased for at least 15 days of infection as previously demonstrated (17, 20) (Fig. 1C). Control mice had no detectable eosinophils in the cavity (Fig. 1A and C). Although heat-killed BCG was capable of recruiting eosinophils to the pleural cavity within 24 h, the intensity of this phenomenon was reduced compared to the same dose of live BCG, suggesting that eosinophil recruitment was partially dependent on the bacterial viability (Fig. 1D).
FIG. 1.
M. bovis BCG-induced eosinophil accumulation in mice. (A) Total and differential recruitment of cells to pleural cavity induced by BCG (5 × 106 bacilli/cavity) within 24 h. (B) Macrophages (arrows), neutrophils (*), and eosinophils (➣) are observed in the pleural cavity from the BCG-infected group within 24 h. (C) Kinetics of BCG-induced eosinophil influx to the pleural cavity. (D) Eosinophil influx induced by the intrapleural injection of live or heat-killed BCG within 24 h. Cells were enumerated after May-Grünwald-Giemsa staining and observed in objective of immersion (×100). The results are expressed as means ± the SEM from at least seven animals. *, P < 0.05 compared to the control group; #, P < 0.05 compared to live BCG.
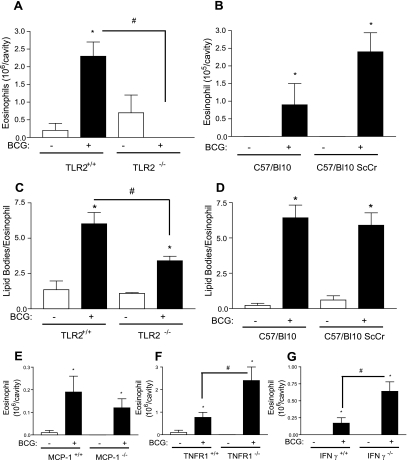
Pattern recognition receptors involved in recognizing mycobacteria include the mannose receptor, complement receptor, and Toll-like receptors (TLRs) (16). TLR2 and TLR4 and, more recently, TLR6 and TLR1 that heterodimerize with TLR2, have been shown to mediate signaling induced by mycobacteria (5, 15). To investigate the role of TLR-mediated pathogen recognition in the mechanism of eosinophil influx and lipid body formation, we infected TLR2 knockout (KO) mice (in a homogeneous C57BL/6 background) and TLR4-deficient mouse strain C57/BL10 ScCr, along with their wild-type controls. We observed that BCG-induced eosinophil recruitment in TLR2-deficient mice was drastically inhibited compared to wild-type mice (Fig. 2A). In contrast, BCG infection in TLR4-deficient mice could induce pleural eosinophil recruitment at levels comparable to that seen in wild-type mice (Fig. 2B).
FIG. 2.
Mechanisms involved in eosinophil recruitment and activation induced by BCG infection. An analysis of pleural eosinophil accumulation and lipid body formation in wild-type (TLR2+/+) and TLR2 KO (TLR2−/−) mice (A and C) and in C57BL/10 (TLR4+/+) and C57BL/10 ScCr (TLR4−/−) mice (B and D) 24 h after BCG infection (5 × 106 bacilli/cavity) was carried out. Eosinophil influx was analyzed in wild-type (MCP-1+/+) and MCP-1 KO (MCP-1−/−) mice (E), wild-type (TNFR1+/+) and TNFR1 knockout (TNFR1−/−) mice (F), and wild-type (IFN-γ+/+) and IFN-γ knockout (IFN-γ−/−) mice (G) 24 h after BCG infection (5 × 106 bacilli/cavity). Lipid body formation was enumerated after osmium staining. Each bar represents the mean ± the SEM from at least eight animals. Differences between control and infected groups are indicated by asterisks (P < 0.05); a “#” symbol indicates differences between BCG-infected wild-type and BCG-deficient mice.
We have recently described how intracellular lipid domains, called lipid bodies, are specific sites involved in the synthesis of lipid mediators during BCG infection (7). In limited numbers, lipid bodies are normal constituents of many cells; however, the numbers and sizes of lipid bodies increase prominently in cells participating in inflammatory responses. It has been demonstrated that the lipid body formation in leukocytes is a highly regulated event that depends on the interaction of cellular receptors with their ligands. Also, these organelles represent specialized intracellular domains whose induced formation is centrally related to activating mechanisms within the cells and are dynamic sites involved in the compartmentalization of proteins (including protein kinases, eicosanoid-forming enzymes, and cytokines), and lipid bodies are markers of leukocyte activation (4). In macrophages, it has been demonstrated that lipopolysaccharide-induced lipid body formation occurs through TLR4 and CD14 recognition, whereas BCG infection in vivo induced lipid body formation in a TLR2-dependent process (7, 18). In vivo the ability of BCG to induce eosinophil activation, as assessed by lipid body formation, through a TLR2-dependent pathway was observed. As shown in Fig. 2C and D, BCG significantly induced lipid body formation in C57BL/6, C57BL/10, and C57BL/10 ScCr eosinophils but not in TLR2−/− eosinophils. These results demonstrate an essential role for TLR2 receptors in BCG recognition and signaling to induce eosinophil recruitment and lipid body formation in eosinophils.
It has been demonstrated that eosinophils do not to express TLR2, TLR4, or CD14, and consequently are not able to directly respond to TLR2 or TLR4 ligands (23). Indeed, it has been demonstrated that the TLR4 ligand lipopolysaccharide induces eosinophil accumulation in vivo through mechanisms that are largely dependent on monocyte/macrophage- and lymphocyte-derived cytokine production (3, 19). Similar monocyte/macrophage-derived cytokine dependent mechanisms could be involved in TLR2-dependent eosinophil recruitment induced by BCG. Mycobacterial infections upregulate genes encoding proteins involved in cell migration and homing (21). Furthermore, it has been demonstrated that mice deficient in IFN-γ or tumor necrosis factor alpha are extremely susceptible to infection by tuberculosis-causing organisms (reviewed in reference 12). The roles of three cytokine/chemokines relevant to cell activation in Mycobacterium-induced responses were evaluated with regard to their involvement in BCG-induced eosinophil influx. In order to determine the involvement of endogenously generated proinflammatory cytokines in eosinophil recruitment induced by BCG, MCP-1/CCL2-deficient mice, TNFRI (p55)-deficient mice (Jackson Laboratories, Bar Harbor, ME), and IFN-γ-deficient mice were infected intrapleurally by BCG.
As shown in Fig. 2E, no inhibition in eosinophil recruitment was observed 24 h after infection when each respective wild-type animal was compared to MCP-1/CCL2-deficient mice. In agreement with previous findings (13), in IFN-γ KO mice (Fig. 2G) and also in tumor necrosis factor receptor 1 (TNFR1) (Fig. 2F)-deficient animals, we observed an increased influx of eosinophils compared to wild-type mice.
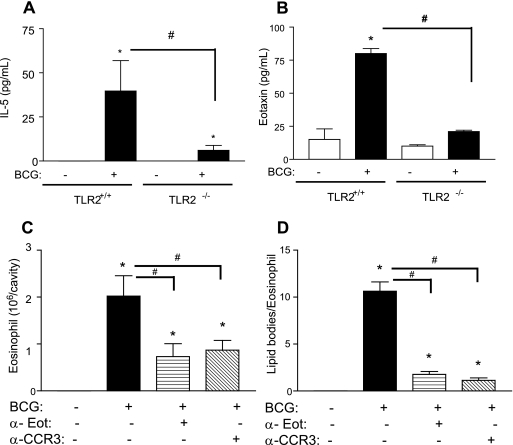
Previous findings have described the role of inteleukin-5 (IL-5) in eosinophil accumulation induced by Mycobacterium bovis BCG in wild-type and in IFN-γ-deficient mice (13, 17). The involvement of TLR2 in IL-5 synthesis after BCG infection was analyzed. TLR2 KO mice produced reduced amounts of IL-5 in the pleural fluid compared to wild-type mice at 24 h after infection (Fig. 3A). Similarly, peritoneal cells from TLR-2 KO mice were unable to produce IL-5 when infected in vitro by BCG under conditions in which cells from wild-type mice produced significant levels of IL-5 when infected (from 7.6 ± 1.35 pg/ml in BCG-infected wild-type mice to undetectable levels in TLR2-deficient mice), indicating that IL-5 production induced by BCG is largely dependent on TLR2.
FIG. 3.
TLR2-mediated endogenous IL-5 and eotaxin production are involved in eosinophil recruitment and activation induced by BCG. IL-5 (A) or eotaxin (B) production were evaluated in the pleural wash from TLR2+/+ and TLR2−/− mice stimulated in vivo by BCG (5 × 106 bacilli/cavity) or vehicle within 24 h by enzyme-linked immunosorbent assay (Duo set kit; R&D Systems, Minneapolis, MN). The effect of neutralization of eotaxin or CCR3 on pleural eosinophil accumulation (C) and lipid body formation (D) induced by BCG infection was also analyzed. Treatments (neutralizing antibodies at 10 μg/animal; R&D Systems) were administered intraperitoneally 30 min before intrapleural injection of BCG (5 × 106 bacilli/cavity), and eosinophil analysis was performed within 24 h. Each bar represents the mean ± the SEM from at least four animals. Differences between control and infected groups are indicated by asterisks (P < 0.05); an “#” symbol represents differences between treated and untreated BCG-infected groups or between wild-type and BCG-deficient mice.
Eotaxin is a key mediator in the eosinophil trafficking into inflamed tissues (22). In addition, eotaxin-mediated CCR3 activation is a potent inducer of lipid body formation in eosinophils (2, 25). As shown in Fig. 3B, BCG significantly induced eotaxin production in vivo detected in the pleural fluid of TLR2+/+ mice but not in TLR2−/− mice (Fig. 3B). Accordingly, eotaxin upregulation has been observed by transcriptome gene array analysis of the macrophage cell line THP-1 infected by M. tuberculosis (21). To investigate the in vivo role of eotaxin activation on BCG-induced lipid body formation and eosinophil recruitment, we pretreated mice with neutralizing antibodies to eotaxin or CCR3 (10 μg/cavity [intraperitoneal] 30 min before infection). Both BCG-induced eosinophil influx and lipid body formation were dramatically inhibited by the pretreatment with neutralizing antibodies (Fig. 3C and D), indicating that endogenous eotaxin, acting via CCR3, elicited in vivo lipid body formation within infiltrating eosinophils. Neutrophil and monocyte recruitment, as well as lipid body formation within these infiltrating cells, were not affected by pretreatment with anti-CCR3 or with anti-eotaxin.
The role of eosinophils in mycobacterial pathogenesis is still not well defined. The bactericidal potential of eosinophils has been demonstrated by their ability to phagocytize, mount a respiratory burst, and mobilize cytotoxic proteins from specific granules after bacterial infection (8, 24), suggesting a protective role of eosinophils in bacterial infections. However, increased numbers of eosinophils at sites of mycobacterial infection has been associated with unrestricted growth of mycobacteria in animals susceptible to infection with tuberculosis-causing organisms (10), suggesting that eosinophils may exaggerate disease severity because eosinophils, which have been shown to phagocytose mycobacteria (6), may provide an intracellular environment in which mycobacteria could proliferate in an unrestricted manner and may also contribute to mycobacteria dissemination (13). Therefore, the role of eosinophils in the host immune response to mycobacterial infection in experimental and clinical tuberculosis remains to be established. The demonstration that eotaxin acting through CCR3 is critically involved in eosinophil recruitment during mycobacterial infection suggests that antibody neutralization or genetically deficient animals to eotaxin and/or CCR3 would be useful tools to address the role of eosinophils in mycobacterial pathogenesis in future studies.
The present findings demonstrate that BCG infection induces pleural eosinophil accumulation in a process requisitely dependent on TLR2 signaling. In addition, our results indicate that BCG-induced eosinophil recruitment and activation is mediated through TLR2-dependent endogenous eotaxin generation and signaling through CCR3.
Acknowledgments
This study was supported by the Howard Hughes Medical Institute (P.T.B.), PRONEX-MCT, CNPq, and FAPERJ.
We thank Bandeira-Melo for helpful comments on this study and the manuscript. We are thank S. Akira, R. Gazzinelli, J. Viola, and C. Gerard for kindly providing the animals used in this study.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Adamko, D. J., S. O. Odemuyiwa, M. Vethanayagam, and R. Moqbel. 2005. The rise of the phoenix the expanding role of the eosinophil in health and disease. Allergy 60:13-22. [DOI] [PubMed] [Google Scholar]
- 2.Bandeira-Melo, C., M. Phoofolo, and P. F. Weller. 2001. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J. Biol. Chem. 276:22779-22787. [DOI] [PubMed] [Google Scholar]
- 3.Bozza, P. T., H. C. Castro-Faria-Neto, C. Penido, A. P. Larangeira, M. das Gracas, M. O. Henriques, P. M. Silva, M. A. Martins, R. R. dos Santos, and R. S. Cordeiro. 1994. Requirement for lymphocytes and resident macrophages in LPS-induced pleural eosinophil accumulation. J. Leukoc. Biol. 56:151-158. [DOI] [PubMed] [Google Scholar]
- 4.Bozza, P. T., R. C. N. Melo, and C. Bandeira-Melo. 2007. Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol. Ther. 113:30-49. [DOI] [PubMed] [Google Scholar]
- 5.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 6.Castro, A. G., N. Esaguy, P. M. Macedo, A. P. Aguas, and M. T. Silva. 1991. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect. Immun. 59:3009-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Avila, H., R. C. Melo, G. G. Parreira, E. Werneck-Barroso, H. C. Castro Faria Neto, and P. T. Bozza. 2006. Mycobacterium bovis BCG induces TLR 2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176:3087-3097. [DOI] [PubMed] [Google Scholar]
- 8.DeChatelet, L. R., P. S. Shirley, L. C. McPhail, C. C. Huntley, H. B. Muss, and D. A. Bass. 1977. Oxidative metabolism of the human eosinophil. Blood 50:525-535. [PubMed] [Google Scholar]
- 9.Elliott, A. M., J. Kyosiimire, M. A. Quigley, J. Nakiyingi, C. Watera, M. Brown, S. Joseph, N. French, C. F. Gilks, and J. A. Whitworth. 2003. Eosinophilia and progression to active tuberculosis in HIV-1-infected Ugandans. Trans. R. Soc. Trop. Med. Hyg. 97:477-480. [DOI] [PubMed] [Google Scholar]
- 10.Erb, K. J., J. Kirman, B. Delahunt, H. Moll, and G. Le Gros. 1999. Infection of mice with Mycobacterium bovis-BCG induces both Th1 and Th2 immune responses in the absence of interferon-gamma signalling. Eur. Cytokine Network 10:147-154. [PubMed] [Google Scholar]
- 11.Flores, M., J. Merino-Angulo, J. G. Tanago, and C. Aquirre. 1983. Late generalized tuberculosis and eosinophilia. Arch. Intern. Med. 143:182. [PubMed] [Google Scholar]
- 12.Flynn, J. L. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 8:1179-1188. [DOI] [PubMed] [Google Scholar]
- 13.Kirman, J., Z. Zakaria, K. McCoy, B. Delahunt, and G. Le Gros. 2000. Role of eosinophils in the pathogenesis of Mycobacterium bovis BCG infection in gamma interferon receptor-deficient mice. Infect. Immun. 68:2976-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasco, T. M., O. C. Turner, L. Cassone, I. Sugawara, H. Yamada, D. N. McMurray, and I. M. Orme. 2004. Rapid accumulation of eosinophils in lung lesions in guinea pigs infected with Mycobacterium tuberculosis. Infect. Immun. 72:1147-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 16.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 17.Menezes-de-Lima-Junior, O., E. Werneck-Barroso, R. S. Cordeiro, and M. G. Henriques. 1997. Effects of inhibitors of inflammatory mediators and cytokines on eosinophil and neutrophil accumulation induced by Mycobacterium bovis bacillus Calmette-Guerin in mouse pleurisy. J. Leukoc. Biol. 62:778-785. [DOI] [PubMed] [Google Scholar]
- 18.Pacheco, P., F. A. Bozza, R. N. Gomes, M. Bozza, P. F. Weller, H. C. Castro-Faria-Neto, and P. T. Bozza. 2002. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J. Immunol. 169:6498-6506. [DOI] [PubMed] [Google Scholar]
- 19.Penido, C., H. C. Castro-Faria-Neto, A. Viera-de-Abreu, R. T. Figueiredo, A. Pelled, M. A. Martins, P. J. Jose, T. J. Williams, and P. T. Bozza. 2001. LPS induced eosinophils migration via CCR3 signaling through a mechanism independent of Rantes and Eotaxin. Am. J. Respir. Cell Mol. Biol. 25:707-716. [DOI] [PubMed] [Google Scholar]
- 20.Penido, C., A. Vieira-de-Abreu, M. T. Bozza, H. C. Castro-Faria-Neto, and P. T. Bozza. 2003. Role of monocyte chemotactic protein-1/CC chemokine ligand 2 on gamma delta T lymphocyte trafficking during inflammation induced by lipopolysaccharide or Mycobacterium bovis bacille Calmette-Guerin. J. Immunol. 171:6788-6794. [DOI] [PubMed] [Google Scholar]
- 21.Ragno, S., M. Romano, S. Howell, D. J. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenberg, M. E., and S. P. Hogan. 2006. The eosinophil. Annu. Rev. Immunol. 24:147-174. [DOI] [PubMed] [Google Scholar]
- 23.Sabroe, I., E. C. Jones, L. R. Usher, M. K. Whyte, and S. K. Dower. 2002. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168:4701-4710. [DOI] [PubMed] [Google Scholar]
- 24.Svensson, L., and C. Wenneras. 2005. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 7:720-728. [DOI] [PubMed] [Google Scholar]
- 25.Vieira-de-Abreu, A., E. F. Assis, G. S. Gomes, H. C. Castro-Faria-Neto, P. F. Weller, C. Bandeira-Melo, and P. T. Bozza. 2005. Allergic challenge-elicited lipid bodies compartmentalize in vivo leukotriene C4 synthesis within eosinophils. Am. J. Respir. Cell Mol. Biol. 33:254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijayan, V. K., A. M. Reetha, M. S. Jawahar, K. Sankaran, and R. Prabhakar. 1992. Pulmonary eosinophilia in pulmonary tuberculosis. Chest 101:1708-1709. [DOI] [PubMed] [Google Scholar]
- 27.Werneck-Barroso, E., A. C. Moura, M. M. Monteiro, O. Menezes de Lima Junior, M. N. de Meirelles, and M. G. Henriques. 2000. Distinct ability to accumulate eosinophils during the inflammatory cellular response to M. bovis BCG in the mouse pleural cavity. Inflamm. Res. 49:206-213. [DOI] [PubMed] [Google Scholar]