Abstract
Flavin-containing monooxygenase (FMO) oxygenates drugs and xenobiotics containing a “soft-nucleophile”, usually nitrogen or sulfur. FMO, like cytochrome P450 (CYP), is a monooxygenase, utilizing the reducing equivalents of NADPH to reduce 1 atom of molecular oxygen to water, while the other atom is used to oxidize the substrate. FMO and CYP also exhibit similar tissue and cellular location, molecular weight, substrate specificity, and exist as multiple enzymes under developmental control. The human FMO functional gene family is much smaller (5 families each with a single member) than CYP. FMO does not require a reductase to transfer electrons from NADPH and the catalytic cycle of the 2 monooxygenases is strikingly different. Another distinction is the lack of induction of FMOs by xenobiotics.
In general, CYP is the major contributor to oxidative xenobiotic metabolism. However, FMO activity may be of significance in a number of cases and should not be overlooked. FMO and CYP have overlapping substrate specificities, but often yield distinct metabolites with potentially significant toxicological/pharmacological consequences.
The physiological function(s) of FMO are poorly understood. Three of the 5 expressed human FMO genes, FMO1, FMO2 and FMO3, exhibit genetic polymorphisms. The most studied of these is FMO3 (adult human liver) in which mutant alleles contribute to the disease known as trimethylaminuria. The consequences of these FMO genetic polymorphisms in drug metabolism and human health are areas of research requiring further exploration.
Keywords: Flavin monooxygenase, Drug metabolism, FMO
Abbreviations: BVMOs, Baeyer–Villiger monooxygenases; CYP, cytochrome P450; DBM, dinucleotide-binding motif; FADPNR, FAD-dependent pyridine nucleotide reductase PRINTS signature; FMO, flavin-containing monooxygenase; FMOXYGENASE, FMO PRINTS signature; GR, glutathione reductase; PAMO, phenylacetone monooxygenase; PNDRDTASEI, pyridine nucleotide disulfide reductase class-I PRINTS signature; ROS, reactive oxygen species; SNP, single-nucleotide polymorphism; TMAU, trimethylaminuria
1. Introduction
Mammalian flavin-containing monooxygenase (FMO, EC 1.14.13.8) was first described by Dr. Daniel Ziegler and colleagues at the University of Texas at Austin (reviewed in Ziegler, 1980, 1988, 1991, 1993, 2002; Poulsen & Ziegler, 1995). This hepatic microsomal enzyme system was demonstrated to utilize oxygen and NADPH to convert N,N′-dimethylaniline to the N-oxide (Ziegler & Pettit, 1964; Mitchell & Zieger, 1969). The location, co-factor requirement and activity of FMO toward xenobiotics were very similar to the then recently characterized cytochrome P450 (CYP) monooxygenase and for a time this enzyme was known as “Ziegler’s enzyme”. In 1971, the enzyme was purified from porcine liver microsomes and definitively demonstrated to be a distinct monooxygenase containing flavin, with no heme (Ziegler et al., 1971a; Ziegler & Mitchell, 1972; Ziegler & Poulsen, 1978). Again, based on studies on the catalytic activity of this enzyme, it was referred to as “mixed-function amine oxidase”, a term that did not capture its wide substrate range.
FMO is a flavoprotein containing a single FAD. Much of the early work describing the structure/function, mechanism, regulation, and substrate specificity of FMO was with the purified porcine liver enzyme, again led by Dr. Dan Ziegler and his colleague Dr. Larry Poulsen (Ziegler et al., 1971a, 1971b, 1992; Poulsen & Ziegler, 1979, 1995). The pig liver enzyme oxygenates a wide range of sulfur- and nitrogen-containing xenobiotics and, in some cases, also oxygenates selenium, iodine, boron, phosphorus and even carbon. An unusual feature of this class of enzymes is that substrate binding has no effect on velocity. In general, any chemical containing a soft nucleophile that gains access to the peroxyflavin intermediate (see Section 2.1 for details) is a potential substrate.
In 1984, the first direct evidence of the existence of multiple forms of FMO was obtained when the enzyme was purified from rabbit lung microsomes independently by 2 laboratories (Williams et al., 1984; Tynes et al., 1985). The “lung” FMO was distinct from the “liver” FMO in having high activity toward primary alkyl amines, restricted substrate specificity related to steric properties, resistance to detergent inhibition and enhanced thermal stability (Williams et al., 1984, 1985, 1990; Tynes&Hodgson, 1985a, 1985b; Tynes et al., 1985, 1986; Poulsen et al., 1986; Nagata et al., 1990; Hodgson & Levi, 1991; Venkatesh et al., 1992). Since those early studies, FMOs have been purified and/or cloned from multiple sources (Table 1). Until recently, it was thought that only 5 genes were expressed in mammals (Hines et al., 1994; Lawton et al., 1994; Cashman, 1995; Phillips et al., 1995). A sixth human gene, FMO6, identified by the Sanger Center chromosome 1 sequencing project, was demonstrated to be a psuedogene (Hines et al., 2002). These FMO genes are located in a cluster on the long arm of chromosome 1 (q23–25). The laboratories of Drs. Ian Phillips and Elizabeth Shephard have recently discovered a second FMO gene cluster, located also on chromosome 1. This second cluster in humans contains an additional 5 genes, all of which appear to be pseudogenes. Interestingly, in the mouse, this second gene cluster contains 3 genes which do not appear to be pseudo genes (Hernandez et al., 2004).
Table 1.
Mammalian FMOs purified or expressed
| Species | FMO | Purified or expressed | Reference |
|---|---|---|---|
| FMO1 | |||
| Dog | 1 | Expressed—baculovirus | Stevens et al., 2003 |
| Human | 1 (wild-type) plus four allelic variants | Expressed—baculovirus | Furnes & Schlenk, 2004 |
| Human | 1 | Expressed—baculovirus | Yeung et al., 2000 |
| Mouse | 1, 3a | Purified—kidney | Venkatesh et al., 1991a, 1991b |
| Mouse | Liver | Purified | Smyser et al., 1985 |
| Mouse | 1 and 5a | Expressed—E. coli | Cherrington et al., 1998 |
| Pig | 1 | Purified | Ziegler & Mitchell, 1972 |
| Pig | 1 | Expressed—E. coli | Lomri et al., 1993 |
| Rabbit | 1, 2, 3, 5 | E. coli | Rettie et al., 1994 |
| Rabbit | 1 | Expressed—Cos-1 cells | Lawton et al., 1991 |
| Rabbit | 1, 3 | Purified—liver | Ozols, 1991, 1994 |
| Rat | 1 | Expressed—yeast | Chiba et al., 1995 |
| Rat | 1 and 3a | Purified—liver | Krause et al., 1996 |
| Rat | 1 | Expressed—yeast | Itoh et al., 1993 |
| Rat | Purified—liver | Kimura et al., 1983 | |
| Sheep | 1, 3a | Purified—liver | Longin-Sauvageon et al., 1998 |
| FMO2 | |||
| Human | 2 | Expressed—baculovirus | Dolphin et al., 1998 |
| Human | 2 (wild-type) plus five allelic variants | Expressed—baculovirus | Krueger et al., 2002a, in press |
| Monkey | 2 | Expressed—baculovirus | Krueger et al., 2001 |
| Mouse | Purified—lung and liver | Tynes et al., 1985 | |
| Mouse | 2 | Expressed—E. coli | Karoly & Rose, 2001 |
| Mouse | Lung | Purified | Venkatesh et al., 1992 |
| Rabbit | 2 | Purified—lung | Williams et al., 1984, 1985 |
| Rabbit | 2 | Purified—lung and liver | Tynes et al., 1985 |
| Rabbit | 2 | Expressed—COS-1 cells | Lawton et al., 1991 |
| Rabbit | 2 | Expressed—baculovirus | Krueger et al., 2001 |
| Rabbit | 3 (1C1) | Expressed—E. coli | Atta-Asafo-Adjei et al., 1993 |
| Rat | 2b | Expressed—E. coli | Lattard et al., 2002 |
| FMO3 | |||
| Human | 3 | Expressed—E. coli fusion | Brunelle et al., 1997 |
| Human | 3 | Expressed—baculovirus | Haining et al., 1997 |
| Human | 3 (wild-type) plus three allelic variants | Expressed—baculovirus | Furnes & Schlenk, 2004 |
| Monkey | 3 | Purified—liver | Sadeque et al., 1993 |
| Monkey | 3 | Purified—liver | Rettie et al., 1995 |
| Mouse | 3 | Expressed—E. coli | Falls et al., 1997 |
| Rat | 3 | Purified—liver | Moroni et al., 1995b |
| FMO4 and FMO5 | |||
| Human | 4 (modified) | Expressed—E. coli | Itagaki et al., 1996 |
| Human | ? | Purified—brain | Bhagwat et al., 1996a |
| Guinea Pig | 5 | Expressed—E. coli | Overby et al., 1995 |
| Human | 5 | Expressed—E. coli | Overby et al., 1995 |
FMO enzyme identity not definitively established.
Rat FMO2 is truncated and enzymatically inactive.
As with the CYP monooxygenase system, over the course of evolutionary plant–animal warfare, FMO developed broad substrate specificity at the expense of turnover. Both monooxygenases are capable of oxidizing thousands of plant alkaloids and other natural products as well as the thousands of synthetic therapeutic drugs. The CYP turnover number for most xenobiotics is 1–20 min−1 and the number is slightly higher for FMO, i.e., 30–60 min−1. Although active toward many of the same substrates, CYP and FMO often produce distinct metabolites. It is recognized in the drug development field that potential concerns can arise if a drug contains a structural feature capable of being bio- activated by CYP to a toxic metabolite, e.g., an epoxide, oxon, or primary aryl N-hydroxy-amine. In general, FMO oxygenation results in metabolites with reduced pharmacological and toxicological properties. As with every rule, there are exceptions. FMO can S-oxygenate thioureas to sulfenic acids which can undergo redox cycling or be further converted to the reactive sulfinic acid (Poulsen et al., 1979; Neal & Halpert, 1982; Ziegler, 1982; Krieter et al., 1984; Sabourin & Hodgson, 1984; Nagata et al., 1990; Decker & Doerge, 1991; Guo & Ziegler, 1991; Guo et al., 1992; Onderwater et al., 1999, 2004; Kim & Ziegler, 2000; Smith & Crespi, 2002; Henderson et al., 2004b).
The structure/function properties of the FMO family are still relatively unknown, as are the physiological function(s) of this enzyme (other than in xenobiotic metabolism), the impact of polymorphic expression, and the importance, relative to CYP, in the metabolism and efficacy of therapeutic drugs. In this review, we have attempted to summarize the current state of that knowledge and suggest possible avenues of further research.
2. Structure/function of flavin-containing monooxygenase
2.1. Catalytic mechanism and reactive oxygen species ROS generation
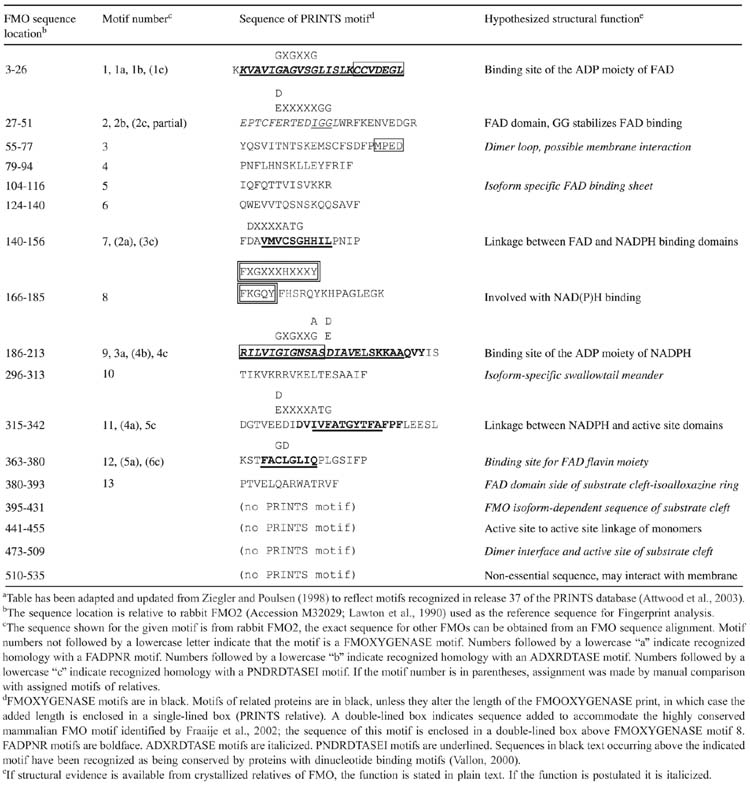
FMO belongs to a class of monooxygenases capable of generating a stable C4a peroxyflavin intermediate (Massey, 1994). This intermediate is stable for minutes to hours at 4 °C and can be observed spectrally (Beaty & Ballou, 1980; Ziegler et al., 1980; Williams et al., 1984; Jones & Ballou, 1986). In the first step of the catalytic cycle, FAD undergoes 2-electron reduction by NADPH (Fig. 1). The reduced flavin reacts rapidly with molecular oxygen to form the peroxyflavin and it is in this state, which the late Henry Kamin of Duke University likened to a “cocked gun,” that FMO may exist predominantly in the cell, waiting for a suitable nucleophile with which to react. This nucleophilic attack by the substrate on the FADOOH results in 1 atom of molecular oxygen being transferred to the substrate and 1 atom to form water. The rate-limiting steps in the catalytic cycle are thought to be the breakdown of the FADOH psuedobase or the release of NADP+. In either case, it is important to note that, unlike the CYP monooxygenase system, substrate binding has no influence on Vmax.
Fig. 1.
Catalytic cycle of flavin-containing monooxygenase (adapted from Ziegler, 1991). (1) FAD reduced by NADPH (fast). (2) FADH2 reacts with O2 (fast). Flavin-hydroperoxide is stable: thought to be the form in which FMO exists in the cell. (3) FAD-OOH reacts with any suitable nucleophile gaining access to active site. No substrate binding required. (4) One atom of O2 is incorporated into substrate and the other into H2O-FMO is a monooxygenase. (5) FAD-OH is converted to FAD via release of H2O (slowest step in the cycle determines Vmax). The final step in the cycle is the release of NADP+ (slow).
The FMO must have evolved a mechanism to protect nucleophilic sites (e.g., methionine, cysteine) from oxidative attack by the peroxyflavin. Furthermore, the structural features of the FAD pocket must be designed to minimize uncoupling/leakage of reactive oxygen species from the breakdown of FADOOH. As FMO is present at high concentrations in the endoplasmic reticulum of some tissues (up to 10% of the total microsomal protein in rabbit lung, Williams et al., 1985), a significant production of superoxide anion radical or hydrogen peroxide from the decomposition of the FADOOH would be detrimental. Rauckman et al. (1979) found that purified pig liver FMO (FMO1) produced superoxide anion radical at the rate of about 4% the total NADPH oxidized. Tynes et al. (1986) found hydrogen peroxide produced by the purified rabbit lung FMO (FMO2) at up to 41% of the total NADPH oxidized. The toxicological consequences of such ROS production by FMOs have not been investigated. The generation of hydrogen peroxide by FMO could also play an important physiological role in control of the overall redox state of the cell and in expression of genes controlled by hydrogen peroxide or the cellular redox potential. However, a caveat to this hypothesis of generation of ROS by FMO is that the purified enzyme may differ markedly from the native enzyme in the endoplasmic reticulum with respect to this “leakage” phenomenon. In the 1970s, Ziegler and Poulsen hypothesized that FMO may play a role in the synthesis of protein disulfide bonds through cysteamine oxidation (Poulsen & Ziegler, 1977; Ziegler et al., 1979). Cysteamine is one of a few endogenous substrates for FMO (see Section 2.4) and is oxidized to the disulfide cystamine. Interestingly, the yeast FMO has been shown to carry out such a cellular function, i.e., it functions in disulfide bond formation in protein synthesis and regulates thiol/disulfide ratios in the cell (Suh et al., 1999; Suh & Robertus, 2002). The yeast FMO gene 5′-promoter region contains an unfolded protein response element and transcription is regulated by this pathway (Suh & Robertus, 2000). In addition, yeast FMO enzyme activity is sensitive to the redox state of the cell (Suh et al., 2000). The mutant yeast FMO exhibits enhanced sensitivity to reductive stress (Suh & Robertus, 2000).
2.2. Substrate specificity of enzyme-restricted substrate access for flavin-containing monooxygenase 2
The initial studies with the purified pig liverFMO(FMO1) established that this enzyme has a very promiscuous substrate specificity. Nitrogen-containing compounds, ranging from the size of trimethylamine to trifluoperazine- and sulfurcontaining compounds ranging from thiourea to sulindac sulfide, can be substrates. Any compound containing a soft nucleophile gaining access to the FADOOH can be a substrate. Compounds containing a single positive charge (e.g., tertiary amines) are excellent substrates. Many compounds containing a negative charge are excluded entirely or are poor substrates with exceptions such as sulindac sulfide and lipoic acid (Taylor & Ziegler, 1987; Hvattum et al., 1991; Attar et al., 2003). Zwitterions and compounds with more than 1 positive charge are typically not substrates, although some, like methionine, can be oxygenated, but the apparent Km is above physiological levels (Park et al., 1992, 1994; Duescher et al., 1994; Elfarra, 1995; Krause et al., 1996, 2002, Ripp et al., 1997, 1999). Diamines, such as cadaverine and spermidine, are not oxygenated by FMO.
Upon purification and characterization of the first extrahepatic FMO, Tynes et al. (1985) and Williams et al. (1984) documented a number of important characteristics that distinguished rabbit lung FMO (FMO2) from the porcine liver enzyme. Among these differences was a markedly distinct substrate specificity. Previous studies employing rabbit lung microsomes had provided suggestive evidence for the existence of an FMO with distinct substrate specificity properties. Chlorpromazine and imipramine, both excellent substrates for porcine liver FMO, were not oxygenated by the rabbit lung enzyme (Ohmiya & Mehendale, 1984). The results with the purified rabbit lung FMO confirmed this lack of turnover with chlorpromazine and imipramine (Williams et al., 1984). Utilizing a series of 10-(N,N-dimethylaminoalkyl)- 2-(trifluoromethyl) phenothiazines with the alkyl side chain varying in length from 2 to 7 carbons, and a series of thioureas varying in size from thiourea to diphenylthiourea (Nagata et al., 1990), further characterized the differences between porcine liver FMO1 and rabbit lung FMO2. With the exception of the shortest side chain (C2), which had a Km 4–5 times higher than the other derivatives, the length of the alkyl tertiary amine side chain made no difference in the kinetics of FMO1 N-oxygenation. In contrast, rabbit lung FMO2 exhibited no detectable activity until the side chain was at least 5 carbons in length. As the length of the side chain increased from 5 to 7 carbons, the Km decreased such that it was equivalent to that of FMO1. With respect to the thioureas of different dimensions, again, the kinetics of porcine FMO1 S-oxygenation did not vary with substrate size. Rabbit FMO2 was incapable of S-oxygenation of 1,3-diphenylthiourea, but was active toward phenylthiourea and naphthylthiourea. Based on these results, Nagata et al. (1990) estimated that the rabbit lung enzyme had a restricted substrate access channel with the FADOOH active site 6–8 Å below the surface of a channel that was no wider than 8 Å. The lack of activity of rabbit FMO2 toward imipramine and chlorpromazine are consistent with this model. Thioureas of various sizes have been used to demonstrate the existence of multiple FMO enzymes in various tissues (Nagata et al., 1990; Guo et al., 1992; Shehin-Johnson et al., 1995; Kim & Ziegler, 2000). The activity of rabbit FMO2 toward alkyl 2-naphthyl sulfides of increasing chain length confirm this restricted substrate access channel in FMO2 (Fisher & Rettie, 1997).
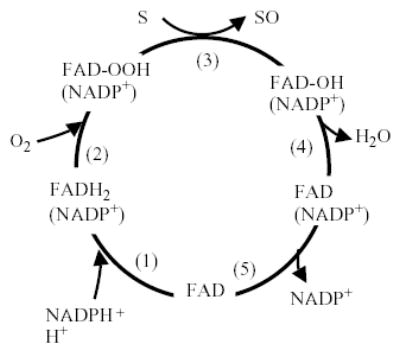
In collaboration with Dr. John Cashman (Human Bio-molecular Research Institute, San Diego, CA), we have recently documented that the size restriction on substrates also applies to human FMO2. No activity is observed with the phenothiazine derivative with a 3 carbon alkyl side chain, but the Km for the 5 and 8 carbon derivatives were 18 and 10 μM, respectively (Fig. 2). In a similar manner, as with rabbit FMO2, the human enzyme is active toward thioureas with small substituents, but is incapable of S-oxygenating 1,3- diphenylthiourea (Henderson et al., 2004b). However, one must be cautious in extrapolating substrate specificity of a particular FMO across species. Kim and Ziegler (2000) demonstrated that the human FMO1 is not as promiscuous as the pig FMO1 and cannot S-oxygenate 1,3-diphenylthiourea. The FADOOH access channel is probably not as deep as FMO2, as soft nucleophiles extending 4.2–5 A° from a bulky ring system (imipramine, orphenadrine, and chlorpromazine) can be oxygenated by human FMO1, but not FMO2 (Kim & Ziegler, 2000). A second precaution with respect to predicting FMO enzyme substrate specificity is that factors other than size and charge must play a role, but these parameters are not well understood. An example is the high selectivity observed with human FMO3, compared to the other FMO enzymes, in the N-oxygenation of the important constitutive substrate trimethylamine (Lang et al., 1998). We also do not know what structure/function factors result in the observed restricted substrate specificity for FMO5 (Rettie et al., 1994; Overby et al., 1995; Cherrington et al., 1998). FMO5 exhibits little activity toward methimazole or other typical FMO substrates, but does carry out the N-oxygenation of short-chain aliphatic primary amines such as N-octylamine. The substrate specificity for FMO4 also appears somewhat restricted, but even less is known about this FMO, probably due in part to difficulties in heterologous expression ofFMO4 (Itagaki et al., 1996). FMO4 exhibits a higher molecular weight than other FMO enzymes, but in some tissues, a smaller protein is present, apparently due to alternative splicing (Lattard et al., 2003a, 2003b). Because of their limited substrate specificity (FMO4 and FMO5) and relatively low expression levels in most tissues (FMO4), these enzymes are currently not thought to play an important role in drug metabolism.
Fig. 2.
Human FMO2 substrate specificity toward phenothiazines with tertiary amine substituents of differing length.
A second striking difference with respect to substrate specificity between purified porcine liver FMO1 and rabbit lung FMO2 is observed with primary aliphatic amines. These amines, typified by N-octylamine are not substrates of the porcine liver FMO1, but serve as positive effectors of catalytic activity (Williams et al., 1984, Tynes et al., 1985, 1986; Poulsen et al., 1986). In contrast, primary alkylamines are readily oxygenated by rabbit FMO2, with the Km decreasing with increasing chain length between C8 and C12. The initial oxygenation results in production of the N-hydroxy primary amine, which is a much better substrate than the parent amine (e.g., the Km for N-hydroxy-dodecylamine is 2 orders of magnitude lower than dodecylamine). The product from this second oxygenation is the oxime which is formed with high stereoselectivity toward production of the cis isomer (Poulsen et al., 1986).
Other notable structure/function distinctions between FMO1 (and to some degree FMO3) and FMO2 can be seen in properties such as thermolability, activation by divalent cations, and inhibition by anionic detergents (Devereux & Fouts, 1974; Devereux et al., 1977; Sabourin & Hodgson, 1984; Kaderlik et al., 1991; Lawton et al., 1991; Venkatesh et al., 1991a, 1992; Williams, 1991; Krueger et al., 2001, 2002a). FMO1 and FMO3 are readily inactivated by incubation at temperatures of 45–50 °C for as few as 5 min and are inhibited at low concentrations of anionic detergents such as sodium cholate or fatty acids. FMO2, on the other hand, is relatively stable at 45–50 °C, especially in the presence of NADPH, and concentrations of sodium cholate as high as 1% do not inhibit catalytic activity. It should be noted that these are general observations on a limited number of enzymes and, again, one must be cautious in extrapolation across species.
In summary, some generalities concerning individual FMO substrate specificity are observed. FMO4 and FMO5 have a very restricted substrate specificity and probably do not contribute significantly to drug metabolism in humans. FMO1 has the broadest substrate specificity of any of the FMOs and FMO2 appears to be the most restricted (based on size); FMO3 is intermediate. An absolute structural requirement for an FMO substrate is a soft-nucleophilic heteroatom, typically a nitrogen or sulfur, with fewer examples of selenium- or phosphorus-containing drugs also known. Uncharged compounds or compounds with a single positive charge are the best substrates. Compounds with a single negative charge can be substrates, but the charge must be located a certain distance from the oxygenation site. Compounds with more than a single charge are almost universally excluded from the FADOOH site. Although some predictions, based on structure, can be made concerning the potential for a particular drug to be oxygenated by FMO, this question still has to be determined empirically.
2.3. Poulsen and Ziegler model predictions for protein structure and functional domains
To date, the tertiary structure of FMO has not been solved; however, Ziegler and Poulsen (1998) proposed a model based on comparison with the solved structures of other flavoenzymes. Using the families of structurally similar proteins (FSSP) database (Holm & Sander, 1994), FMOs are members of the flavocytochrome c sulfide dehydrogenase (41.7.1.1.1.1) subfamily of flavoenzymes, which includes a number of NAD(P)H-dependent oxidases and reductases for which the tertiary structure has been solved. The members of this family catalyze either 2 electron reduction of dioxygen or disulfides. Although the primary structure of family members is not highly conserved, tertiary structures elucidated to date are similar and all members are dimers composed of identical subunits.
Since structural similarities abound among family members, Ziegler and Poulsen (1998) applied the known structure of family member Escherichia coli glutathione reductase (GR; Thieme et al., 1981) to pig FMO1 and subsequently human FMO3 (Ziegler, 2002). An alignment of primary structures was built around regions of high conservation, which serve as anchor points in the structure. The function known for a given segment of GR was assigned the same function in FMO for the aligned region. The PRINTS database (Attwood et al., 2003) catalogs protein fingerprints, which are conserved motifs that characterize a protein family. This database currently recognizes 13 motifs characterizing FMOs (FMO PRINTS signature [FMOXYGENASE] motifs; Table 2), rather than the 7 originally documented by Ziegler and Poulsen (1998). Using the amino acid sequence for rabbit FMO2 (Accession M32029; Lawton et al., 1990) as the model and the current PRINTS database release, other common elements include FAD-dependent pyridine nucleotide reductase (FADPNR), pyridine nucleotide disulfide reductase class-I (PNDRDTASEI) and adrenodoxin reductase (ADXRDTASE) signature homologies. GR is classified with both the FADPNR and PDRDTASEI signature groups. GR and all of the members in these groups have an N-terminal FAD-binding domain followed by an NAD(P)H-binding domain, a central domain and a C-terminal interface domain.
Table 2.
Postulated function of conserved sequence motifsa
The basic elements of dinucleotide-binding motifs (DBMs) have been known for many years. The classic Rossmann (Rossmann et al., 1974; βαβ) fold of DBMs has been described in detail (Wierenga et al., 1985) and contains the GXGXXG and GXGXX(G/A) motifs which bind the ADP moiety of FAD and NAD(P)H, respectively (FMOXYGENASE motifs 1 and 9), in addition to other conserved residues. Other, more recently recognized, motifs common to flavoproteins with 2 DBMs (Vallon, 2000) include the GG motif which stabilizes FAD binding (FMOXYGENASE motif 2), 2 ATG motifs (FMOXGENASE motifs 7 and 11) that form the linkage between the FAD and NADPH domains, respectively, and the linkage between the NADPH and active site domains. The final common element identified by Vallon is the GD motif (FMOXYGENASE motif 12) that binds the FAD flavin moiety; it should be noted, however, that with this motif, conservation is limited and requires experimental evidence to confirm FAD binding by this region. All of these motifs are shown with the PRINTS database information cataloged in Table 2. The ATG motifs are composed of an acid residue, 4 to 6 variable amino acids and the ATG; only the G shows complete conservation among FMO relatives. The FATGY motif recognized for years as a conserved component of nearly all mammalian FMOs (FMO4 proteins use FTTGY) has at its core this second ATG motif. The ATG fingerprint, D(X)4ATG, retains only the D and G residues in many of its flavoenzyme relatives. However, N-hydroxylating siderphore enzymes from bacteria and fungi have retained the closest reiteration of the FATGY motif (Stehr et al., 1998) which can be written as D(X)3(L/F)ATGY(X)4P, where L can be substituted for F among siderphore enzymes. Like other FMO relatives these enzymes also bind FAD (via a modified GXGXXP motif) and NADPH.
Another fingerprint has been identified among FMOs upon comparison of sequences with those from Baeyer–Villiger monooxygenases (BVMOs; Fraaije et al., 2002), bacterial or fungal flavoenzymes that use dioxygen and NAD(P)H as an electron donor to convert a ketone to an ester. The fingerprint thus identified among mammalian FMOs is indicated above the expanded version of FMOOXYGENASE motif 8 (Table 2), as FXGXXXHXXXY. This fingerprint occurs just before the NAD(P)H-binding GXGXX(G/A) motif. BVMOs have a slightly modified motif that substitutes W for Y. When Fraaije et al. (2002) used site-directed mutagenesis of 4-hydroxyacetophenone monooxygenase, a BMVO enzyme, to convert the central H→A or the terminal W→Y of this motif, they found that the W→Y protein was both insoluble and inactive. The H→A protein was a soluble dimer, but prone to losing some of the FAD cofactor during purification; furthermore, the mutant had almost no activity. Cheesman et al. (2003) mutated the homologous H of cyclohexanone monooxygenase, another BVMO, to Q. These authors found only limited evidence of impaired FAD binding with the H→Q mutant, but did find that enzyme activity was only 10% that of the wild-type enzyme. Kinetic studies indicated an 11- fold decrease in enzyme efficiency (Vmax/Km) that was only partially explainable as a decreased binding affinity for NADPH. Thus, FMOXYGENASE motif 8, contributes to NADPH binding and appears to have an additional function as well.
There are a number of studies that have sought to understand why particular mutations made to FMO proteins alter the function of the protein, and some of these studies verify aspects of the proposed structural model. Obvious examples of this are experiments that demonstrate that site-directed mutagenesis of the G residues of the conserved GXGXXG from the putative DBM for FAD, does, in fact, cause the loss of FAD binding and concomitant loss of enzyme activity (Lawton & Philpot, 1993; Kubo et al., 1997). More recently, Zhang et al. (2003) determined that an individual presenting with trimethylaminuria (TMAU) had 2 mutations, one of which encoded p.E32K. The E residue corresponds to the conserved (E/D) that is part of the GG motif (Table 2, FMOXYGENASE motif 2) that stabilizes FAD binding. Although Zhang et al. (2003) did not confirm that expressed p.E32K had suboptimal FAD content, they were unable to detect either N- or S-oxidation with FMO3 substrates, which would be the expected outcome in the absence of FAD binding. There are 2 further causative mutations of TMAU that occur just downstream of the GXGXX(G/A) motif, but within the βαβ-fold for NAD(P)H, p.I199T (Zschocke et al., 1999) and p.R205C (Fujieda et al., 2003). Both of these mutations are at positions described by Wierenga et al. (1985) as being tolerant of any amino acid. While the relevant determinations of NAD(P)H binding and Km were not given for the TMAU mutations, due to the location of the mutations, it is likely that these alterations are interfering with NADPH binding, although the possibility of a second FMO-specific function occurring in this motif cannot be eliminated at present. A final example of a mutation that verifies part of the proposed structural model is the p.M434I single-nucleotide polymorphism (SNP) that also causes TMAU (Dolphin et al., 2000). N- and S-oxygenations performed by this protein are drastically reduced, despite the fact that FMO1 enzymes utilize an I residue at this location and FMO2 enzymes utilize L at the same position. The p.M434I residue is expected to lie in or near to the FMO enzyme specific substrate cleft (Table 2), so it is not surprising that a residue tolerated by FMO1 would alter the activity of FMO3.
The proposed structural model may be useful in preliminary predictions of the general 3-dimensional structure of FMO, assignment of function to specific regions of the sequence and generation of hypotheses that can be tested on expressed mutant proteins; however, the model also has serious limitations. For example, while we can with fair precision assign a function such as stabilization of FAD binding to FMOXYGENASE motif 2, that cannot be interpreted to mean that no other function exists for that sequence. The comparable motif of GR, in addition to FAD binding stabilization, also contains 2 C residues involved in the redox-active disulfide bond of this protein, but disulfide bonds do not exist for mammalian FMO. Dual-function fingerprints, and interactions between residues that are distant in the sequence alignment but interact in the tertiary structure, will abound. Because structural features that are unique to FMO, and yet define FMO as a family, are least amenable to modeling a priori, crystallization of an FMO remains essential to gaining an in-depth understanding of the structure and function of this family. The only bacterial flavin monooxygenase whose crystal structure has been solved is phenylacetone monooxygenase (PAMO; Malito et al., 2004). The authors found significant homology with human GR (ca. 20% identity). PAMO may provide important insights into mammalian FMO structure/function. Again, only the acquisition of a structure derived from crystals of a mammalian FMO will settle the issue definitively.
2.4. Physiological function(s) of flavin-containing monooxygenase: endogenous substrates and association with disease (Table 3)
Table 3.
Endogenous FMO substrates
| Substrate | FMO | Km (μM) | Product | Reference |
|---|---|---|---|---|
| Sulfur-containing | ||||
| Cysteamine | pFMO1a | 120 | Disulfide | Poulsen, 1981 |
| hFMO2 | 175 | Unpublished | ||
| S-cysteine conjugates | pFMO1 | 344–1538 | S-oxide (diastereometric excess 10–78%) | Park et al., 1992 |
| S-allyl-l-cysteine | Rat in vivo | Sulfoxide | Krause et al., 2002 | |
| S-farnesylcysteine and | pFMO1 | Park et al., 1994 | ||
| S-farnesylcysteine methyl ester | 23–34 | S-oxide | Park et al., 1994 | |
| Dihydrolipoic acid and | pFMO1 | 1700 | Taylor & Ziegler, 1987 | |
| Lipoic acid | pFMO1 | 120 | Taylor & Ziegler, 1987 | |
| hFMO2 | 71 | Unpublished | ||
| Methionine | hFMO3 | 20,000 | (d>l)-sulfoxide | Ripp et al., 1999 |
| rat FMO3 | 3400 | (d>l)-sulfoxide | Krause et al., 1996 | |
| Rabbit FMO1 | 48,000 | (d/l=1.5)-sulfoxide | Duescher et al., 1994 | |
| Rabbit FMO2 | 30,000 | (d/l=0.7)-sulfoxide | ||
| Rabbit FMO3 | 6500 | (d/l=8)-sulfoxide | ||
| Nitrogen-containing | ||||
| Trimethylamine | hFMO3 | 28 | N-oxide | Lang et al., 1998 |
| pFMO1 | 617 | Sabourin & Hodgson, 1984 | ||
| Mouse liver FMO | 2340 | Sabourin & Hodgson, 1984 | ||
p = Pig; h = human.
As discussed previously, the charge restrictions for access to the substrate channel leading to FADOOH exclude many potential nucleophilic endogenous substrates from FMO-dependent oxygenation. There are a number of notable exceptions, however. For the purpose of the present discussion, “endogenous substrate” will refer to a chemical synthesized in the body or as a result of conjugation of a xenobiotic with an endogenous nucleophile such as cysteine. Dietary constituents, such as alkaloids, allyl sulfides, amines, etc., are not considered to be “endogenous” substrates. In some cases, this distinction is blurred and admittedly somewhat arbitrary. For example, we will consider the biogenic amine phenethylamine as an endogenous substrate, although a significant amount of human exposure can also be dietary.
2.4.1. Nitrogen-containing endogenous substrates
The biogenic amines, phenethylamine and tyramine, are N-oxygenated by FMO to produce the N-hydroxy metabolite, followed by a rapid second oxygenation to produce the trans-oximes (Lin & Cashman, 1997a, 1997b). This stereoselective N-oxygenation to the trans-oxime is also seen in the FMO-dependent N-oxygenation of amphetamine (Cashman et al., 1999), but differs from that seen with 10-N-(n-octylamino)- 2-(trifluoromethyl) phenothiazine (Lin et al., 1996) and of straight-chain aliphatic amines discussed previously (Poulsen et al., 1986), for which the product is the cis-oxime. Interestingly, FMO2, which very efficiently N-oxygenates N-dodecylamine, is a poor catalyst of phenethylamine N-oxygenation. The most efficient human FMO in phenethylamine N-oxygenation is FMO3, the major FMO present in adult human liver; the Km is between 90 and 200 μM (Lin & Cashman, 1997b). The oximes appear to have little pharmacological activity, and therefore FMO-mediated N-oxygenation of biogenic amines appears to be a mechanism for inactivation. Brain contains active FMO (Bhamre et al., 1993, 1995; Bhagwat et al., 1996a, 1996b; Blake et al., 1996; Kawaji et al., 1997) and conversion of biogenic amines in the brain by FMO (Lin & Cashman, 1997a) could be of clinical significance.
Trimethylamine is another example of an endogenous compound that is also present in the diet (primarily as trimethylamine N-oxide from fish, which is reduced by gut bacteria to the parent amine). Trimethylamine is primarily formed in vivo from the breakdown of choline and is N-oxygenated by FMO (Gut & Conney, 1993; Lang et al., 1998; Lambert et al., 2001; Cashman et al., 2004). The product, as with all FMO-mediated oxygenations of tertiary amines, is the N-oxide. Trimethylamine is extremely odorous, whereas, the N-oxide has little or no detectable odor. Failure to efficiently N-oxygenate trimethylamine leads to the genetic disease known as trimethylaminuria (see Section 3.1).
2.4.2. Sulfur-containing endogenous substrates
Sulfur-containing endogenous substrates for FMO have also been documented (Poulsen, 1981). Sulfhydryls, such as cysteamine, are S-oxygenated to the disulfide (cystamine). Cysteamine has been used therapeutically as a radioprotector (Biaglow et al., 1984) and is the only approved treatment for pediatric cystinosis (Belldina et al., 2003). Cysteamine also can regulate various hormones; it is a potent inhibitor of somatostatin and has been shown to affect circulating growth hormone levels in pigs (McElwain et al., 1999). The physiological role of cysteamine S-oxygenation by FMO is not known. A number of years ago, Ziegler and Poulsen hypothesized a role for FMO in protein disulfide bond formation through oxidation of cysteamine (Ziegler et al., 1979). FMO oxygenation of cysteamine may serve to help control the overall thiol/disulfide redox state of the cell which, in turn, controls many important pathways. However, this is entirely speculation at this point. Another function may simply be a protective one. Cysteamine, at concentrations as low as 39 μM, is toxic to cells, apparently through the transition metal-dependent formation of hydrogen peroxide (Jeitner & Lawrence, 2001). It has been postulated that cysteamine can reach and exceed these concentrations (perhaps as high as mM concentrations) through transport into the cell as a mixed disulfide with cysteine (required to maintain high intracellular glutathione levels), followed by reduction intracellularly. FMO oxygenation of cysteamine to cystamine and transport out of the cell may represent a detoxication mechanism. By this indirect mechanism, FMO may control H2O2 levels in the cell and the expression of genes regulated by H2O2, sulfhydryl/disulfide ratios and general redox state (Khomenko et al., 2004).
Lipoic acid is another example of an “endogenous” substrate also available through the diet as a dietary antioxidant supplement (Bustamante et al., 1998) and as a therapeutic agent (Smith et al., 2004). Lipoic acid is a cofactor for α-ketoglutarate and pyruvate dehydrogenases. Recent evidence suggests that lipoic acid can play an important role in protection of mitochondria during aging or even in reversal of age-associated declines in mitochondrial function (Hagen et al., 2002). FMO catalyzes the S-oxygenation of the disulfide lipoic acid, as well as lipoamide. Recently, we have found that human FMO2 exhibits activity toward lipoic acid with a Km of 71 μM (Henderson et al., unpublished). The major urinary metabolites of lipoic acid in mice, rats, dogs and humans appear to be derived from mitochondrial β-oxidation or reduction to lipoamide followed by methylation and sulfoxidation (Schupke et al., 2001). The potential role of FMO in the sulfoxidation of the methyl sulfides of lipoic acid is not known at this time.
As discussed previously, FMO-mediated oxygenation of a number of potential endogenous compounds are prohibited due to charge restrictions in the FADOOH-substrate access channel. However, FMO3 will oxygenate methionine, but the Km is at mM concentrations which are probably not physiologically relevant. Many xenobiotics are metabolized by glutathione S-transferases and these compounds are then excreted as the cysteine S-conjugates or mercapturic acids. A number of cysteine S-conjugates of xenobiotics are S-oxygenated by FMO (Table 3), but again the Km values are in the low mM range which may call into question the importance of such metabolism in vivo. The Se of selenocysteine conjugates is also oxygenated by FMO (Rooseboom et al., 2001). Interestingly, the sulfoxidation of farnesyl cysteine is catalyzed by FMO with a Km in the low μM range (Park et al., 1994).
2.4.3. Association of flavin-containing monooxygenase with human disease
As discussed in Section 3.1 on genetic variants, currently the association of FMO expression and human disease is only established for the genetically inherited disease known as trimethylaminuria. Numerous studies have correlated altered susceptibility to a number of diseases, the existence of particular alleles and the expression of certain phase 1 and/or phase 2 drug metabolizing enzymes (e.g., Rosvold et al., 1995; Taioli et al., 1998; Ford et al., 2000; Wu et al., 2001). In the case of CYP, the wide interindividual variation in expression is due to both genetic and environmental factors. As FMO is not markedly regulated by such environmental factors, one could predict interindividual variability in FMO enzyme expression would be predominantly genetic in origin and it may be possible to more clearly delineate correlations (or lack thereof) between expression of wild-type FMO or particular FMO allelic variants and susceptibility to disease. Hepatic total FMO activity is enhanced in mouse models of type I and type II diabetes (Rouer et al., 1987, 1988). Conversely, expression of FMO5 is markedly down-regulated in the liver of humans with type II diabetes (Takamura et al., 2004). Alterations in FMO expression and activity in diabetic patients could be of significance in drug therapy (Section 4). A global gene expression approach with patients diagnosed with atrial fibrillation documented a significant increase in the expression of FMO1 (Kim et al., 2003). There has also been suggestive evidence presented that FMO may be associated with sideroblastic anemia (Barber et al., 2000). Finally, expression of certain FMO enzymes in some neoplastic tissues has been observed (Rebhan et al., 1997). Our knowledge of the physiological function of FMOs and the relationship of FMO deficiency or over expression to disease susceptibility is still in its infancy.
3. Genetic variants and polymorphisms
3.1. Genetic variants in flavin-containing monooxygenase 3 and trimethylaminuria
Trimethylaminuria (TMAU), previously referred to as “Fish-Odor Syndrome”, is manifested clinically by a body odor resembling rotten fish and is accompanied by various psychosocial abnormalities (Ayesh & Smith, 1990; Treacy et al., 1998; Mitchell & Smith, 2001). This disease is classified by NIH as a rare disease and has been the focus of 2 international meetings sponsored by the NIH Office of Rare Diseases. The actual incidence of TMAU, however, may be underreported. Due to the paucity of information available to physicians, this disease is rarely diagnosed in a timely fashion and the symptoms for patients are often attributed to poor hygiene.
This disease has probably been around for centuries. In the Mahabharata, in an epic tale perhaps dating back to as early as 1400 BC, Satyavata is described as a young woman ostracized from society because she smelled like rotting fish (Mitchell & Smith, 2001). Shakespeare likely was referring to a TMAU patient in a scene from The Tempest (II. ii. 26–29). As the name implies, the condition is caused by an excess of the extremely odorous trimethylamine in the urine, sweat, and breath of patients. Trimethylamine is formed endogenously from the breakdown of choline and the reduction of trimethylamine N-oxide present in high concentrations in diets rich in fish. Trimethylamine is normally metabolized to the N-oxide, a metabolite with no detectable offensive odor, by FMO in the liver (Sardas et al., 1996; Mitchell et al., 1997). Studies in vitro with expressed FMO enzymes have shown FMO3 to be selective in the N-oxygenation of trimethylamine (Lang et al., 1998). A number of allelic variants have been associated with the incidence and severity of TMAU in patients (Zhang et al., 1996, 2003; Cashman et al., 1997, 2000, 2001, 2003; Dolphin et al., 1997; Kang et al., 2000; Murphy et al., 2000; Forrest et al., 2001; Cashman, 2002; Cashman & Zhang, 2002; Park et al., 2002; Hernandez et al., 2003; Lattard et al., 2003a, 2003b). These individuals can be diagnosed by urinary ratios of trimethylamine N-oxide to trimethylamine following a challenge dose of choline. Most individuals excrete 95% or more of the trimethylamine as the N-oxide. The severity of the disease can be predicted to be greater with a lower ratio, which appears to be related to the type of FMO3 gene mutation. Interestingly, the incidence of TMAU appears to be higher in females (although there could be a bias at diagnosis) and patients often report the symptoms to be worse during menstruation. Once diagnosed, the most effective treatments appear to be dietary restriction (low choline, fish) and/or antibiotic treatment (inhibits bacterial reduction of trimethylamine N-oxide in the intestine). Recently, treatment with charcoal or the deodorant copper chlorophyllin has been demonstrated to be effective in some patients (Yamazaki et al., 2004). Interestingly, reports of transient TMAU in young children have been reported. This phenomenon may be explained by the observation that the FMO with high activity toward trimethylamine, FMO3, is not expressed in fetal liver, but is induced after birth (0–8 years of age; Koukouritaki et al., 2002).
Of particular significance for this review is that individuals homozygous for certain FMO3 allelic variants (e.g., null variants) also demonstrate impaired metabolism toward other FMO substrates including ranitidine, nicotine, thio-benzamide, and phenothiazine derivatives (Table 4; Cashman et al., 1995, 2000; Kang et al., 2000; Cashman, 2002; Park et al., 2002; Lattard et al., 2003a, 2003b). The rare null variants are present in patients with TMAU, whereas variants resulting in a less efficient enzyme (e.g., E158K, E308G) may not exhibit a phenotype. In at least one study, in vivo phenotyping of impaired drug metabolism using ranitidine as the substrate correlated to genotyping for such FMO3 allelic variants (Kang et al., 2000). These results support an important role for FMO in human drug metabolism and highlight the need for further studies on pharmacogenetics of FMOs and drug metabolism. Other FMO3 allelic variants which produce inactive FMO3 (Table 4) include P153L (Dolphin et al., 1997; Treacy et al., 1998); E305X (Treacy et al., 1998); M66I (Treacy et al., 1998; Akerman et al., 1999a); R492W (Akerman et al., 1999a); A52T, R387L, E314X (Akerman et al., 1999b); I199T (Zschocke et al., 1999); V143E (Basarab et al., 1999); N61S, M434I (Dolphin et al., 2000); V58I (Kubota et al., 2002); D198I and R205C (Fujieda et al., 2003).
Table 4.
Enzymatic activity of human FMO1, FMO2 and FMO3 allelic variants
| Variant | Substrate | Km (μM) | Vmax (min−1) | Reference |
|---|---|---|---|---|
| FMO1 | ||||
| Wild type | Methimazole | 7 | 64 | Furnes & Schlenk, 2004 |
| p.H97Q | 14 | 104 | ||
| p.I303V | 7 | 71 | ||
| p.I303T | 16 | 113 | ||
| p.R502X | – | – | ||
| Wild type | Imipramine | 14 | 51 | |
| p.H97Q | 15 | 66 | ||
| p.I303V | 20 | 48 | ||
| p.I303T | 14 | 71 | ||
| p.R502X | 16 | 25 | ||
| Wild type | Fenthion | 340 | 93 | |
| p.H97Q | 320 | 125 | ||
| p.I303V | 351 | 99 | ||
| p.I303T | 240 | 149 | ||
| p.R502X | 300 | 51 | ||
| Wild-type | Methyl p-tolyl sulfide | 284 | 46 | |
| p.H97Q | 251 | 66 | ||
| p.I303V | 300 | 42 | ||
| p.I303T | 245 | 84 | ||
| p.R502X | 200 | 32 | ||
| Note: Some of the variants reported above may be rare alleles. See Hines et al. (2003) for a description of a common allelic variant (FMO1*6) that effects transcription levels. | ||||
| FMO2 | ||||
| Wild type | Ethylenethiourea | 16 | 41 | Krueger et al., in press |
| p.N413K | 40 | 124 | Krueger et al., in press | |
| FMO variant alleles with no demonstrable activity toward ethylenethiourea include p.71D dup, p.V113X, p.S1195L, p.X472. | Krueger et al., in review | |||
| FMO3 | ||||
| Wild type | Fenthion | 145 | 11 | Furnes & Schlenk, 2004 |
| p.D132H | 150 | 10 | Furnes & Schlenk, 2004 | |
| p.E158K | 200 | 12 | Furnes & Schlenk, 2004 | |
| Wild type | 5-DPT | 155 | 51 | Cashman et al., 2000 |
| 929 | 65 | Lattard et al., 2003a, 2003b | ||
| p.E158K | 187 | 44 | Cashman et al., 2000 | |
| 763 | 20 | Lattard et al., 2003a, 2003b | ||
| p.V257M | 82 | 13 | Cashman et al., 2000 | |
| p.F510X | 62 | 0.1 | Cashman et al., 2000 | |
| p.E132H | 562 | 37 | Lattard et al., 2003a, 2003b | |
| p.E132H/E158K | 1517 | 41 | Lattard et al., 2003a, 2003b | |
| p.E308G | 2280 | 33 | Lattard et al., 2003a, 2003b | |
| p.E158K/E308G | 2285 | 26 | Lattard et al., 2003a, 2003b | |
| p.L360P | 993 | 130 | Lattard et al., 2003a, 2003b | |
| Wild type | Trimethylamine | 32 | 189 | Cashman et al., 2000 |
| 8 | 40 | Lattard et al., 2003a, 2003b | ||
| p.E158K | 206 | 105 | Cashman et al., 2000 | |
| 17 | 30 | Lattard et al., 2003a, 2003b | ||
| p.V257M | 1151 | 28 | Cashman et al., 2000 | |
| p.F510X | 1034 | 35 | Cashman et al., 2000 | |
| p.E132H | 26 | 40 | Lattard et al., 2003a, 2003b | |
| p.E132H/E158K | 11 | 20 | Lattard et al., 2003a, 2003b | |
| p.E308G | 42 | 18 | Lattard et al., 2003a, 2003b | |
| p.E158K/E308G | 23 | 8 | Lattard et al., 2003a, 2003b | |
| p.L360P | 11 | 220 | Lattard et al., 2003a, 2003b | |
| Wild type | Tyramine | 231 | 110 | Cashman et al., 2000 |
| p.E158K | 941 | 106 | Cashman et al., 2000 | |
| p.V257M | 2164 | 41 | Cashman et al., 2000 | |
| p.F510X | 1384 | 11 | Cashman et al., 2000 | |
| Wild type | Methimazole | 12 | 59 | Lattard et al., 2003a, 2003b |
| p.E158K | 10 | 27 | Lattard et al., 2003a, 2003b | |
| p.E132H | 15 | 48 | Lattard et al., 2003a, 2003b | |
| Wild type | Methimazole | 12 | 59 | Lattard et al., 2003a, 2003b |
| p.E132H/E158K | 10 | 19 | Lattard et al., 2003a, 2003b | |
| p.E308G | 17 | 15 | Lattard et al., 2003a, 2003b | |
| p.E158K/E308G | 12 | 11 | Lattard et al., 2003a, 2003b | |
| p.L360P | 8 | 184 | Lattard et al., 2003a, 2003b | |
| p.N61S | ~15 | Dolphin et al., 2000 | ||
| Wild type | Ranitidine | 3 | 27 | Park et al., 2002 |
| p.E158K | .1 | 13 | Park et al., 2002 | |
| p.E308G | 1 | 13 | Park et al., 2002 | |
| p.E158K/E308G | 0.7 | 3 | Park et al., 2002 | |
Variant alleles with no activity toward 5-DPT, tyramine or trimethylamine include p.M66I, p.P153L, p.E305X (Treacy et al., 1998; Dolphin et al., 1997); p.R492W (Akerman et al., 1999a); p.A52T; p.387L; p.E314X (Akerman et al., 1999b); p.I199T (Zschocke et al., 1999); p.M82T (Murphy et al., 2000); p.E32K (Zhang et al., 2003); p.V143E (Basarab et al., 1999); p.N61S; p.M434I (Dolphin et al., 2000); p.V58I (Kubota et al., 2002); p.D198I; p.R205C (Fujieda et al., 2003). Note: expressed p.N61S is fully active toward methimazole but is incapable of N-oxygenation of trimethylamine (Dolphin et al., 2000).
Inherited genetic defects in hepatic FMO metabolism of trimethylamine have also been documented in livestock. A serious problem developed in the UK with certain breeds of chickens, notably Brown Leghorn and Rhode Island Red, fed diets containing high levels of choline. These chickens laid a high percentage of eggs with a “fishy taint” that were unmarketable (Butler & Fenwick, 1984). This malodor was attributed to high levels of trimethylamine deposition in the eggs. Fortunately, the problem has largely been solved by diet modification and breeding programs. The second example is with some dairy cows that produce milk with a definitive off-flavor and smell, again attributed to high levels of trimethylamine deposition (Lunden et al., 2002). It should be noted here that humans may be particularly susceptible because, unlike most other animals, FMO3 is the major hepatic FMO in the adult.
3.2. Genetic polymorphisms of flavin-containing monooxygenase 2
FMO2 is the major lung FMOexpressed in most mammals including non-human primates (Yueh et al., 1997; Dolphin et al., 1998; Krueger et al., 2001). As discussed in Section 2.2, FMO2 exhibits a number of distinct properties of substrate specificity, thermostability, resistance to detergent inhibition, etc. Our initial attempts to characterize FMO2 protein expression in human lung were unsuccessful. Subsequently, Dolphin et al. (1998) published the sequence of human FMO2 and documented a very interesting genetic polymorphism in expression. All of the Caucasians and Asians genotyped to date have 2 alleles with a c.1414C→T transition replacing a Gln (CAG, FMO2*1, p.Q472) with a premature stop codon (TAG, FMO2*2A, p.X472; Dolphin et al., 1998; Whestine et al., 2000; Krueger et al., 2002b; Furnes et al., 2003). This truncated protein is not found in human lung samples; expressed p.X472 does not incorporate FAD, is catalytically inactive, and is probably rapidly destroyed due to incorrect folding (Whestine et al., 2000; Krueger et al., 2002a). Interestingly, 27% of individuals of African descent (Dolphin et al., 1998; Whestine et al., 2000) and 5% of Hispanics (Krueger et al., 2004) possess at least 1 allele coding for the full length, enzymatically active protein (FMO2.1, p.Q472). Individuals genotyped as possessing at least 1 FMO2*1 allele express full length, catalytically active protein which can be detected by immunoquantitation. Furnes et al. (2003) have documented additional variants in FMO2 (as well as FMO1, FMO4, and FMO5) in African–Americans. We have recently evaluated the occurrence of 4 common FMO2 variants (c.211_213dup- GAC, c.337delG, c.584C→T, and c.1239T→G) identified in African–Americans by Furnes et al. (2003) in Hispanics and expressed each to determine the probable impact on drug metabolism (Krueger et al., in press). Only the expressed p.N413K (c.1239T→G) variant had full catalytic activity (Table 4). The allelic frequency of these variants was lower in the Hispanic population we studied, compared to the African–American population in the Furnes et al. (2003) study, although this latter study examined fewer individuals (50). Furthermore, we have recently demonstrated through haplotype determination and inferred haplotype determination that these variants are located on the major c.1414T allele and thus would not significantly impact the activity of the full-length functional enzyme produced by the c.1414C allele (Krueger et al., in press). Interestingly, the FMO2 gene is also truncated in laboratory, but not in wild, strains of rats (Lattard et al., 2002), and the use of R. norvegicus as a model for the human FMO2 genetic polymorphism has been proposed (Hugonnard et al., 2004).
Our laboratory is now addressing the hypothesis that individuals with the FMO2*1 allele metabolize drugs and xenobiotics differently than individuals with no functional FMO2 in the lung. FMOs bioactivate thioureas through S-oxygenation to toxic sulfenic and/or sulfinic acid metabolites (Poulsen et al., 1979; Neal & Halpert, 1982; Ziegler, 1982; Sabourin & Hodgson, 1984; Nagata et al., 1990; Decker & Doerge, 1991; Decker et al., 1992; Guo et al., 1992; Kim & Ziegler, 2000; Smith & Crespi, 2002). The sulfenic acid is capable of undergoing redox cycling following conjugation with glutathione, resulting in oxidative stress due to depletion of reduced glutathione and NADPH (Hardwick et al., 1991; Decker & Doerge, 1992; Onderwater et al., 1998, 1999, 2004; Smith & Crespi, 2002; Henderson et al., 2004b). This is thought to be an important mechanism of thiourea toxicity in lung as well as in liver (Boyd & Neal, 1976; Lee et al., 1980; Scott et al., 1990; Hardwick et al., 1991; Ziegler-Skylakakis et al., 1998). Human FMO2 is very active toward bioactivation of small molecular weight thioureas (Henderson et al., 2004b) and one would predict that individuals expressing the FMO2*1 allele would be at enhanced risk of toxicity following exposure.
In the case of thioether-containing organophosphate pesticides, the presence of the FMO2*1 allele would be predicted to have the opposite (protective) effect. Organophosphates are bioactivated by desulfuration of the phosphorothionate sulfur to produce the oxon, a metabolite that is orders of magnitude more potent in cholinesterase inhibition (Neal & Halpert, 1982; Jokanovic, 2001). FMO-dependent oxidation of the thioether moiety produces the sulfoxide, a pathway that represents detoxication compared to oxon production (Hajjar & Hodgson, 1980, 1982; Kulkarni & Hodgson, 1984; Osimitz & Kulkarni, 1985; Smyser et al., 1985; Tynes & Hodgson, 1985b; Kinsler et al., 1988, 1990; Levi & Hodgson, 1988; Hodgson & Levi, 1992; Buronfosse et al., 1995; Hodgson et al., 1998; Usmani et al., 2004). Again, we have found human FMO2 to be very effective at S-oxygenation of the thioether of phorate and disulfoton (Henderson et al., 2004a), as has been previously shown for other FMO enzymes and for the mouse FMO2 (Karoly & Rose, 2001). Thus, in individuals expressing full-length, functional FMO2, we hypothesize that the metabolite profile should be shifted in favor of formation of the sulfoxide, rather than the more toxic oxon, resulting in reduced toxicity.
3.3. Genetic variants and polymorphisms in flavin-containing monooxygenase 1
In humans, FMO1 is the major FMO in fetal liver and adult kidney and intestine. The human is a very interesting species with respect to developmental regulation of FMO1 and FMO3. FMO1, the major FMO in liver of most adult mammals, is expressed at relatively high concentrations in human fetal liver, but shortly after birth, expression is reduced to almost zero; the signal for termination of expression is related to the parturition and not to gestational age (Hines & McCarver, 2002; Hines et al., 2003). FMO1 may play an important role in extrahepatic drug metabolism in humans as well as in the liver of the fetus exposed to numerous potential xenobiotic substrates in utero. Currently, there are ~20 allelic variants of human FMO1 described by Furnes et al. (2003) and Hines et al. (2003) and the GeneSNPs database. The enzymatic activity of some of the FMO1 variants are given in Table 4. Based on the work of Hines et al. (2003), some of these allelic variants are probably rare alleles (H97Q, I303V, I303T, R502X) and may not contribute significantly to interindividual differences in FMO1 expression. On the other hand, a much more common variant (FMO1*6) caused by a C→A transversion in the upstream promoter region (−9,536), may impact FMO1 expression as it renders a Yin Yang basal promoter incapable of binding YY1 (Hines et al., 2003). The allelic frequency of FMO1*6 is 13, 11, and 30% in African–Americans, northern European–Americans, and Hispanic–Americans, respectively (Hines et al., 2003).
4. Role of flavin-containing monooxygenase in drug metabolism
FMO and CYP share a number of similarities with respect to tissue, cellular, and organelle expression, the utilization of oxygen and NADPH as cofactors, and activity toward many of the same drugs. In order to determine in vitro the relative contribution of FMO versus CYP in the oxygenation of a particular drug, a number of approaches have been used. The activity of any and all microsomal CYPs can be inhibited by titration with antibody to NADPH CYP reductase, carbon monoxide, or addition of detergent. There are also CYP-selective antibodies and chemical inhibitors to dissect out the contribution of individual CYPs. Unfortunately, antibodies directed toward FMOs typically inhibit catalytic activity weakly, if at all, and the only chemical inhibitors are competitive substrates. The most commonly used alternative substrate to inhibit FMO in vitro (Mani et al., 1993; Narimatsu et al., 1999; Rawden et al., 2000; Pike et al., 2001; Attar et al., 2003; Wynalda et al., 2003; Virkel et al., 2004) and in vivo (Ruse & Waring, 1991; Nace et al., 1997; Wang et al., 2000) has been methimazole (see next section for further discussion of methimazole). FMO activity is unaffected by non-ionic detergent or carbon monoxide, but most FMOs are readily inhibited by short incubations at temperatures of 45–50 °C. In order to estimate the relative contribution of FMO versus CYP, the standard approach is to utilize a combination of these approaches, e.g., preincubation at elevated temperature and incubation with and without a competing FMO substrate. If the results are consistent, one can get a fairly accurate estimate of the relative contributions of these 2 monooxygenases. A more direct approach is to perform incubations of the test drug with individual expressed CYP and FMO enzymes now available commercially. By this approach, one can determine catalytic efficacy for individual FMOs. Another useful approach is to utilize banks of human liver microsomes with known levels of each CYP and FMO and correlate metabolism with the level of the individual enzyme. Finally, the relative importance of FMO and CYP in oxidative metabolism of a particular drug will depend not only upon the catalytic efficiency of that particular enzyme but also upon the relative abundance in the organ being studied. For example, if FMO3 and CYP2D6 had similar kinetics toward a drug, based on the relative abundance in liver, one would predict a greater contribution from FMO3 (perhaps even first pass hepatic metabolism).
It is somewhat more difficult to get an accurate assessment of the relative contribution of FMO in metabolism of a given drug in vivo (Damani & Nnane, 1996). Measurements of FMO-specific metabolites of nicotine (Berkman et al., 1995) and caffeine (Chung & Cha, 1997; Park et al., 1999; Chung et al., 2000) have both been proposed as possible approaches to phenotyping individuals. In the case of nicotine, the N-oxide represents a minor metabolite in urine and the fact that tertiary amine N-oxides are readily reduced in vivo complicates the interpretation of such assays with respect to phenotyping of individuals. Caffeine is very attractive as a potential substrate for phenotyping as one could theoretically simultaneously phenotype an individual for FMO, CYP1A2, xanthine oxidase, and N-acetyltransferase. However, subsequent examination has raised serious doubts about the utility of caffeine for FMO phenotyping (Lang & Rettie, 2000; Rettie & Lang, 2000). Ranitidine metabolism correlates with FMO3 phenotype (Park et al., 2002) and may provide promise in phenotyping individuals for metabolism of other FMO3 substrates.
In animal models, pretreatment with methimazole to inhibit FMO-mediated drug metabolism has been the most common approach (Ruse & Waring, 1991; Nace et al., 1997; Wang et al., 2000). Methimazole is not specific for inhibition of FMO and, in fact, is used clinically as an anti-thyroid drug as it inhibits thyroid peroxidase (Engler et al., 1982). In addition, methimazole can exhibit toxicological properties itself that may confound interpretation of the data (Brittebo, 1995). With respect to distinguishing FMO-from CYP-mediated metabolism, results with methimazole should be interpreted with caution as methimazole can be a substrate for CYPs (Kedderis & Rickert, 1985) and FMO-mediated S-oxygenation of methimazole produces the reactive sulfenic acid which inhibits CYP activity (Kedderis & Rickert, 1985). Thiourea has also been used in vitro to inhibit FMO-mediated activity, but again, due to the reactivity of the sulfenic acid of thiourea, interpretation of such results is not straightforward.
The relative contribution of individual FMO enzymes in the metabolism of a given drug is difficult to determine as, to date, there are few if any FMO family-specific or even selective substrates. Thus, one cannot phenotype an individual for a given FMO, as is possible for many of the CYPs. Possible exceptions to this rule are trimethylamine or ranitidine N-oxygenation (Lang et al., 1998; Kang et al., 2000; FMO3-selective) and the stereoselective N-oxygenation of nicotine to trans-(S)-( −)-nicotine N-1′-oxide (Damani et al., 1988; Cashman et al., 1992a; Park et al., 1993; Berkman et al., 1995; Cashman, 2002).
4.1. Role of flavin-containing monooxygenase in metabolism of N-containing xenobiotics
A number of therapeutic and recreational drugs are substrates for FMO. Table 5 provides a partial listing of nitrogen-containing substrates. Unlike the case with CYP, there are few pharmaceutical agents for which FMO has been shown to be the primary determinant of efficacy and/or toxicity. Tertiary amines, produced by FMO-mediated N-oxygenation, are highly lipophilic, easily excreted, and typically exhibit markedly less pharmacological and/or toxicological properties than the parent amine or CYP-mediated metabolites. For this reason, FMO-mediated N-oxygenation of tertiary amines usually represents detoxication. For example, the neurotoxicant 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP) is readily N-oxygenated by FMO to the N-oxide (Weissman et al., 1985; Cashman, 1986; Cashman & Ziegler, 1986; Chiba et al., 1988, 1995; DiMonte et al., 1988; Ottoboni et al., 1990; Wu et al., 1992; Wu & Ichikawa, 1994, 1995; Narimatsu et al., 1996), a non-toxic easily excreted metabolite. If not N-oxygenated by FMO, MPTP can undergo CYP and monoamine oxidase-catalyzed metabolism to the potent neurotoxins MPDP+ and MPP+ resulting in parkinsonism. The activity of FMO in the N-oxygenation of MPTP in brain is probably of critical importance and may explain species differences in toxicity (Mushiroda et al., 2001). The contribution of FMO N-oxygenation may be underestimated as the N-oxide can be reduced back to the parent amine by CYP or other reductases. Such is certainly the case for tamoxifen, nicotine, and many other drugs with tertiary amines subject to N-oxygenation. By this mechanism, FMO N-oxygenation provides a reservoir of available parent drug, prolonging its action. It should be emphasized again that aliphatic tertiary amine oxidation by CYP and FMO typically yields distinct products; CYP oxidation results in N-dealkylation, whereas FMO produces exclusively the N-oxide. Aryl amines and primary aryl amines are seldom N-oxygenated by FMO, probably due to electron delocalization into the aromatic ring (Frederick et al., 1982; Hammons et al., 1985; Hlavica et al., 1997). In the case of N-oxygenation of amines in heterocycles to N-oxides, this is almost exclusively catalyzed by CYP.
Table 5.
N-containing drugs and xenobiotics oxygenated by FMOa
| Substrate | FMO | Km (μM) | Kcat | Product | Reference |
|---|---|---|---|---|---|
| Primary amines | |||||
| 2-Aminofluorene | pFMO1b | 5b | N-hydroxy | Frederick et al., 1982 | |
| Amphetamine | hFMO3 | trans:cis oxime 5:1 | Cashman et al., 1999 | ||
| m-Amylamine | rFMO2 | >50,000 | Tynes et al., 1986 | ||
| n-Heptylamine | rFMO2 | 27,000 | Tynes et al., 1986 | ||
| N-octylamine | rFMO2 | 6700 | Tynes et al., 1986 | ||
| N-octylamine | rFMO2 | 12,000 | Poulsen et al., 1986 | ||
| N-nonylamine | rFMO2 | 1800 | Tynes et al., 1986 | ||
| N-decylamine | rFMO2 | 400 | Tynes et al., 1986 | ||
| N-undecylamine | rFMO2 | 90 | Tynes et al., 1986 | ||
| N-dodecylamine | rFMO2 | 33 | Tynes et al., 1986 | ||
| N-dodecylamine | rFMO2 | 290 | cis-oxime | Poulsen et al., 1986 | |
| N-tridecylamine | rFMO2 | 13 | Tynes et al., 1986 | ||
| C8 and C10 N- | hFMO3 | cis-oximes | Lin et al., 1996 | ||
| (n-octylamino-phenethylamine) | hFMO3 | 90–197 | trans-oxime | Lin & Cashman, 1997b | |
| 2-(Trifluoromethyl)phenothiazines | hFMO3 | cis-oxime | Lin et al., 1996 | ||
| Tyramine | hFMO3 | 220–950 | trans-oxime | Lin & Cashman, 1997a | |
| Secondary amines | |||||
| p-Chloro-N-methylaniline | pFMO1 | Prough & Ziegler, 1977 | |||
| N-deacetyl ketoconazole | hFMO1 and rFMO1 | N-hydroxy/nitrone | Rodriguez & Miranda, 2000 | ||
| r-FMO2 | Rodriguez & Miranda, 2000 | ||||
| Human and rabbit FMO3 | |||||
| pFMO1 | 35 | Rodriguez & Acosta, 1997a, 1997b | |||
| Desipramine | pFMO1 | 250 | Ziegler, 1988 | ||
| Desmethyltrifluperazine | pFMO1 | 9 | Ziegler, 1988 | ||
| Ephedrine | pFMO1 | Ziegler, 1988 | |||
| N-fluoro-N-methylaniline | Rat liver FMO | 19 | C4-hydroxy | Boersma et al., 1993 | |
| Rat liver FMO | 53 | N-hydroxyl | Boersma et al., 1993 | ||
| Rat liver FMO | 93 | N-demethyl | Boersma et al., 1993 | ||
| 3,3′-Iminodipropionitrile | Rat in vivo | N-hydroxy | Nace et al., 1997 | ||
| Methamphetamine | Guinea pig in vitro
Rat in vitro |
Amphetamine | Yamada et al., 1984 | ||
| Methamphetamine | hFMO3 | Phenylpropanone | Cashman et al., 1999 | ||
| N-methylaniline | pFMO1 | 30 | N-hydroxy | Ziegler, 1988 | |
| Mouse liver FMO | 1060 | Sabourin & Hodgson, 1984 | |||
| N-methyloctylamine | rFMO2 | 120 | Poulsen et al., 1986 | ||
| Nortryptyline | pFMO1 | 500 | Ziegler, 1988 | ||
| Propranolol | pFMO1 | 210 | N-hydroxy | Wu et al., 2004 | |
| pFMO1 | 400 | Ziegler, 1988 | |||
| Tertiary amines | |||||
| ABT-418 | pFMO1 | 100% trans N-oxide | Rodrigues et al., 1995 | ||
| rFMO2 | 100% trans N-oxide | Rodrigues et al., 1995 | |||
| hFMO3 | 1:1 cis:trans N-oxide | Rodrigues et al., 1995 | |||
| Acetylpromazine | pFMO1 | 53 | Ziegler et al., 1971a, 1971b | ||
| Amitriptyline | pFMO1 | 100 | N-oxide | Ziegler, 1980 | |
| Benzphetamine | pFMO1 | 74 | Sabourin & Hodgson, 1984 | ||
| Mouse liver FMO | 1670 | Sabourin & Hodgson, 1984 | |||
| Mouse liver FMO | 130 | N-oxide | Ziegler, 1988 | ||
| Benzydaminec | hFMO1 | 60 | 46 | N-oxide | Lang & Rettie, 2000 |
| hFMO1 | 24 | N-oxide | Stormer et al., 2000 | ||
| hFMO3 | 80 | 36 | Lang & Rettie, 2000 | ||
| hFMO3 | 40 | Stormer et al., 2000 | |||
| Bromdiphenhydramine | pFMO1 | 47 | Ziegler et al., 1971a | ||
| Brompheniramine | pFMO1 | Prough & Ziegler, 1977 | |||
| pFMO1 | Cashman et al., 1993a | ||||
| pFMO1 | 200 | N-oxide | Ziegler, 1988 | ||
| Bupivacaine | pFMO1 | Wu et al., 2004 | |||
| Butytyriptyline | pFMO1 | 100 | Ziegler et al., 1971a | ||
| Chlorimipramine | pFMO1 | 40 | Ziegler et al., 1971a | ||
| (d)-Chlorpheniramine | pFMO1 | 76 | N-oxide | Cashman et al., 1992b | |
| Tertiary amines | |||||
| (l)-Chlorpheniramine | pFMO1 | 300 | N-oxide | Cashman et al., 1992b | |
| Chlorpromazine | pFMO1 | 9 | N-oxide | Sofer & Ziegler, 1978 | |
| Chlorprothixene | pFMO1 | 30 | Ziegler et al., 1971a | ||
| Clorgyline | pFMO1 | 2 | N-oxide | Wu & Ichikawa, 1995 | |
| Clozapine | hFMO3 | 324 | N-oxide | Tugnait et al., 1997 | |
| Deprenyl | pFMO1 | 65 | N-oxide | Cashman & Ziegler, 1986 | |
| pFMO1 | 14 | Wu & Ichikawa, 1995 | |||
| Diethazine | pFMO1 | 600 | Ziegler et al., 1971a | ||
| N,N-diethylaniline | pFMO1 | 130 | Ziegler et al., 1971a | ||
| Mouse liver FMO | 144 | Sabourin & Hodgson, 1984 | |||
| N,N-dimethyloctylamine | rFMO2 | 46 | Poulsen et al., 1986 | ||
| Diphenhydramine | pFMO1 | 160 | N-oxide | Ziegler, 1988 | |
| N-ethyl-N-methylaniline | pFMO1 | 59 | Ziegler et al., 1971a | ||
| pFMO1 | 20 | N-oxide (S/R=4) | Hadley et al., 1994 | ||
| Ethylmorphine | pFMO1 | 284 | Sabourin & Hodgson, 1984 | ||
| Mouse liver FMO | 1650 | Sabourin & Hodgson, 1984 | |||
| Fluphenazine | pFMO1 | 12 | Sofer & Ziegler, 1978 | ||
| Guanethidine | pFMO1 | 310 | N-oxide | McManus et al., 1983 | |
| pFMO1 | 170 | Ziegler, 1988 | |||
| Imipramine | pFMO1 | 22 | N-oxide | Cashman & Ziegler, 1986 | |
| dFMO1 | 5 | Stevens et al., 2003 | |||
| Mouse liver FMO | 27 | Sabourin & Hodgson, 1984 | |||
| Human brain | N-oxide | Bhagwat et al., 1996b | |||
| Itopride | hFMO1 and FMO3 | N-oxide | Mushiroda et al., 2000 | ||
| K11777 | hFMO3 | 109 | N-oxide | Jacobsen et al., 2000 | |
| Lidocaine | pFMO1 | Wu et al., 2004 | |||
| Methaphenilene | pFMO1 | 42 | Ziegler et al., 1971a | ||
| Methapyrilene | pFMO1 | 93 | Ziegler et al., 1971a | ||
| Methdilazine | pFMO1 | 55 | Ziegler et al., 1971a | ||
| Methotrimeprazine | pFMO1 | 9 | Ziegler et al., 1971a | ||
| Methoxypromazine | pFMO1 | 33 | Ziegler et al., 1971a | ||
| Methyapyrilene | pFMO1 | 93 | N-oxide | Ziegler, 1988 | |
| MPTP | rFMO1 | 2 | N-oxide | Chiba et al., 1995 | |
| pFMO1 | 32 | N-oxide | Cashman & Ziegler, 1986 | ||
| pFMO1 | 38 | N-oxide | Wu & Ichikawa, 1995 | ||
| (S)-nicotine | Monkey FMO2 | 1700 | 0.6 | N-oxide | Krueger et al., 2001 |
| rFMO2 | 500 | 7 | N-oxide | Krueger et al., 2001 | |
| pFMO | trans:cis 57:43 | N-oxide | Park et al., 1993 | ||
| hFMO3 | trans only | N-oxide | Park et al., 1993 | ||
| rFMO2 | trans only | N-oxide | Park et al., 1993 | ||
| pFMO1 | 181 | 37-1:1 cis:trans | N-oxide | Damani et al., 1988 | |
| pFMO1 | 380 | 57 | Williams et al., 1990 | ||
| rFMO2 | 1030 | <1 | Williams et al., 1990 | ||
| (R)-(+)-nicotine | pFMO1 | 70 | 41-trans | N-oxide only | Damani et al., 1988 |
| Olopatidine | hFMO1 and FMO3 | N-oxide | Kajita et al., 2002 | ||
| Pargyline | pFMO1 | 65 | N-oxide | Cashman & Ziegler, 1986 | |
| pFMO1 | 12 | Wu & Ichikawa, 1995 | |||
| Phenethiazine | pFMO1 | 120 | Ziegler et al., 1971a | ||
| Pheniramine | pFMO1 | 1300 | Ziegler et al., 1971a | ||
| Prochlorperazine | pFMO1 | 3 | Sofer & Ziegler, 1978 | ||
| rFMO2 | 3 | Poulsen et al., 1986 | |||
| Promazine | pFMO1 | 66 | N-oxide | Ziegler, 1988 | |
| Promethazine | pFMO1 | 120 | Ziegler et al., 1971a | ||
| Propiomazine | pFMO1 | 100 | Ziegler et al., 1971a | ||
| Prothipendyl | pFMO1 | 1 | Ziegler et al., 1971a | ||
| S 16020 | hFMO3 | N-oxide | Pichard-Garcia et al., 2004 | ||
| SnI-2011 | hFMO1 | N-oxide | Washio et al., 2001 | ||
| Tamoxifen | Mouse FMO1 | 1200 | N-oxide | Hodgson et al., 2000 | |
| hFMO3 | 1400 | N-oxide | |||
| Thioperazine | pFMO1 | 11 | N-oxide | Sofer & Ziegler, 1978 | |
| Thioridazine | Mouse liver | N-oxide | Blake et al., 1995 | ||
| Tertiary amines | |||||
| Trifluoperazine | pFMO1 | 13 | Sofer & Ziegler, 1978 | ||
| rFMO2 | 31 | Poulsen et al., 1986 | |||
| Triflupromzaine | pFMO1 | 20 | Ziegler, 1988 | ||
| Trimeprazine | pFMO1 | 20 | Ziegler et al., 1971a | ||
| Trimethylamine | mFMO2 | 1686 | N-oxide | Karoly & Rose, 2001 | |
| Verapamil | pFMO1 | 64 | N-oxide (S/R=10) | Cashman, 1989 | |
| Rat liver | 51 | N-oxide (S/R=7) | Cashman, 1989 | ||
| Xanomeline | hFMO1 and FMO3 | N-oxide | Ring et al., 1999 | ||
| Zimeldine | Rat liver | N-oxide | Cashman et al., 1988 | ||
| Pig liver | N-oxide | Cashman et al., 1988 | |||
| Hydrazines | |||||
| N-aminohomopiperdine | pFMO1 | 170 | Ziegler, 1988 | ||
| N-aminomorpholine | pFMO1 | 610 | Ziegler, 1988 | ||
| N-aminopiperdine | pFMO1 | 30 | Hydrazone | Ziegler, 1988 | |
| N-aminopyrrolidine | pFMO1 | 100 | Ziegler, 1988 | ||
| 1,1-Dimethylhydrazine | pFMO1 | 430 | Ziegler, 1988 | ||
| 1,2-Dimethylhydrazine | pFMO1 | 5600 | Ziegler, 1988 | ||
| 1-Methyl-1-phenylhydrazine | pFMO1 | 80 | Hydrazone | Ziegler, 1988 | |
| 1-Methyl-2-phenylhydrazine | pFMO1 | 2000 | Ziegler, 1988 | ||
| Monobenzylhydrazine | pFMO1 | 7000 | Ziegler, 1988 | ||
| Monobutylhydrazine | pFMO1 | 6900 | Ziegler, 1988 | ||
| Monomethylhdrazine | Rat liver in vitro | Gomez & Castro, 1986 | |||
| pFMO1 | 35,000 | Prough, 1973 | |||
| Monophenylhydrazine | pFMO1 | 3000 | Ziegler, 1988 | ||
| Procarbazine | pFMO1 | 1800 | Ziegler, 1988 | ||
N,N-dimethyaniline is not included as it has been used by many researchers to assay FMO activity.
p = pig; h = human; d = dog; r= rabbit; m= mouse.
Drugs for which FMO-mediated metabolism may predominate in humans is shown in italics.
The contribution of FMO to the metabolism of secondary amines, such as methamphetamine (Yamada et al., 1984; Cashman et al., 1999), may also be underestimated. In this case, it is not reduction back to the parent amine, such as with tertiary amines, but rather the production of metabolites that may be attributed to CYP action. Secondary amines are oxygenated by FMO initially to the N-hydroxy amine followed by a second oxygenation to nitrones. The nitrones are typically rapidly hydrolyzed to yield a hydroxylated primary amine and an aldehyde (Ziegler, 1980, 1988). The hydroxylated primary amine is then reduced enzymatically to the primary amine, producing the same product as CYP-mediated N-dealkylation. Unlike the N-oxygenation of tertiary amines, FMO-mediated metabolism of secondary amines does not always result in detoxication. For example, 3,3′-iminodipropionitrile is a secondary amine that is bioactivated by FMO to the neurotoxic N-hydroxy-3,3′iminodipropionitrile (Nace et al., 1997). Another example is N-deacetyl ketoconazole which is N-hydroxylated by a number of FMOs (Table 5; Rodriguez & Acosta, 1997a; Rodriguez et al., 1999; Rodriguez & Miranda, 2000). FMO-mediated metabolism of N-deacetyl ketoconazole appears to be involved in hepatotoxicity following administration as a antifungal agent (Rodriguez & Acosta, 1997b; Rodriguez & Buckholz, 2003). In addition, evidence exists that FMO-dependent production of hydroxylated secondary (and primary) amines can lead to complexation with hemoproteins, including CYP, resulting in inhibition and perhaps subsequent toxicity (Jonsson & Lindeke, 1992).
Primary alkyl amines can be positive effectors of certain FMO enzymes or serve as substrates for other FMOs. In the latter case, the initial N-hydroxylamine is a markedly better substrate than the parent amine and is rapidly oxygenated a second time to give the oxime, often in a stereospecific fashion (e.g., the trans-oxime with FMO2; Williams et al., 1984; Tynes et al., 1985, 1986; Poulsen et al., 1986). Oxime formation from primary alkyl amines can thus usually be attributed to FMO. Oximes typically have little pharmacological or toxicological activity and are readily excreted.
Hydrazines can also be excellent substrates for FMO. The initial product observed is the hydrazone, which is subsequently hydrolyzed to form an aldehyde and a dealkylated hydrazine (Prough et al., 1981; Ziegler, 1984, 1988; Gomez & Castro, 1986; Jenner & Timbrell, 1995). Both CYP and FMO are capable of carrying out this reaction; however, it appears that CYP oxygenates 1,2-disubstituted hydrazines, whereas FMO exclusively oxygenates 1,1- disubstituted hydrazines (Ziegler, 1984, 1988). In addition to industrial uses, hydrazines have been used as pharmaceutical agents for a number of years. The use of hydrazines in therapeutics has been limited by toxicity in liver and other organs. The contribution of FMO-mediated oxygenation of 1,1-disubstituted hydrazines to the risk of developing hydrazine toxicities is not known.
It is possible, based on in vitro studies with human hepatocytes or hepatic microsomes, to predict for which N-containing drugs FMO-mediated N-oyxgenation should play a predominant role in pharmacokinetics (Table 5). The nitrogen-containing drugs most likely to be metabolized predominantly, if not exclusively, in vivo by FMO include chlor- and bromo-pheniramine, zimeldine, ranitidine, benzydamine, olopatadine, xanomeline, pargyline, and itopride.
4.2. Role of flavin-containing monooxygenase in metabolism of S-containing xenobiotics
FMO readily catalyzes the S-oxygenation of numerous drugs and xenobiotics (Table 6). These include sulfides, thiols and disulfides, thiocarbamides and thioamides, and mercapto-purines, pyrimidines, and imidazoles (Ziegler, 1980, 1982, 1988, 1991; Poulsen, 1981). Thiols are oxygenated to disulfides through an intermediate sulfenic acid. Disulfides can be further oxygenated to S-oxides (e.g., butyldisulfide S-oxide). The endogenous substrate cysteamine is oxygenated to the disulfide cystamine. Cystamine is not further oxygenated by FMO. Another endogenous substrate, the disulfide lipoic acid, appears to be a better substrate than dihydrolipoamide.
Table 6.
S-containing drugs and xenobiotics oxygenated by FMOa
| Substrate | FMO | Km (μM) | Kcat (min−1) | Product | Reference |
|---|---|---|---|---|---|
| Thiols | |||||
| Benzylmercaptan | pFMO1b | 55 | Ziegler, 1980 | ||
| n-Butylmercaptan | pFMO1 | 33 | Ziegler, 1980 | ||
| Dithiothreitol | pFMO1 | 465 | Ziegler, 1980 | ||
| Ethanethiol | pFMO1 | 58 | Disulfide | Poulsen, 1981 | |
| n-Heptylmercaptan | pFMO1 | 15 | Ziegler, 1980 | ||
| β-Mercaptoethanol | pFMO1 | 2800 | Ziegler, 1980 | ||
| Thiophenol | pFMO1 | 33 | Ziegler, 1980 | ||
| Thioglycerol | pFMO1 | 721 | Ziegler, 1980 | ||
| Disulfides | |||||
| Butyldisulfide | pFMO1 | 6 | S-oxide | Poulsen, 1981 | |
| Dibenyldisulfide | pFMO1 | 45 | Ziegler, 1980 | ||
| o-Dithiane | pFMO1 | <3 | Ziegler, 1980 | ||
| trans-4,5-Dihydroxy-o-dithiane | pFMO1 | 270 | S-oxide | Poulsen, 1981 | |
| Sulfides/thioethers | |||||
| Albendazolec | Rat liver FMO | Sulfoxide | Moroni et al., 1995a | ||
| Aldicarb | mFMO liver | 607 | Usmani et al., 2004 | ||
| pFMO1 | 312 | Usmani et al., 2004 | |||
| 2-Aryl-1,3-dithiolanes | rFMO2 | trans-S-oxides | Cashman & Williams, 1990 | ||
| (+)-4-Bromophenyl-1,3-oxathiolane | pFMO1 | 100% trans-S-oxide | Cashman et al., 1995 | ||
| (−)-4-Bromophenyl-1,3-oxathiolane | pFMO1 | 24% trans-S-oxide | Cashman et al., 1995 | ||
| Benzyl methyl sulfide | pFMO1 | 1 | 49 | Sulfoxide | Light et al., 1982 |
| 4-Chlorophenyl allyl sulfide | pFMO1 | 53 | Sulfoxide | Light et al., 1982 | |
| 4-Chlorophenyl methyl sulfide | pFMO1 | 185 | Nnane & Damani, 2003b | ||
| Cimetidine | pFMO1 | S-oxide | Cashman et al., 1993b | ||
| Dazomet | mFMO liver | 399 | Usmani et al., 2004 | ||
| pFMO1 | 244 | Usmani et al., 2004 | |||
| Demeton-S | mFMO liver | 110 | Levi & Hodgson, 1988 | ||
| pFMO1 | 40 | Levi & Hodgson, 1988 | |||
| Demeton-O | mFMO liver | 59 | Levi & Hodgson, 1988 | ||
| Dimethylsulfide | pFMO1 | <3 | DMSO | Poulsen, 1981 | |
| Mouse liver FMO | 34 | Sabourin & Hodgson, 1984 | |||
| DMSO | pFMO1 | 90,000 | S-dioxide | Poulsen, 1981 | |
| Diphenyl sulfide | pFMO1 | 68 | Nnane & Damani, 2003a, 2003b | ||
| pFMO1 | 74 | Sulfoxide | Poulsen, 1981 | ||
| Disulfoton | mFMO liver | 3 | Levi & Hodgson, 1988 | ||
| pFMO1 | 2 | Levi & Hodgson, 1988 | |||
| rFMO2 | 71 | 44 | Sulfoxide | Henderson et al., 2004a | |
| hFMO2 | 32 | 20 | Sulfoxide | Henderson et al., 2004a | |
| hFMO1 | 7 | 37 | Sulfoxide | Usmani et al., 2004 | |
| Ethylene sulfide | pFMO1 | 60 | Ziegler, 1980 | ||
| Ethyl methyl sulfide | pFMO1 | 1380 | Sulfoxide | Nnane & Damani, 2003a, 2003b | |
| Fenthion | mFMO liver | 12 | Levi & Hodgson, 1988 | ||
| pFMO1 | 9 | Levi & Hodgson, 1988 | |||
| pFMO1 | 18 | Levi & Hodgson, 1988 | |||
| Flosequinan sulfide | ratFMO1 | 33 | R(+)-sulfoxide | Kashiyama et al., 1994 | |
| Fonofos | pFMO1 | 33 | Smyser & Hodgson, 1985 | ||
| Metam-sodium | mFMO liver | 572 | Usmani et al., 2004 | ||
| pFMO1 | 175 | Usmani et al., 2004 | |||
| Methiocarb | mFMO liver | 130 | Usmani et al., 2004 | ||
| pFMO1 | 115 | Usmani et al., 2004 | |||
| hFMO1 | 30 | 86 | Sulfoxide | Usmani et al., 2004 | |
| 4-Methoxyphenyl allyl sulfide | pFMO1 | 60 | Sulfoxide | Light et al., 1982 | |
| 2-Methyl-1,3-benzodithiol | pFMO1 | 100% cis (−)-(1S,2R) | Cashman et al., 1995 | ||
| S-methyl-N-N-dithiocarbamate | hFMO1 | 7.6 | S-oxide | Pike et al., 2001 | |
| S-methyl-esonarimod | hFMO1,3,5 | 2.71 (FMO5) | Sulfoxide | Ohmi et al., 2003 | |
| Sulfides/thioethers | |||||
| Methylphenylsulfide | pFMO1 | 13 | Ziegler, 1980 | ||
| pFMO1 | 16 | 62 | Sulfoxide | Light et al., 1982 | |
| Mouse liver FMO | 6 | Sabourin & Hodgson, 1984 | |||
| MK-0767 methyl sulfide | hFMO1,3,5 | FMO1 99% stereoselective ee | Sulfoxide | Karanam et al., 2004 | |
| 4-Nitrophenyl allyl sulfide | pFMO1 | 53 | Sulfoxide | Light et al., 1982 | |
| Phenothiazine | pFMO1 | 12 | Nagata et al., 1990 | ||
| S-phenyl diethyl-phosphinothiolothionate | pFMO1 | 48 | Smyser & Hodgson, 1985 | ||
| Phorate | mFMO liver | 32 | 80 | (−) Sulfoxide | Levi & Hodgson, 1988 |
| pFMO1 | 12 | Levi & Hodgson, 1988 | |||
| hFMO1 | 49 | 26 | Sulfoxide | Usmani et al., 2004 | |
| rFMO2 | 71 | 39 | Sulfoxide | Henderson et al., 2004a | |
| hFMO2 | 57 | 41 | Sulfoxide | Henderson et al., 2004a | |
| mFMO2 | 107 | Sulfoxide | Karoly & Rose, 2001 | ||
| Phorate oxon | mFMO liver | 462 | Levi & Hodgson, 1988 | ||
| pFMO1 | 256 | Levi & Hodgson, 1988 | |||
| SM-12502 | rat FMO1 | S-oxide | Nunoya et al., 1995 | ||
| Sodium dimethyldithio-carbamate | mFMO liver | 761 | Usmani et al., 2004 | ||
| pFMO1 | 1360 | Usmani et al., 2004 | |||
| Sodium diethyldithio-carbamate | mFMO liver | 739 | Usmani et al., 2004 | ||
| pFMO1 | 1099 | Usmani et al., 2004 | |||
| Sulindac sulfide | rFMO2, hFMO3 | (R)-sulfoxide | Hamman et al., 2000 | ||
| pFMO1 | 3 | 80>90% (R)-sulfoxide | Light et al., 1982 | ||
| Sulprofos | mFMO liver | 1 | Levi & Hodgson, 1988 | ||
| pFMO1 | 1 | Levi & Hodgson, 1988 | |||
| hFMO1 | 14 | 16 | Sulfoxide | Usmani et al., 2004 | |
| Tazarotenic acid | hFMO1,3 | Sulfoxide | Attar et al., 2003 | ||
| Thiobenzamide sulfoxide | pFMO1 | 250 | S-dioxide | Poulsen, 1981 | |
| Thiofanox | mFMO liver | 575 | Usmani et al., 2004 | ||
| pFMO1 | 1307 | Usmani et al., 2004 | |||
| p-Tolyl methyl sulfide | hFMO1 | (R) sulfoxide | Yeung et al., 2000 | ||
| hFMO1 | 87 | Stevens et al., 2003 | |||
| dFMO1 | 99 | Stevens et al., 2003 | |||
| hFMO2 | 63 | (R)-sulfoxide | Unpublished | ||
| p-Tolyl ethyl sulfide | pFMO1 | 13 | 48 | 95% (R)-sulfoxide | Light et al., 1982 |
| p-Tolyl substituted sulfides (methyl-n-butyl) | rFMO3 | <10 | 100% (R) sulfoxide decreasing slightly with chain length | Rettie et al., 1994 | |
| rFMO3 | <10 | 100% (R) sulfoxide decreasing markedly with chain length | Rettie et al., 1994 | ||
| rFMO3 | 100–280 | 90% (R) with n-butyl-decreasing to no stereoselectivity with methyl or ethyl | Rettie et al., 1994 | ||
| hFMO3 | 80–90% (R) for propyl and butyl | Haining et al., 1997 | |||
| rFMO5 | 97–98% (S) with methyl and ethyl Little or no activity with propyl | Fisher et al., 1995 | |||
| Straight-chain and branched alkyl p-tolyl sulfides | rFMO2 | Stereoselectivity of sulfoxide function of steric factors | Rettie et al., 1990, 1995 | ||
| Monkey FMO3, rFMO1 and rFMO3 | Primarily (R)-sulfoxide | Rettie et al., 1995 | |||
| Methy–hexyl 2-naphthyl sulfides | rFMO2 | <0.2–66 97–98% (R)-sulfoxide | Fisher & Rettie, 1997 | ||
| rFMO1 | 1–37 83–92% (R)-sulfoxide | Fisher & Rettie, 1997 | |||
| rFMO5 | >92% (S)-sulfoxide with methyl | Fisher et al., 1995 | |||
| rFMO5 | >85% (S) with ethyl | Fisher et al., 1995 | |||
| rFMO5 | Little or no activity with propyl | Fisher et al., 1995 | |||
| Thiocarbamides and related | |||||
| Ethionamide | hFMO2 | 107 | 9 | Sulfoxide | Unpublished |
| hFMO3 | 184 | 33 | Sulfoxide | Unpublished | |
| Ethylene thiourea | hFMO2 | 14 | 50 | Krueger et al., 2002a, 2002b | |
| rFMO2 | 21 | 149 | Henderson et al., 2004b | ||
| α-Naphthylthiourea | pFMO1 | 4 | Nagata et al., 1990 | ||
| rFMO2 | 25 | 60 | Henderson et al., 2004b | ||
| hFMO2 | 42 | 33 | Sulfoxide | Henderson et al., 2004b | |
| Thiocarbamides and related | |||||
| 1-Phenylthiourea | pFMO1 | 4 | Poulsen et al., 1979 | ||
| hFMO1 | 3 | Henderson et al., 2004b | |||
| rFMO2 | 82 | 327 | Henderson et al., 2004b | ||
| hFMO2 | 29 | 24 | Sulfoxide | Henderson et al., 2004b | |
| Thioacetamide | pFMO1 | 65 | S-oxide | Ziegler, 1980 | |
| Thioacetanilide | hFMO2 | Krueger et al., 2002a | |||
| Thiobenzamide | hFMO2 | Krueger et al., 2002a | |||
| Thiocarbanilide | pFMO1 | 7 | Poulsen et al., 1979 | ||
| Thionicotinamide | pFMO1 | 460 | S-oxide | Ziegler, 1980 | |
| Thiourea | pFMO1 | 23 | Nagata et al., 1990 | ||
| hFMO1 | 4 | Kim & Ziegler, 2000 | |||
| rFMO2 | 36 | 134 | Henderson et al., 2004b | ||
| hFMO2 | 27 | 51 | Henderson et al., 2004b | ||
| mFMO2 | 11 | Karoly & Rose, 2001 | |||
| pFMO1 | 34 | Poulsen et al., 1979 | |||
| Sulfenic acids | |||||
| Benzimidazoline-2-thiones | pFMO1 | Sulfenic/sulfinic acids | Decker et al., 1992 | ||
| Deacetylated spironolactone | pFMO1 | Sulfenic acid | Decker et al., 1991 | ||
| Diethylcarbodithioic acid | pFMO1 | 75 | Poulsen, 1981 | ||
| 1,3-Diphenylformadidine-sulfenic acid | pFMO1 | 7 | Ziegler, 1980 | ||
| Dithiobenzoic acids and esters | pFMO1 | 3–7500 | Taylor & Ziegler, 1987 | ||
| Ethylene formadidine–sulfenic acid | pFMO1 | 190 | Ziegler, 1980 | ||
| Formamidinesulfenic acid | pFMO1 | 140 | Ziegler, 1980 | ||
| 2-Mercaptobenzimidazole | pFMO1 | 13 | Poulsen, 1981 | ||
| Mercaptobenzoic acid | pFMO1 | 67 | Taylor & Ziegler, 1987 | ||
| N-methylimidazole-2-sulfenic acid | pFMO1 | 22 | Sulfinic acid | Ziegler, 1980 | |
| Phenylcarbodithioic acid | pFMO1 | <3 | Poulsen, 1981 | ||
| Phenylformamidine–sulfenic acid | pFMO1 | 34 | Sulfinic acid | Ziegler, 1980 | |
| Propylthiouracil | pFMO1 | 950 | Poulsen, 1981 | ||
| Thiolacetic acid | pFMO1 | 200 | Taylor & Ziegler, 1987 | ||
Methimazole is not included as it has been used by many investigators to assay FMO activity.
p = pig; h = human; m = mouse; r = rabbit; d = dog.
Drugs for which FMO-mediated metabolism may predominate in humans is shown in italics.
Many therapeutic drugs possess a sulfide pharmacophore. Sulfides are typically excellent substrates for FMO and are readily converted to the sulfoxide (Ziegler, 1980, 1982, 1984; Poulsen, 1981). The S-oxygenation of thioureas, thioether-containing insecticides such as phorate and disulfoton, and FMO-mediated sulfoxidation of cysteine S-conjugates have already been discussed. FMO readily S-oxygenates simple sulfides such as methyl-, ethyl-, ethyl methyl, 4-chlorophenyl methyl, and diphenyl sulfides (Poulsen & Ziegler, 1995; Nnane & Damani, 2003a, 2003b). Sulfide-containing drugs, for which FMO is active in S-oxygenation to sulfoxides, include cimetidine, rantidine, thioridazine, 7-(1,3-thiazolidin-2-methyl) theophylline, (+)-cis-3,5-dimethyl-2-(3-pyridyl)-thiazolidin-4-one, sulindac sulfide, clindamycin, albendazole, and fenbendazole (Eriksson & Bostrom, 1988; Grosa et al., 1992; Cashman et al., 1993b; Lanusse et al., 1993; Moroni et al., 1995a; Nunoya et al., 1995; Poulsen & Ziegler, 1995; Hamman et al., 2000; Rawden et al., 2000; Nnane & Damani, 2003a, 2003b; Wynalda et al., 2003; Virkel et al., 2004). Although not employed as a therapeutic drug, the cyclic sulfide tetrahydrothiophene is the best substrate to date, as evidenced by sub-micromolar Km values, found by us with FMO. FMO can catalyze the further oxygenation of the sulfoxide to the sulfone, but often this reaction is more efficiently catalyzed by CYP. In the case of chiral sulfides, the sulfoxygenation is often stereoselective (Cashman & Williams, 1990; Rettie et al., 1990, 1994, 1995; Cashman et al., 1993b; Kashiyama et al., 1994; Buronfosse et al., 1995; Moroni et al., 1995a; Hamman et al., 2000). Interestingly, this stereoselectivity is often distinct from that of CYP sulfoxygenation of the same achiral substrate.
Related to the thioureas, or thiocarbamides, are the thioamides such as thioacetamide and thiobenzamide. Thioacetamide and thiobenzamide are potent liver toxicants (Hunter et al., 1977; Hanzlik et al., 1980; Grossman et al., 1991), and the hepatotoxicity appears primarily due to FMO-mediated S-oxygenation to sulfines and sulfenes (Cashman & Hanzlik, 1981; Dyroff & Neal, 1983; Chieli & Malvaldi, 1984; Ziegler, 1984; Ruse & Waring, 1991; Lee et al., 2003).
Ethionamide (2-ethyl-4-thiocarbamoylpyridine) is structurally related to thiobenzamide and is being utilized in the treatment of tuberculosis. Recently, the laboratory of Dr. Paul Ortiz de Montellano demonstrated that ethionamide is a prodrug that must be S-oxygenated by an FMO in the target organism, Mycobacterium tuberculosis, to exert its effect (Vannelli et al., 2002). The metabolic activation of ethionamide by the bacterial FMO is the same as the mammalian FMO activation of thiobenzamide to produce hepatotoxic sulfinic and sulfinic acid metabolites. Not surprisingly, Dr. Ortiz de Montellano’s laboratory and our own have found ethionamide to be a substrate for human FMO1, FMO2, and FMO3 (unpublished observations). Approximately 25% of individuals have to discontinue ethionamide due to toxicity, primarily in liver, thought to be due to FMO3-dependent S-oxygenation. It would be of interest to determine if particular FMO3 allelic variants correlated to susceptibility to ethionamide hepatotoxicity.
Sulfur-containing drugs for which FMO may be the primary determinant of metabolism in vivo include cimetidine, albendazole, sulindac sulfide, 7-(1,3-thiazolidin-2- ylmethyl) theophylline, (+)-cis-3,5-dimethyl-2-(3-pyridyl)- thiazolidin-4-one hydrochloride (SM-12502), methimazole, and ethionamide (Table 6). Xenobiotics with “soft nucleophile” substituents other than nitrogen and sulfur, can also be FMO substrates (Table 7).
Table 7.
FMO activities not involving S- or N-oxygenation
| Substrate | Product | FMO | Km (μM) | Reference |
|---|---|---|---|---|
| Carbon | ||||
| 5,6-Dimethylxanthenone-4-acetic acid | 6-Methyl hydroxyl | hFMO3a | Zhou et al., 2002 | |
| Salicylaldehyde | Pyrocatechol/formate | pFMO1 | 500–600 | Chen et al., 1995 |
| Selenium | ||||
| Benzeneselenol | pFMO1 | 18 | Ziegler et al., 1992 | |
| Benzylselenide | pFMO1 | 0.7 | Chen & Ziegler, 1994 | |
| Dimethylselenide | Selenoxide | pFMO1 | Goeger & Ganther, 1994 | |
| Methylphenylselenide | Selenoxide | pFMO1 | 0.3 | Chen & Ziegler, 1994 |
| 2-(Methylseleno)benanilide | Selenoxide | pFMO1 | 5 | Ziegler et al., 1992 |
| 2-(Methylseleno)cinnamonitrile | pFMO1 | 3 | Ziegler et al., 1992 | |
| N-[2-(methylseleno)ethyl]benzamide | pFMO1 | 1 | Ziegler et al., 1992 | |
| Phenylselenomethyl)trimethylsilane | pFMO1 | 10 | Ziegler et al., 1992 | |
| 2-Selenylbenzanilide | pFMO1 | Chen & Ziegler, 1994 | ||
| Selenocyteine Se-conjugates | Selenoxide | pFMO1 | Rooseboom et al., 2001 | |
| Selenourea | pFMO1 | 3 | Ziegler et al., 1992 | |
| Others | ||||
| Diphenylmethylphosphine | Oxide | pFMO1 | Smyser & Hodgson, 1985 | |
| Boronic acids | pFMO1 | Jones & Ballou, 1986 | ||
| Iodine | pFMO1 | Ziegler et al., 1980 | ||
p = pig; h = human.
5. Conclusions and suggestions for future research
The flavin-containing monooxygenase (FMO) is a family of enzymes that like the cytochrome P450 (CYP) superfamily evolved during plant–animal warfare to metabolize a plethora of xenobiotics. There are numerous similarities between these monooxygenase systems. Both utilize reducing equivalents from NADPH to reduce the second atom of molecular oxygen to water and incorporate the other atom into the substrate. FMO and CYP are between 50 and 60 kDa, located in the endoplasmic reticulum and are present in highest amounts in liver with significant amounts also present in other portals of entry for xenobiotics or in excretory organs (nasal, lung, GI, skin, kidney). CYP and FMO are active toward many of the same xenobiotics. There are, however, numerous differences. Unlike the CYP gene superfamily, FMO has relatively few genes (11 in humans, 6 of which are pseudogenes). FMO is a flavoprotein with a single tightly but not covalently-bound FAD. The catalytic cycle for FMO is markedly distinct from CYP, the major differences being that with FMO there is no real substrate binding and electrons are transferred in a single 2-electron step. Although FMO and CYP share many of the same substrates, the products are usually distinct, e.g., N-oxides from tertiary amines rather than the N-dealkylation seen with CYPs. In the case of chiral compounds, the stereoselectivity is often distinct. There are no identified mechanism-based (suicide substrate) inhibitors of FMO, and polyclonal antibodies inhibit catalytic activity weakly, if at all. Many of the CYP families are involved in endobiotic synthesis or catabolism pathways, but the “physiological function” of FMOs, other than in xenobiotic metabolism is uncertain. One theory already discussed and supported by the properties of the yeast FMO is that mammalian FMO can function in control of the cellular redox state. Most FMOs tend to be inhibited by preincubation at 45–50 °C in the absence of NADPH or by anionic detergents, conditions which do not affect CYP activity. FMOs, like CYPs, exhibit tissue- and developmental-dependent expression, but are not inducible by xenobiotics as are the CYPs. In part, owing to the relatively fewer number of FMOs compared to the drug-metabolizing CYPs, there are fewer examples of significant effects of FMO genetic polymorphisms or inhibition on adverse drug–drug interactions leading to clinical failures of therapy or to toxicity. In drug development, however, this may be an advantage, especially given that, with the exception of the thioamides, thiocarbamides, and thiocarbamates where FMO oxygenation of sulfur results in bio-activation, FMO produces relatively fewer toxic metabolites than CYP. The contribution of FMOs in the metabolism of drugs and other xenobiotics is still an incomplete picture in many cases. Relative to the CYPs, for most, but not all, drugs, FMOs probably play a secondary role. However, in some cases (see Tables 5 and 6), FMO activity may predominate. Further efforts to understand the role of FMO in drug metabolism and the potential for incorporation of such information in development of new drugs are important areas. Another critical area of future research is in furthering our knowledge of the structure/function of FMOs. To date, unlike CYPs, there is only a single potential bacterial (PAMO) and no mammalian X-ray structures upon which to model the FMO protein. As a result, many unanswered questions remain including the dimensions of the substrate access channel and the mechanism by which many endogenous nucleophiles are excluded, differences between FMO enzymes that result in exclusion of certain bulkier substrates, access channels for O2 and NADPH to the FAD, determinations of FMO stereospecificity, and explanations for how the C4a hydroperoxy FAD (FADOOH) is stabilized and the protein is protected from oxidative damage from reactive oxygen “leakage”. The structure of bacterial analogues such as PAMO, or the engineering of a more soluble mammalian FMO, amendable to crystallization, may in the future enable researchers to address these and other questions on FMO structure/function. Finally, a critical area of future research is in increasing our understanding of how genetic variation impacts individual susceptibility to xenobiotics and therapeutic responses to drugs.
References
- Akerman BR, Forrest S, Chow L, Youil R, Knight M, Treacy EP. Two novel mutations of the FMO3 gene in a proband with trimethylaminuria. Hum Mutat. 1999;13:376–379. doi: 10.1002/(SICI)1098-1004(1999)13:5<376::AID-HUMU5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Akerman BR, Lemass H, Chow LM, Lambert DM, Greenberg C, Bibeau C, et al. Trimethylaminuria is caused by mutations of the FMO3 gene in a North American cohort. Mol Genet Metab. 1999;68:24–31. doi: 10.1006/mgme.1999.2885. [DOI] [PubMed] [Google Scholar]
- Atta-Asafo-Adjei E, Lawton MP, Philpot RM. Cloning, sequencing, distribution, and expression in Escherichia coli of flavin-containing monooxygenase 1C1. Evidence for a third gene subfamiliy in rabbits. J Biol Chem. 1993;268:9681–9689. [PubMed] [Google Scholar]
- Attar M, Dong D, Ling KH, Tang-Liu DD. Cytochrome P450 2C8 and flavin-containing monooxygenases are involved in the metabolism of tazarotenic acid in humans. Drug Metab Dispos. 2003;31:476–481. doi: 10.1124/dmd.31.4.476. [DOI] [PubMed] [Google Scholar]
- Attwood TK, Bradley P, Flower DR, Gaulton A, Maudling N, Mitchell AL, et al. PRINTS and its automatic supplement, prePRINTS. Nucleic Acids Res. 2003;31:400–402. doi: 10.1093/nar/gkg030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayesh R, Smith RL. Genetic polymorphism of trimethylamine N-oxidation. Pharmacol Ther. 1990;45:387–401. doi: 10.1016/0163-7258(90)90074-c. [DOI] [PubMed] [Google Scholar]
- Barber M, Conrad ME, Umbreit JN, Barton JC, Moore EG. Abnormalities of flavin monooxygenase as an etiology for sideroblastic anemia. Am J Hematol. 2000;65:149–153. doi: 10.1002/1096-8652(200010)65:2<149::aid-ajh10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Basarab T, Ashton GH, Menage HP, McGrath JA. Sequence variations in the flavin-containing monooxygenase 3 gene (FMO3) in fish odour syndrome. Br J Dermatol. 1999;140:164–167. doi: 10.1046/j.1365-2133.1999.02693.x. [DOI] [PubMed] [Google Scholar]
- Beaty N, Ballou D. Microsomes, drug oxidation, and chemical carcinogenesis. NY: Academic Press; 1980. A kinetic investigation of liver microsomal mixed function amine oxidase; –299.pp. 302 [Google Scholar]
- Belldina EB, Huang MY, Schneider JA, Brundage RC, Tracy TS. Steady-state pharmacokinetics and pharmacodynamics of cysteamine bitartrate in paediatric nephropathic cystinosis patients. Br J Clin Pharmacol. 2003;56:520–525. doi: 10.1046/j.1365-2125.2003.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman CE, Park SB, Wrighton SA, Cashman JR. In vitro-in vivo correlations of human (S)-nicotine metabolism. Biochem Pharmacol. 1995;50:565–570. doi: 10.1016/0006-2952(95)00168-y. [DOI] [PubMed] [Google Scholar]
- Bhagwat SV, Bhamre S, Boyd MR, Ravindranath V. Further characterization of rat brain flavin-containing monooxygenase. Biochem Pharmacol. 1996a;51:1469–1475. doi: 10.1016/0006-2952(96)00088-3. [DOI] [PubMed] [Google Scholar]
- Bhagwat SV, Bhamre S, Boyd MR, Ravindranath V. Cerebral metabolism of imipramine and a purified flavin-containing monooxygenase from human brain. Neuropsychopharmacology. 1996b;15:133–142. doi: 10.1016/0893-133X(95)00175-D. [DOI] [PubMed] [Google Scholar]
- Bhamre S, Bhagwat SV, Shankar SK, Williams DE, Ravindranath V. Cerebral flavin-containing monooxygenase-mediated metabolism of antidepressants in brain: immunochemical properties and immunocytochemical localization. J Pharmacol Exp Ther. 1993;267:555–559. [PubMed] [Google Scholar]
- Bhamre S, Bhagwat SV, Shankar SK, Boyd MR, Ravindranath V. Flavin-containing monooxygenase mediated metabolism of psychoactive drugs by human brain microsomes. Brain Res. 1995;672:276–280. doi: 10.1016/0006-8993(94)01135-5. [DOI] [PubMed] [Google Scholar]
- Biaglow JE, Issels RW, Gerweck LE, Varnes ME, Jacobson JB, Mitchell J, et al. Factors influencing the oxidation of cysteamine and other thiols: implications for hyperthermic sensitization and radiation protection. Radiat Res. 1984;100:298–301. [PubMed] [Google Scholar]
- Blake BL, Rose RL, Mailman RB, Levi PE, Hodgson E. Metabolism of thioridazine by microsomal monooxygenases: relative roles of P450 and flavin-containing monooxygenase. Xenobiotica. 1995;25:377–393. doi: 10.3109/00498259509061859. [DOI] [PubMed] [Google Scholar]
- Blake BL, Philpot RM, Levi PE, Hodgson E. Xenobiotic biotransforming enzymes in the central nervous system: an isoform of flavin-containing monooxygenase (FMO4) is expressed in rabbit brain. Chem Biol Interact. 1996;99:253–261. doi: 10.1016/0009-2797(95)03679-2. [DOI] [PubMed] [Google Scholar]
- Boersma MG, Cnubben NHP, Van Berkel WJH, Blom M, Vervoort J, Rietjens IMCM. Role of cytochromes P-450 and flavin-containing monooxygenase in the biotransformation of 4- fluoro-N-methylaniline. Drug Metab Dispos. 1993;21:218–230. [PubMed] [Google Scholar]
- Boyd MR, Neal RA. Studies on the mechanism of toxicity and of development of tolerance to the pulmonary toxin, alphanaphthylthiourea (ANTU) Drug Metab Dispos. 1976;4:314–322. [PubMed] [Google Scholar]
- Brittebo EB. Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol Toxicol. 1995;76:76–79. doi: 10.1111/j.1600-0773.1995.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Brunelle A, Bi Y-A, Russel B, Luy L, Berkman C, Cashman J. Characterization of two human flavin-containing monooxygenase (form 3) enzymes expressed in Escherichia coli as maltose binding protein fusions. Drug Metab Dispos. 1997;25:1001–1007. [PubMed] [Google Scholar]
- Buronfosse T, Moroni P, Benoit E, Riviere JL. Stereoselective sulfoxidation of the pesticide methiocarb by flavin-containing monooxygenase and cytochrome P450-dependent monooxygenases of rat liver microsomes. Anticholinesterase activity of the two sulfoxide enantiomers. J Biochem Toxicol. 1995;10:179–189. doi: 10.1002/jbt.2570100402. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. Alpha-lipoic acid in liver metabolism and disease. Free Radic Biol Med. 1998;24:1023–1039. doi: 10.1016/s0891-5849(97)00371-7. [DOI] [PubMed] [Google Scholar]
- Butler EJ, Fenwick GR. Trimethylamine and fishy taint in eggs. World Poult Sci J. 1984;40:38–51. [Google Scholar]
- Cashman JR. MPTP: a Neurotoxin producing a parkinsonian syndrome. Academic Press; 1986. Biotransformation of MPTP by the flavoprotein monooxygenase; –511.pp. 516 [Google Scholar]
- Cashman JR. Enantioselective N-oxygenation of verapamil by the hepatic flavin-containing monooxygenase. Mol Pharmacol. 1989;36:497–503. [PubMed] [Google Scholar]
- Cashman JR. Structural and catalytic properties of the mammalian flavin-containing monooxygenase. Chem Res Toxicol. 1995;8:165–181. doi: 10.1021/tx00044a001. [DOI] [PubMed] [Google Scholar]
- Cashman JR. Human flavin-containing monooxygenase (form 3): polymorphisms and variations in chemical metabolism. Pharmacogenomics. 2002;3:325–339. doi: 10.1517/14622416.3.3.325. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Hanzlik RP. Microsomal oxidation of thiobenzamide. A photometric assay for the flavin-containing monooxygenase. Biochem Biophys Res Commun. 1981;98:147–153. doi: 10.1016/0006-291x(81)91881-7. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Williams DE. Enantioselective S-oxygenation of 2-aryl-1,3-dithiolanes by rabbit lung enzyme preparations. Mol Pharmacol. 1990;37:333–339. [PubMed] [Google Scholar]
- Cashman JR, Zhang J. Interindividual differences of human flavin-containing monooxygenase 3: genetic polymorphisms and functional variation. Drug Metab Dispos. 2002;30:1043–1052. doi: 10.1124/dmd.30.10.1043. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Ziegler DM. Contribution of N-oxygenation to the metabolism of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) by various liver preparations. Mol Pharmacol. 1986;29:163–167. [PubMed] [Google Scholar]
- Cashman JR, Proudfoot J, Pate DW, Hogberg T. Stereoselective N-oxygenation of zimeldine and homozimeldine by the flavin-containing monooxygenase. Drug Metab Dispos. 1988;16:616–622. [PubMed] [Google Scholar]
- Cashman JR, Park SB, Yang ZC, Wrighton SA, Jacob P, III, Benowitz N. Metabolism of nicotine by human liver microsomes: stereoselective formation of trans-nicotine N′-oxide. Chem Res Toxicol. 1992a;5:639–646. doi: 10.1021/tx00029a008. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Celestial JR, Leach AR. Enantioselective N-oxygenation of chlorpheniramine by the flavin-containing monooxygenase from hog liver. Xenobiotica. 1992b;22:459–469. doi: 10.3109/00498259209046658. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Celestial JR, Leach A, Newdoll J, Park SB. Tertiary amines related to brompheniramine: preferred conformations for N-oxygenation by the hog liver flavin-containing monooxygenase. Pharm Res. 1993a;10:1005–1097. doi: 10.1023/a:1018947714030. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Park SB, Yang ZC, Washington CB, Gomez DY, Giacomini KM, et al. Chemical, enzymatic, and human enantioselective S-oxygenation of cimetidine. Drug Metab Dispos. 1993b;21:587–597. [PubMed] [Google Scholar]
- Cashman JR, Park SB, Berkman CE, Cashman LE. Role of hepatic flavin-containing monooxygenase in drug and chemical metabolism in adult humans. Chem Biol Interact. 1995;96:33–46. doi: 10.1016/0009-2797(94)03581-r. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Bi Y-A, Lin J, Youil R, Knight M, Forrest S, et al. Human flavin-containing monooxygenase form 3: cDNA expression of the enzymes containing amino acid substitutions observed in individuals with trimethylaminuria. Chem Res Toxicol. 1997;10:837–841. doi: 10.1021/tx9700533. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Xiong YN, Xu L, Janowsky A. N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication. J Pharmacol Exp Ther. 1999;288:1251–1260. [PubMed] [Google Scholar]
- Cashman JR, Akerman BR, Forrest SM, Treacy EP. Population-specific polymorphisms of the human FMO3 gene: significance for detoxication. Drug Metab Dispos. 2000;28:169–173. [PubMed] [Google Scholar]
- Cashman JR, Zhang J, Leushner J, Braun S. Population distribution of human flavin-containing monooxygenase form 3: gene polymorphisms. Drug Metab Dispos. 2001;29:1629–1637. [PubMed] [Google Scholar]
- Cashman JR, Camp K, Fakharzadeh SS, Fennessey PV, Hines RN, Mamer OA, et al. Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr Drug Metab. 2003;4:151–170. doi: 10.2174/1389200033489505. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Lattard V, Lin J. Effect of total parenteral nutrition and choline on hepatic flavin-containing and cytochrome P-450 monooxygenase activity in rats. Drug Metab Dispos. 2004;32:222–229. doi: 10.1124/dmd.32.2.222. [DOI] [PubMed] [Google Scholar]
- Cheesman MJ, Kneller MB, Rettie AE. Critical role of histidine residues in cyclohexanone monooxygenase expression, cofactor binding and catalysis. Chem Biol Interact. 2003;146:157–164. doi: 10.1016/s0009-2797(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Chen G-P, Ziegler DM. Liver microsome and flavin-containing monooxygenase catalyzed oxidation of organic selenium compounds. Arch Biochem Biophys. 1994;312:566–572. doi: 10.1006/abbi.1994.1346. [DOI] [PubMed] [Google Scholar]
- Chen G-P, Poulsen LL, Ziegler DM. Oxidation of aldehydes catalyzed by pig liver flavin-containing monooxygenase. Drug Metab Dispos. 1995;23:1390–1393. [PubMed] [Google Scholar]
- Cherrington NJ, Falls JG, Rose RL, Clements KM, Philpot RM, Levi PE, et al. Molecular cloning, sequence, and expression of mouse flavin-containing monooxygenase 1 and 5 (FMO1 and FMO5) J Biochem Mol Toxicol. 1998;12:205–212. doi: 10.1002/(sici)1099-0461(1998)12:4<205::aid-jbt2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Chiba K, Kubota E, Miyakawa T, Dato Y, Ishizaki T. Characterization of hepatic microsomal metabolism as an in vivo detoxication pathway of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Pharmacol Exp Ther. 1988;246:1108–1115. [PubMed] [Google Scholar]
- Chiba K, Kobayashi K, Itoh K, Itoh S, Chiba T, Ishizaki T, et al. N-oxygenation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by the rat liver flavin-containing monooxygenase expressed in yeast cells. Eur J Pharmacol. 1995;293:97–100. doi: 10.1016/0926-6917(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Chieli E, Malvaldi G. Role of the microsomal FAD-containing monooxygenase in the liver toxicity of thioacetamide S-oxide. Toxicology. 1984;31:41–52. doi: 10.1016/0300-483x(84)90154-9. [DOI] [PubMed] [Google Scholar]
- Chung WG, Cha YN. Oxidation of caffeine to theobromine and theophylline is catalyzed primarily by flavin-containing monooxygenase in liver microsomes. Biochem Biophys Res Commun. 1997;235:685–688. doi: 10.1006/bbrc.1997.6866. [DOI] [PubMed] [Google Scholar]
- Chung WG, Kang JH, Park CS, Cho MH, Cha YN. Effect of age and smoking on in vivo CYP1A2, flavin-containing monooxygenase, and xanthine oxidase activities in Koreans: determination by caffeine metabolism. Clin Pharmacol Ther. 2000;67:258–266. doi: 10.1067/mcp.2000.104617. [DOI] [PubMed] [Google Scholar]
- Damani LA, Nnane IP. The assessment of flavin-containing monooxygenase activity in intact animals. Drug Metab Drug Interact. 1996;13:1–28. doi: 10.1515/dmdi.1996.13.1.1. [DOI] [PubMed] [Google Scholar]
- Damani LA, Pool WF, Crooks PA, Kaderlik RK, Ziegler DM. Stereoselctivity in the N′-oxidation of nicotine isomers by flavin-containing monooxygenase. Mol Pharmacol. 1988;33:702–705. [PubMed] [Google Scholar]
- Decker CJ, Doerge DR. Rat hepatic microsomal metabolism of ethylenethiourea. Contributions of the flavin-containing monooxygenase and cytochrome P-450 isozymes. Chem Res Toxicol. 1991;4:482–489. doi: 10.1021/tx00022a013. [DOI] [PubMed] [Google Scholar]
- Decker CJ, Doerge DR. Covalent binding of 14C- and 35S-labelled thiocarbamides in rat hepatic microsomes. Biochem Pharmacol. 1992;43:881–888. doi: 10.1016/0006-2952(92)90256-i. [DOI] [PubMed] [Google Scholar]
- Decker CJ, Cashman JR, Sugiyama K, Maltby D, Correia MA. Formation of flutathinyl-spironolactone disulfide by rat liver cytochromes P450 or hog liver flavin-containing monooxygenases: a functional probe of two-electron oxidations of the thiosteroid? Chem Res Toxicol. 1991;4:669–677. doi: 10.1021/tx00024a012. [DOI] [PubMed] [Google Scholar]
- Decker CJ, Doerge DR, Cashman JR. Metabolism of benzimidazoline-2-thiones by rat hepatic microsomes and hog liver flavin-containing monooxygenase. Chem Res Toxicol. 1992;5:726–733. doi: 10.1021/tx00029a021. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Fouts JR. N-oxidation and demethylation of N,N-dimethylaniline by rabbit liver and lung microsomes: effects of age and metals. Chem Biol Interact. 1974;8:91–105. doi: 10.1016/0009-2797(74)90055-6. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Philpot RM, Fouts JR. The effect of Hg2+ on rabbit hepatic and pulmonary solubilized partially purified N, N-dimethylaniline N-oxidases. Chem Biol Interact. 1977;18:254–258. doi: 10.1016/0009-2797(77)90014-x. [DOI] [PubMed] [Google Scholar]
- DiMonte D, Shinka T, Sandy MS, Castagnoli N, Jr, Smith MT. Quantitative analysis of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine metabolism in isolated rat hepatocytes. Drug Metab Dispos. 1988;16:250–255. [PubMed] [Google Scholar]
- Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing monooxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- Dolphin CT, Beckett DJ, Janmohamed A, Cullingford TE, Smith RL, Shephard EA, et al. The flavin-containing monooxygenase 2 gene (FMO2) of humans, but not of other primates, encodes a truncated, nonfunctional protein. J Biol Chem. 1998;273:30599–30607. doi: 10.1074/jbc.273.46.30599. [DOI] [PubMed] [Google Scholar]
- Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Compound heterozygosity for missense mutations in the flavin-containing monooxygenase 3 (FMO3) gene in patients with fish-odour syndrome. Pharmacogenetics. 2000;10:799–807. doi: 10.1097/00008571-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Duescher RJ, Lawton MP, Philpot RM, Elfarra AA. Flavin-containing monooxygenase (FMO)-dependent metabolism of methionine and evidence for FMO3 being the major FMO involved in methionine sulfoxidation in rabbit liver and kidney microsomes. J Biol Chem. 1994;269:17525–17530. [PubMed] [Google Scholar]
- Dyroff MC, Neal RA. Studies on the mechanism of metabolism of thioacetamide S-oxide by rat liver microsomes. Mol Pharmacol. 1983;23:219–227. [PubMed] [Google Scholar]
- Elfarra AA. Potential role of the flavin-containing monooxygenases in the metabolism of endogenous compounds. Chem Biol Interact. 1995;96:47–55. doi: 10.1016/0009-2797(94)03582-s. [DOI] [PubMed] [Google Scholar]
- Engler H, Taurog A, Nakashima T. Mechanism of inactivation of thyroid peroxidase by thioureylene drugs. Biochem Pharmacol. 1982;31:3801–3806. doi: 10.1016/0006-2952(82)90296-9. [DOI] [PubMed] [Google Scholar]
- Eriksson L-O, Bostrom H. Deactivation of sulindac-sulphide by human renal microsomes. Pharmacol Toxicol. 1988;62:177–183. doi: 10.1111/j.1600-0773.1988.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Falls JG, Cherrington NJ, Clements KM, Philpot RM, Levi PE, Rose RL, et al. Molecular cloning, sequencing, and expression in Escherichia coli of mouse flavin-containing monooxygenase 3 (FMO3): comparison with the human isoform. Arch Biochem Biophys. 1997;347:9–18. doi: 10.1006/abbi.1997.0322. [DOI] [PubMed] [Google Scholar]
- Fisher MB, Rettie AE. Prochiral sulfide probes for the active-site topography of rabbit flavin-containing monooxygenase. Tetrahedron: Asymmetry. 1997;8:613–618. [Google Scholar]
- Fisher MB, Lawton MP, Atta-Asafo-Adjei E, Philpot RM, Rettie AE. Selectivity of flavin-containing monooxygenase 5 for the (S)-sulfoxidation of short-chain aralkyl sulfides. Drug Metab Dispos. 1995;23:1431–1433. [PubMed] [Google Scholar]
- Ford JG, Li Y, O’Sullivan MM, Demopoulos R, Garte S, Taioli E, et al. Glutathione S-transferase M1 polymorphism and lung cancer risk in African–Americans. Carcinogenesis. 2000;21:1971–1975. doi: 10.1093/carcin/21.11.1971. [DOI] [PubMed] [Google Scholar]
- Forrest SM, Knight M, Akerman BR, Cashman JR, Treacy EP. A novel deletion in the flavin-containing monooxygenase gene (FMO3) in a Greek patient with trimethylaminuria. Pharmacogenetics. 2001;11:169–174. doi: 10.1097/00008571-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Fraaije MW, Kamerbeek NM, van Berkel WJH, Janssen DB. Identification of a Baeyer–Villiger monooxygenase sequence motif. FEBS Lett. 2002;518:43–47. doi: 10.1016/s0014-5793(02)02623-6. [DOI] [PubMed] [Google Scholar]
- Frederick CB, Mays JB, Ziegler DM, Guengerich FP, Kadlubar FF. Cytochrome P-450- and flavin-containing monooxygenase-catalyzed formation of the carcinogen N-hydroxy-2- aminofluorene and its covalent binding to nuclear DNA. Cancer Res. 1982;42:2671–2677. [PubMed] [Google Scholar]
- Fujieda M, Yamazake H, Togashi M, Saito T, Kamataki T. Two novel single nucleotide polymorphisms (SNPs) of the FMO3 gene in Japanese. Drug Metab Pharmacokinet. 2003;18:SNP35333–SNP37335. doi: 10.2133/dmpk.18.333. [DOI] [PubMed] [Google Scholar]
- Furnes B, Schlenk D. Evaluation of xenobiotic N- and S-oxidation by variant flavin-containing monooxygenase 1 (FMO1) enzymes. Toxicol Sci. 2004;78:196–203. doi: 10.1093/toxsci/kfh079. [DOI] [PubMed] [Google Scholar]
- Furnes B, Feng J, Sommer SS, Schlenk D. Identification of novel variants of the flavin-containing monooxygenase gene family in African Americans. Drug Metab Dispos. 2003;31:187–193. doi: 10.1124/dmd.31.2.187. [DOI] [PubMed] [Google Scholar]
- Goeger DE, Ganther HE. Oxidation of dimethylselenide to dimethylselenoxide by microsomes from rat liver and lung and by flavin-containing monooxygenase from pig liver. Arch Biochem Biophys. 1994;310:448–451. doi: 10.1006/abbi.1994.1191. [DOI] [PubMed] [Google Scholar]
- Gomez MID, Castro JA. Effect of inhibitors of the FAD-containing monooxygenase system from rat liver microsomes on monomethylhydrazine metabolism and activation to reactive metabolites. Arch Toxicol. 1986;59:64–66. doi: 10.1007/BF00263961. [DOI] [PubMed] [Google Scholar]
- Grosa G, Caputo O, Ceruti M, Biglino G. Metabolism of 7- (1,3-thiazolindin-2-ylmethyl) theophylline by rat liver microsomes. Evidence for a monooxygenase-dependent step in 1,3-thiazolidine ring cleavage. Drug Metab Dispos. 1992;20:742–746. [PubMed] [Google Scholar]
- Grossman SJ, Patrick DH, Kornbrust D, Smith PF, Herold EG, DeLuca JG, et al. Thiobenzamide-induced hepatotoxicity: effects of substituents and route of administration on the nature and extent of liver injury. Toxicol Appl Pharmacol. 1991;111:388–408. doi: 10.1016/0041-008x(91)90245-a. [DOI] [PubMed] [Google Scholar]
- Guo WXA, Ziegler DM. Estimation of flavin-containing monooxygenase activities in crude tissue preparations by thiourea-dependent oxidation of thiocholine. Anal Biochem. 1991;198:143–148. doi: 10.1016/0003-2697(91)90519-y. [DOI] [PubMed] [Google Scholar]
- Guo WX, Poulsen LL, Ziegler DM. Use of thiocarbamides as selective substrate probes for isoforms of flavin-containing monooxygenases. Biochem Pharmacol. 1992;44:2029–2037. doi: 10.1016/0006-2952(92)90106-s. [DOI] [PubMed] [Google Scholar]
- Gut I, Conney AH. Trimethylamine N-oxygenation and N-demethylation in rat liver microsomes. Biochem Pharmacol. 1993;46:239–244. doi: 10.1016/0006-2952(93)90409-p. [DOI] [PubMed] [Google Scholar]
- Hadley MR, Oldham HG, Damani LA, Hutt AJ. Asymmetric metabolic N-oxidation of N-ethyl-N-methylaniline by purified flavin-containing monooxygenase. Chirality. 1994;6:98–104. doi: 10.1002/chir.530060210. [DOI] [PubMed] [Google Scholar]
- Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-l-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Haining RL, Hunter AP, Sadeque AJM, Philpot RM, Rettie AE. Baculovirus-mediated expression and purification of human FMO3. Catalytic, immunochemical, and structural characterization. Drug Metab Dispos. 1997;25:790–797. [PubMed] [Google Scholar]
- Hajjar NP, Hodgson E. Flavin adenine dinucleotide-dependent monooxygenase: its role in the sulfoxidation of pesticides in mammals. Science. 1980;209:1134–1136. doi: 10.1126/science.7403873. [DOI] [PubMed] [Google Scholar]
- Hajjar NP, Hodgson E. Sulfoxidation of thioether-containing pesticides by the flavin–adenine dinucleotide-dependent monooxygenase of pig liver microsomes. Biochem Pharmacol. 1982;31:745–752. doi: 10.1016/0006-2952(82)90458-0. [DOI] [PubMed] [Google Scholar]
- Hamman MA, Haehner-Daniels BD, Wrighton SA, Rettie AE, Hall SD. Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochem Pharmacol. 2000;60:7–17. doi: 10.1016/s0006-2952(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Hammons GJ, Guengerich FP, Weis CC, Beland FA, Kadlubar FF. Metabolic oxidation of carcinogenic arylamines by rat, dog, and human hepatic microsomes and by purified flavin-containing and cytochrome P-450 monooxygenases. Cancer Res. 1985;45:3578–3585. [PubMed] [Google Scholar]
- Hanzlik RP, Cashman JR, Traiger GJ. Relative hepatoxtoxicity of thiobenzamides and thiobenzamides-S-oxides in the rat. Toxicol Appl Pharmacol. 1980;55:260–272. doi: 10.1016/0041-008x(80)90088-5. [DOI] [PubMed] [Google Scholar]
- Hardwick SJ, Skamarauskas JT, Smith LL, Upshall DG, Cohen GM. Protection of rats against the effects of alpha-naphthylthiourea (ANTU) by elevation of non-protein sulphydryl levels. Biochem Pharmacol. 1991;42:1203–1208. doi: 10.1016/0006-2952(91)90255-4. [DOI] [PubMed] [Google Scholar]
- Henderson MC, Krueger SK, Siddens LK, Stevens JF, Williams DE. S-oxygenation of the thioether organo-phosphate insecticides phorate and disulfoton by human lung flavin-containing monooxygenase 2. Biochem Pharmacol. 2004a;68:959–967. doi: 10.1016/j.bcp.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Henderson MC, Krueger SK, Stevens JF, Williams DE. Human flavin-containing monooxygenase form 2 S-oxygenation: sulfenic acid formation from thioureas and oxidation of glutathione. Chem Res Toxicol. 2004b;17:633–640. doi: 10.1021/tx034253s. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Addou S, Lee D, Orengo C, Shephard EA, Phillips IR. Trimethylaminuria and a human FMO3 mutation database. Hum Mutat. 2003;22:209–213. doi: 10.1002/humu.10252. [DOI] [PubMed] [Google Scholar]
- Hernandez D, Janmohamed A, Chandan P, Phillips IR, Shephard EA. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004;14:117–130. doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase 1 oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- Hines RN, Cashman JR, Philpot RM, Williams DE, Ziegler DM. The mammalian flavin-containing monooxygenases: molecular characterization and regulation of expression. Toxicol Appl Pharmacol. 1994;125:1–6. doi: 10.1006/taap.1994.1042. [DOI] [PubMed] [Google Scholar]
- Hines RN, Hopp KA, Franco J, Saeian K, Begun FP. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol Pharmacol. 2002;62:320–325. doi: 10.1124/mol.62.2.320. [DOI] [PubMed] [Google Scholar]
- Hines RN, Luo Z, Hopp KA, Cabacungan ET, Koukouritaki SB, McCarver DG. Genetic variability at the human FMO1 locus: significance of a basal promoter Yin Yang 1 element polymorphism (FMO1*6) J Pharmacol Exp Ther. 2003;306:1210–1218. doi: 10.1124/jpet.103.053686. [DOI] [PubMed] [Google Scholar]
- Hlavica P, Golly I, Lehnerer M, Schulze J. Primary aromatic amines: their N-oxidative bioactivation. Hum Exp Toxicol. 1997;16:441–448. doi: 10.1177/096032719701600805. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Levi PE. Species, organ, and cellular variation in the flavin-containing monooxygenase. Drug Metab Drug Interact. 1991;6:219–233. doi: 10.1515/dmdi.1988.6.3-4.219. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Levi PE. The role of the flavin-containing monooxygenase (EC 1.14.13.8) in the metabolism and mode of action of agricultural chemicals. Xenobiotica. 1992;22:1175–1183. doi: 10.3109/00498259209051871. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Cherrington N, Coleman SC, Liu S, Falls JG, Cao Y, et al. Flavin-containing monooxygenase and cytochrome P450 mediated metabolism of pesticides: from mouse to human. Rev Toxicol. 1998;2:231–243. [Google Scholar]
- Hodgson E, Rose RL, Cao Y, Dehal SS, Kupfer D. Flavin-containing monooxygenase isoform specificity for the N-oxidaiton of tamoxifen determined by product measurement and NADPH oxidation. J Biochem Mol Toxicol. 2000;14:118–120. doi: 10.1002/(sici)1099-0461(2000)14:2<118::aid-jbt8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Holm L, Sander C. The FSSP database of structurally aligned protein fold families. Nucleic Acids Res. 1994;22:3600–3609. [PMC free article] [PubMed] [Google Scholar]
- Hugonnard M, Benoit E, Longin-Sauvageon C, Lattard V. Identification and characterization of the FMO2 gene in Rattus norvegicus: a good model to study metabolic and toxicological consequences of the FMO2 polymorphism. Pharmacogenetics. 2004;14:647–655. doi: 10.1097/00008571-200410000-00002. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Holscher MA, Neal RA. Thioacetamide induced hepatic necrosis: I. Involvement of the mixed-function oxidase enzymes system. J Pharmacol Exp Ther. 1977;200:439–448. [PubMed] [Google Scholar]
- Hvattum E, Bergseth S, Pedersen CN, Bremer J, Aarsland A, Berge RK. Microsomal oxidation of dodecylthioacetic acid (a 3-thia fatty acid) in rat liver. Biochem Pharmacol. 1991;41:945–953. doi: 10.1016/0006-2952(91)90200-o. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Carver GT, Philpot RM. Expression and characterization of a modified flavin-containing monooxygenase 4 from humans. J Biol Chem. 1996;271:20102–20127. doi: 10.1074/jbc.271.33.20102. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kimura T, Yokoi T, Itoh S, Kamataki T. Rat liver flavin-containing monooxygenase (FMO): cDNA cloning and expression in yeast. Biochim Biophys Acta. 1993;1173:165–171. doi: 10.1016/0167-4781(93)90177-f. [DOI] [PubMed] [Google Scholar]
- Jacobsen W, Christians U, Benet LZ. In vitro evaluation of the disposition of a novel cysteine protease inhibitor. Drug Metab Dispos. 2000;28:1343–1351. [PubMed] [Google Scholar]
- Jeitner TM, Lawrence DA. Mechanisms for the cytotoxicity of cysteamine. Toxicol Sci. 2001;62:57–64. doi: 10.1093/toxsci/63.1.57. [DOI] [PubMed] [Google Scholar]
- Jenner AM, Timbrell JA. In vitro microsomal metabolism of hydrazine. Xenobiotica. 1995;25:599–609. doi: 10.3109/00498259509061878. [DOI] [PubMed] [Google Scholar]
- Jokanovic M. Biotransformation of organophosphorus compounds. Toxicology. 2001;166:139–160. doi: 10.1016/s0300-483x(01)00463-2. [DOI] [PubMed] [Google Scholar]
- Jones KC, Ballou DP. Reactions of the 4a-hydroperoxide of liver microsomal flavin-containing monooxygenase with nucleophilic and electrophilic substrates. J Biol Chem. 1986;261:2553–2559. [PubMed] [Google Scholar]
- Jonsson K-H, Lindeke B. Cytochrome P-455 nm complex formation in the metabolism of phenylalkylamines: XII. Enantioselectivity and temperature dependence in microsomes and reconstituted cytochrome P-450 systems from rat liver. Chirality. 1992;4:469–477. doi: 10.1002/chir.530040803. [DOI] [PubMed] [Google Scholar]
- Kaderlik KR, Poulsen LL, Ziegler DM. Studies on the mechanism for the thermal inactivation of hog liver flavin-containing monooxygenase. Prog Pharmacol Clin Pharmacol. 1991;8:85–94. [Google Scholar]
- Kajita J, Inano K, Fuse E, Kuwabara T, Kobayashi H. Effects of olopatiadine, a new antiallergic agent, on human liver microsomal cytochrome P450 activities. Drug Metab Dispos. 2002;30:1504–1511. doi: 10.1124/dmd.30.12.1504. [DOI] [PubMed] [Google Scholar]
- Kang J-H, Chung W-G, Lee K-H, Park C-S, Kang J-S, Shin I-C, et al. Phenotypes of flavin-containing monooxygenase activity determined by ranitidine N-oxidation are positively correlated with genotypes of linked FMO3 gene mutations in a Korean population. Pharmacogenetics. 2000;10:67–78. doi: 10.1097/00008571-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Karanam BV, Welch CJ, Reddy VG, Chilenski J, Biba M, Vincent S. Species differential stereoselective oxidation of a methylsulfide metabolite of MK-0767 [(±)-5-[(2,4-dioxothiazolidin-5- yl)methyl]-2-methoxy-N-[[(4-trifluoromethyl)phenyl]methyl]benzamide], a peroxisome proliferator-activated receptor dual agonist. Drug Metab Dispos. 2004;32:1061–1068. doi: 10.1124/dmd.104.000224. [DOI] [PubMed] [Google Scholar]
- Karoly ED, Rose RL. Sequencing, expression, and characterization of cDNA expressed flavin-containing monooxygenase 2 from mouse. J Biochem Mol Toxicol. 2001;15:300–308. doi: 10.1002/jbt.10009. [DOI] [PubMed] [Google Scholar]
- Kashiyama E, Yokoi T, Itoh K, Itoh S, Odomi M, Kamataki T. Stereoselective S-oxidation of flosequinan sulfide by rat hepatic flavin-containing monooxygenase 1A1 in yeast. Biochem Pharmacol. 1994;47:1357–1363. doi: 10.1016/0006-2952(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Kawaji A, Isobe M, Takabatake E. Differences in enzymatic properties of flavin-containing monooxygenase in brain microsomes of rat, mouse, hamster, guinea pig and rabbit. Biol Pharm Bull. 1997;20:917–919. doi: 10.1248/bpb.20.917. [DOI] [PubMed] [Google Scholar]
- Kedderis GL, Rickert DE. Loss of rat liver microsomal cytochrome P-450 during methimazole metabolism. Role of flavin-containing monooxygenase. Drug Metab Dispos. 1985;13:58–61. [PubMed] [Google Scholar]
- Khomenko T, Deng X, Sandor Z, Tarnawski AS, Szabo S. Cysteamine alters redox state, HIF-1alpha transcriptional interactions and reduces duodenal mucosal oxygenation: novel insight into the mechanisms of duodenal ulceration. Biochem Biophys Res Commun. 2004;317:121–127. doi: 10.1016/j.bbrc.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Kim YM, Ziegler DM. Size limits of thiocarbamides accepted as substrates by human flavin-containing monooxygenase 1. Drug Metab Dispos. 2000;28:1003–1006. [PubMed] [Google Scholar]
- Kim YH, Lee JY, Lim DS, Shim WJ, Ro YM, Park GH, et al. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med. 2003;35:336–349. doi: 10.1038/emm.2003.45. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kodama M, Nagata C. Purification of mixed-function amine oxidase from rat liver microsomes. Biochem Biophys Res Commun. 1983;110:640–645. doi: 10.1016/0006-291x(83)91197-x. [DOI] [PubMed] [Google Scholar]
- Kinsler S, Levi PE, Hodgson E. Hepatic and extrahepatic microsomal oxidation of phorate by the cytochrome P-450 and FAD-containing monooxygenase systems in the mouse. Pestic Biochem Physiol. 1988;31:54–60. [Google Scholar]
- Kinsler S, Levi PE, Hodgson E. Relative contributions of the cytochrome P450 and flavin-containing monooxygenases to the microsomal oxidation of phorate following treatment of mice with phenobarbital, hydrocortisone, acetone, and piperonyl butoxide. Pestic Biochem Physiol. 1990;37:174–181. [Google Scholar]
- Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res. 2002;51:236–243. doi: 10.1203/00006450-200202000-00018. [DOI] [PubMed] [Google Scholar]
- Krause RJ, Ripp SL, Sausen PJ, Overby LH, Philpot RM, Elfarra AA. Characterization of the methionine S-oxidation activity of rat liver and kidney microsomes: immunochemical and kinetic evidence for FMO3 being the major catalyst. Arch Biochem Biophys. 1996;333:109–116. doi: 10.1006/abbi.1996.0370. [DOI] [PubMed] [Google Scholar]
- Krause RJ, Glocke SC, Elfarra AA. Sulfoxides as urinary metabolites of S-allyl-l-cysteine in rats: evidence for the involvement of flavin-containing monooxygenases. Drug Metab Dispos. 2002;30:1137–1142. doi: 10.1124/dmd.30.10.1137. [DOI] [PubMed] [Google Scholar]
- Krieter PA, Ziegler DM, Hill KE, Burk RF. Increased biliary GSSG efflux from rat livers perfused with thiocarbamide substrates for the flavin-containing monooxygenase. Mol Pharmacol. 1984;26:122–127. [PubMed] [Google Scholar]
- Krueger SK, Yueh M-F, Martin SR, Pereira CB, Williams DE. Characterization of expressed full-length and truncated FMO2 from rhesus monkey. Drug Metab Dispos. 2001;29:693–700. [PubMed] [Google Scholar]
- Krueger SK, Martin SR, Yueh M-F, Pereira CB, Williams DE. Identification of active flavin-containing monooxygenase isoform 2 in human lung and characterization of expressed protein. Drug Metab Dispos. 2002a;30:34–41. doi: 10.1124/dmd.30.1.34. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Williams DE, Yueh M-F, Martin SR, Hines RN, Raucy JL, et al. Genetic polymorphisms of flavin-containing monooxygenase (FMO) Drug Metab Rev. 2002b;34:523–532. doi: 10.1081/dmr-120005653. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Siddens LK, Martin SR, Yu Z, Pereira CB, Cabacungan ET, et al. Differences in FMO2*1 allelic frequency between Hispanics of Puerto Rican and Mexican Descent. Drug Metab Dispos. 2004;32:1337–1340. doi: 10.1124/dmd.104.001099. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Siddens LK, Henderson MC, Andreasen EA, Tanguay RL, Pereira CB, Cabacungan ET, Hine RN, Ardlie K, Williams DE. Haplotype and functional analysis of four flavin-containing monooxygenase isoform 2 (FMO2) polymorphisms in Hispanics. Pharmacogenetics and Genomics. 2005 doi: 10.1097/01213011-200504000-00008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Itoh S, Itoh K, Kamataki T. Determination of FAD-binding domain in flavin-containing monooxygenase 1 (FMO1) Arch Biochem Biophys. 1997;345:271–277. doi: 10.1006/abbi.1997.0242. [DOI] [PubMed] [Google Scholar]
- Kubota M, Hakamoto Y, Nakayama K, Ujjin P, Satarug S, Muchiroda T, et al. A mutation in the flavin-containing monooxygenase 3 gene and its effects on catalytic activity for N-oxidation of trimethylamine in vitro. Drug Metab Pharmacokinet. 2002;17:207–213. doi: 10.2133/dmpk.17.207. [DOI] [PubMed] [Google Scholar]
- Kulkarni AP, Hodgson E. The metabolism of insecticides: the role of monooxygenase enzymes. Annu Rev Pharmacol Toxicol. 1984;24:19–42. doi: 10.1146/annurev.pa.24.040184.000315. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Mamer OA, Akerman BR, Choiniere L, Gaudet D, Hamet P, et al. In vivo variability of TMA oxidation is partially mediated by polymorphisms of the FMO3 gene. Mol Genet Metab. 2001;73:224–229. doi: 10.1006/mgme.2001.3189. [DOI] [PubMed] [Google Scholar]
- Lang DH, Rettie AE. In vitro evaluation of potential in vivo probes for human flavin-containing monooxygenase (FMO): metabolism of benzydamine and caffeine by FMO and P450 isoforms. Br J Clin Pharmacol. 2000;50:311–314. doi: 10.1046/j.1365-2125.2000.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Lanusse CE, Nare B, Prichard RK. Comparative sulphoxidation of albendazole by sheep and cattle liver microsomes and the inhibitory effect of methimazole. Xenobiotica. 1993;23:285–295. doi: 10.3109/00498259309059382. [DOI] [PubMed] [Google Scholar]
- Lattard V, Longin-Sauvageon C, Krueger SK, Williams DE, Benoit E. The FMO2 gene of laboratory rats, as in most humans, encodes a truncated protein. Biochem Biophys Res Commun. 2002;292:558–563. doi: 10.1006/bbrc.2002.6656. [DOI] [PubMed] [Google Scholar]
- Lattard V, Longin-Sauvageon C, Benoit E. Cloning, sequencing and tissue distribution of rat flavin-containing monooxygenase 4: two different forms are produced by tissue-specific alternative splicing. Mol Pharmacol. 2003a;63:253–261. doi: 10.1124/mol.63.1.253. [DOI] [PubMed] [Google Scholar]
- Lattard V, Zhang J, Tran Q, Furnes B, Schlenk D, Cashman JR. Two new polymorphisms of the FMO3 gene in Caucasian and African–American populations: comparative genetic and functional studies. Drug Metab Dispos. 2003b;31:854–860. doi: 10.1124/dmd.31.7.854. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Philpot RM. Functional characterization of flavin-containing monooxygenase 1B1 expressed in Saccharomyces cerevisiae and Escherichia coli and analysis of proposed FAD- and membrane-binding domains. J Biol Chem. 1993;268:5728–5734. [PubMed] [Google Scholar]
- Lawton MP, Gasser R, Tynes RE, Hodgson E, Philpot RM. The flavin-containing monooxygenase enzymes expressed in rabbit liver and lung are products of related but distinctly different genes. J Biol Chem. 1990;265:5855–5861. [PubMed] [Google Scholar]
- Lawton MP, Kronbach T, Johnson EF, Philpot RM. Properties of expressed and native flavin-containing monooxygenases: evidence of multiple forms in rabbit liver and lung. Mol Pharmacol. 1991;40:692–698. [PubMed] [Google Scholar]
- Lawton M, Cashman J, Cresteil T, Dolphin C, Elfarra A, Hines RN, et al. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch Biochem Biophys. 1994;308:254–257. doi: 10.1006/abbi.1994.1035. [DOI] [PubMed] [Google Scholar]
- Lee PW, Arnau T, Neal RA. Metabolism of alphanaphthylthiourea by rat liver and rat lung microsomes. Toxicol Appl Pharmacol. 1980;53:164–173. doi: 10.1016/0041-008x(80)90393-2. [DOI] [PubMed] [Google Scholar]
- Lee JW, Shin KD, Lee M, Kim EJ, Han SS, Han MY, et al. Role of metabolism by flavin-containing monooxygenase in thioacetamide-induced immunosuppression. Toxicol Lett. 2003;136:163–172. doi: 10.1016/s0378-4274(02)00333-8. [DOI] [PubMed] [Google Scholar]
- Levi PE, Hodgson E. Stereospecificity in the oxidation of phorate and phorate sulphoxide by purified FAD-containing monooxygenase and cytochrome P-450 isozymes. Xenobiotica. 1988;18:29–39. doi: 10.3109/00498258809055134. [DOI] [PubMed] [Google Scholar]
- Light DR, Waxman DJ, Walsh C. Studies on the chirality of sulfoxidation catalyzed by bacterial flavoenzyme cyclohexanone monooxygenase and hog liver flavin adenine dinucleotide containing monooxygenase. Biochemistry. 1982;21:2490–2498. doi: 10.1021/bi00539a031. [DOI] [PubMed] [Google Scholar]
- Lin J, Cashman JR. Detoxication of tyramine by the flavin-containing monooxygenase: stereoselective formation of the trans oxime. Chem Res Toxicol. 1997a;10:842–852. doi: 10.1021/tx970030o. [DOI] [PubMed] [Google Scholar]
- Lin J, Cashman JR. N-oxygenation of phenethylamine to the trans-oxime by adult human liver flavin-containing monooxygenase and retroreduction of phenethylamine hydroxylamine by human liver microsomes. J Pharmacol Exp Ther. 1997b;282:1269–1279. [PubMed] [Google Scholar]
- Lin J, Berkman CE, Cashman JR. N-oxygenation of primary amines and hydroxylamines and retroreduction of hydroxylamines by adult human liver microsomes and adult human flavin-containing monooxygenase 3. Chem Res Toxicol. 1996;9:1183–1193. doi: 10.1021/tx9600614. [DOI] [PubMed] [Google Scholar]
- Lomri N, Thomas J, Cashman JR. Expression in Escherichia coli of the cloned flavin-containing monooxygenase from pig liver. J Biol Chem. 1993;268:5048–5054. [PubMed] [Google Scholar]
- Longin-Sauvageon C, Lattard V, Lilaz-Michel C, Buronfosse T, Benoit E. Expression of two different FMOs in sheep liver. Drug Metab Dispos. 1998;26:284–287. [PubMed] [Google Scholar]
- Lunden A, Marklund S, Gustafsson V, Andersson L. A nonsense mutation in the FMO3 gene underlies fishy off-flavor in cow’s milk. Genome Res. 2002;12:1885–1888. doi: 10.1101/gr.240202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malito E, Alfieri A, Fraaije MW, Mattevi A. Crystal structure of a Baeyer Villiger monooxygenase. Proc Natl Acad Sci U S A. 2004;110:13157–13162. doi: 10.1073/pnas.0404538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani C, Hodgson E, Kupfer D. Metabolism of the antimammary cancer antiestrogenic agent tamoxifen: II. Flavin-containing monooxygenase-mediated N-oxidation. Drug Metab Dispos. 1993;21:657–661. [PubMed] [Google Scholar]
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269:22459–22462. [PubMed] [Google Scholar]
- McElwain KV, Estienne MJ, Barb CR. Effect of cysteamine hydrochloride on secretion of growth hormone in male swine. Life Sci. 1999;64:2233–2238. doi: 10.1016/s0024-3205(99)00183-6. [DOI] [PubMed] [Google Scholar]
- McManus ME, Grantham PH, Cone JL, Roller PP, Wirth PJ, Thorgeirsson SS. Guanethidine N-oxide formation as a measure of cellular flavin-containing monooxygenase activity. Biochem Biophys Res Commun. 1983;112:437–443. doi: 10.1016/0006-291x(83)91483-3. [DOI] [PubMed] [Google Scholar]
- Mitchell SC, Smith RL. Trimethylaminuria: the fish malodor syndrome. Drug Metab Dispos. 2001;29:517–521. [PubMed] [Google Scholar]
- Mitchell CH, Zieger DM. A quantitative micro method for the estimation of amine oxides. Anal Biochem. 1969;28:261–268. doi: 10.1016/0003-2697(69)90177-8. [DOI] [PubMed] [Google Scholar]
- Mitchell SC, Zhang AQ, Barrett T, Ayesh R, Smith RL. Studies on the discontinuous N-oxidation of trimethylamine among Jordanian. Ecuadorian and New Guinean populations. Pharmacogenetics. 1997;7:45–50. doi: 10.1097/00008571-199702000-00006. [DOI] [PubMed] [Google Scholar]
- Moroni P, Buronfosse T, Longin-Sauvageon C, Delatour P, Benoit E. Chiral sulfoxidation of albendazole by the flavin adenine dinucleotides-containing and cytochrome P450-dependent monooxygenases from rat liver microsomes. Drug Metab Dispos. 1995a;23:160–165. [PubMed] [Google Scholar]
- Moroni P, Longin-Sauvageon C, Benoit E. The flavin-containing monooxygenases in rat liver: evidence for the expression of a second form different from FMO1. Biochem Biophys Res Commun. 1995b;212:820–826. doi: 10.1006/bbrc.1995.2042. [DOI] [PubMed] [Google Scholar]
- Murphy HC, Dolphin CT, Janmohamed A, Holmes HC, Michelakakis H, Shephard EA, et al. A novel mutation in the flavin-containing monooxygenase 3 gene, FMO3, that causes fish-odour syndrome: activity of the mutant enzyme assessed by proton NMR spectroscopy. Pharmacogenetics. 2000;10:439–451. doi: 10.1097/00008571-200007000-00007. [DOI] [PubMed] [Google Scholar]
- Mushiroda T, Douya R, Takahara E, Nagata O. The involvement of flavin-containing monooxygenase but not CYP3A4 in metabolism of itopride hydrochloride, a gastroprokinetic agent: comparison with cisapride and mosapride citrate. Drug Metab Dispos. 2000;28:1231–1237. [PubMed] [Google Scholar]
- Mushiroda T, Ariyoshi N, Yokoi T, Takahara E, Nagata O, Kato H, et al. Accumulation of the 1-methyl-4-phenylpyridinium ion in suncus (Suncus murinus) brain: implication for flavin-containing monooxygenase activity in brain microvessels. Chem Res Toxicol. 2001;14:226–232. doi: 10.1021/tx0001225. [DOI] [PubMed] [Google Scholar]
- Nace CG, Genter MB, Sayre LM, Crofton KM. Effect of methimazole, an FMO substrate and competitive inhibitor, on the neurotoxicity of 3,3′-iminodipropionitrile in male rats. Fundam Appl Toxicol. 1997;37:131–140. doi: 10.1006/faat.1997.2307. [DOI] [PubMed] [Google Scholar]
- Nagata T, Williams DE, Ziegler DM. Substrate specificities of rabbit lung and porcine liver flavin-containing monooxygenase: differences due to substrate size. Chem Res Toxicol. 1990;3:372–376. doi: 10.1021/tx00016a016. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Tachibana M, Masubuchi Y, Suzuki T. Cytochrome P4502D and -2C enzymes catalyze the oxidative N-demethylation of the parkinsonism-inducing substance 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine in rat liver microsomes. Chem Res Toxicol. 1996;9:93–98. doi: 10.1021/tx9500540. [DOI] [PubMed] [Google Scholar]
- Narimatsu S, Yamamoto S, Kato R, Masubuchi Y, Horie T. Contribution of flavin-containing monooxygenase and cytochrome P450 to imipramine N-oxidation in rat hepatic microsomes. Biol Pharmacol Bull. 1999;22:567–571. doi: 10.1248/bpb.22.567. [DOI] [PubMed] [Google Scholar]
- Neal RA, Halpert J. Toxicology of thiono-sulfur compounds. Annu Rev Pharmacol Toxicol. 1982;22:321–339. doi: 10.1146/annurev.pa.22.040182.001541. [DOI] [PubMed] [Google Scholar]
- Nnane IP, Damani LA. Involvement of cytochrome P450 and the flavin-containing monooxygenases(s) in the sulphoxidation of simple sulphides in human liver microsomes. Life Sci. 2003a;73:359–369. doi: 10.1016/s0024-3205(03)00290-x. [DOI] [PubMed] [Google Scholar]
- Nnane IP, Damani LA. Sulphoxidation of ethyl methyl sulphide, 4-chlorophenyl methyl sulphide and diphenyl sulphide by purified pig liver flavin-containing monooxygenase. Xenobiotica. 2003b;33:83–91. doi: 10.1080/0049825021000022339. [DOI] [PubMed] [Google Scholar]
- Nunoya K, Yokio T, Itoh K, Itoh S, Kimura K, Kamataki T. S-oxidation of (+)-cis-3,5-dimethyl-2-(3-pyridyl)-thiazolidin-4- one hydrochloride by rat hepatic flavin-containing monooxygenase 1 expressed in yeast. Xenobiotica. 1995;25:1283–1291. doi: 10.3109/00498259509061917. [DOI] [PubMed] [Google Scholar]
- Ohmi N, Yoshida H, Endo H, Hasegawa M, Akimoto M, Higuchi S. S-oxidation of S-methyl-esonarimod by flavin-containing monooxygenases in human liver microsomes. Xenobiotica. 2003;33:1221–1231. doi: 10.1080/00498250310001624627. [DOI] [PubMed] [Google Scholar]
- Ohmiya Y, Mehendale HM. Species differences in pulmonary N-oxidation of chlorpromazine and imipramine. Pharmacology. 1984;28:289–295. doi: 10.1159/000137976. [DOI] [PubMed] [Google Scholar]
- Onderwater RC, Commandeur JN, Groot EJ, Sitters A, Menge WM, Vermeulen NP. Cytotoxicity of a series of mono- and di-substituted thioureas in freshly isolated rat hepatocytes: a preliminary structure-toxicity relationship study. Toxicology. 1998;125:117–129. doi: 10.1016/s0300-483x(97)00169-8. [DOI] [PubMed] [Google Scholar]
- Onderwater RCA, Commandeur JNM, Menge WMPB, Vermeulen NPE. Activation of microsomal glutathione S-transferase and inhibition of cytochrome P450 1A1 activity as a model system for detecting protein alkylation by thiourea-containing compounds in rat liver microsomes. Chem Res Toxicol. 1999;12:396–402. doi: 10.1021/tx980198p. [DOI] [PubMed] [Google Scholar]
- Onderwater RCA, Commandeur JNM, Vermeulen NPE. Comparative cytotoxicity of N-substituted N′-(4-imidazoleethyl) thiourea in precision-cut rat liver slices. Toxicology. 2004;197:81–91. doi: 10.1016/j.tox.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Osimitz TG, Kulkarni AP. Hepatic microsomal oxidative metabolism of pesticides and other xenobiotics in pregnant CD1 mice. Pestic Biochem Physiol. 1985;23:328–334. [Google Scholar]
- Ottoboni S, Carlson TJ, Trager WF, Castagnoli K, Castagnoli N., Jr Studies on the cytochrome P-450 catalyzed ring α-carbon oxidation of the nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Chem Res Toxicol. 1990;3:423–427. doi: 10.1021/tx00017a006. [DOI] [PubMed] [Google Scholar]
- Overby LH, Buckpitt AR, Lawton MP, Atta-Asafo-Adjei E, Schulze J, Philpot RM. Characterization of flavin-containing monooxygenase 5 (FMO5) cloned from human and guinea pig: evidence that the unique catalytic properties of FMO5 are not confined to the rabbit orthologs. Arch Biochem Biophys. 1995;317:275–284. doi: 10.1006/abbi.1995.1163. [DOI] [PubMed] [Google Scholar]
- Ozols J. Multiple forms of liver microsomal flavin-containing monooxygenases: complete covalent structure of form 2. Arch Biochem Biophys. 1991;290:103–115. doi: 10.1016/0003-9861(91)90596-b. [DOI] [PubMed] [Google Scholar]
- Ozols J. Isolation and structure of a third form of liver microsomal flavin monooxygenase. Biochemistry. 1994;33:3751–3757. doi: 10.1021/bi00178a035. [DOI] [PubMed] [Google Scholar]
- Park SB, Osterloh JD, Vamvakas S, Hashmi M, Anders MW, Cashman JR. Flavin-containing monooxygenase-dependent stereoselective S-oxygenation and cytotoxicity of cysteine S-conjugates and mercapurates. Chem Res Toxicol. 1992;5:193–201. doi: 10.1021/tx00026a008. [DOI] [PubMed] [Google Scholar]
- Park SB, Jacob P, III, Benowitz NL, Cashman JR. Stereoselective metabolism of (S)-( −)-nicotine in humans: formation of trans-(S)-( −)-nicotine N-1′-oxide. Chem Res Toxicol. 1993;6:880–888. doi: 10.1021/tx00036a019. [DOI] [PubMed] [Google Scholar]
- Park SB, Howald WN, Cashman JR. S-oxidative cleavage of farnesylcysteine and farnesylcysteine methyl ester by the flavin-containing monooxygenase. Chem Res Toxicol. 1994;7:191–198. doi: 10.1021/tx00038a012. [DOI] [PubMed] [Google Scholar]
- Park CS, Chung WG, Kang JH, Roh HK, Lee KH, Cha YN. Phenotyping of flavin-containing monooxygenase using caffeine metabolism and genotyping of FMO3 gene in a Korean population. Pharmacogenetics. 1999;9:155–164. [PubMed] [Google Scholar]
- Park C-S, Kang J-H, Chung W-G, Yi H-G, Pie J-E, Park D-L, et al. Ethnic differences in allelic frequency of two flavin-containing monooxygenase 3 (FMO3) polymorphisms: linkage and effect on in vivo and in vitro FMO activities. Pharmacogenetics. 2002;12:77–80. doi: 10.1097/00008571-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Phillips IR, Dolphin CT, Clair P, Hadley MR, Hutt AJ, McCombie RR, et al. The molecular biology of the flavin-containing monooxygenase of man. Chem Biol Interact. 1995;96:17–32. doi: 10.1016/0009-2797(94)03580-2. [DOI] [PubMed] [Google Scholar]
- Pichard-Garcia L, Weaver RJ, Eckett N, Scarfe G, Fabre J-M, Lucas C, et al. The olivacine derivative S 16020 (9- hydroxy-5,6-dimethyl-N-[2-(dimethylamino)ethyl)-6H-pyrido(4,3-B) -carbazole-1-carboxamide) induces CYP1A and its own metabolism in human hepatocytes in primary culture. Drug Metab Dispos. 2004;32:80–88. doi: 10.1124/dmd.32.1.80. [DOI] [PubMed] [Google Scholar]
- Pike MG, Mays DC, Macomber DW, Lipsky JJ. Metabolism of a disulfiram metabolite, S-methyl N,N-diethyldithiocarbamate, by flavin monooxygenase in human renal microsomes. Drug Metab Dispos. 2001;29:127–132. [PubMed] [Google Scholar]
- Poulsen LL. Organic sulfur substrates for the microsomal flavin-containing monooxygenase. In: Hodgson E, Bend JR, Philpot RM, editors. Rev Biochem Toxicol. North Holland: Elsevier Press; 1981. –33.pp. 49 [Google Scholar]
- Poulsen LL, Ziegler DM. Microsomal mixed-function oxidase-dependent renaturation of reduced ribonuclease. Arch Biochem Biophys. 1977;183:565–570. doi: 10.1016/0003-9861(77)90391-5. [DOI] [PubMed] [Google Scholar]
- Poulsen LL, Ziegler DM. The liver microsomal FAD-containing monooxygenase. Spectral characterization and kinetic studies. J Biol Chem. 1979;254:6449–6455. [PubMed] [Google Scholar]
- Poulsen LL, Ziegler DM. Multisubstrate flavin-containing monooxygenase: application of mechanism to specificity. Chem Biol Interact. 1995;96:57–73. doi: 10.1016/0009-2797(94)03583-t. [DOI] [PubMed] [Google Scholar]
- Poulsen LL, Hyslop RM, Ziegler DM. S-oxygenation of N-substituted thioureas catalyzed by the pig liver microsomal FAD-containing monooxygenase. Arch Biochem Biophys. 1979;198:78–88. doi: 10.1016/0003-9861(79)90397-7. [DOI] [PubMed] [Google Scholar]
- Poulsen LL, Taylor K, Williams DE, Masters BSS, Ziegler DM. Substrate specificity of the rabbit lung flavin-containing monooxygenase for amines: oxidation products of primary alkylamines. Mol Pharmacol. 1986;30:680–685. [PubMed] [Google Scholar]
- Prough RA. The N-oxidation of alkylhydrazines catalyzed by the microsomal mixed-function amine oxidase. Arch Biochem Biophys. 1973;158:442–444. doi: 10.1016/0003-9861(73)90641-3. [DOI] [PubMed] [Google Scholar]
- Prough RA, Ziegler DM. The relative participation of liver microsomal amine oxidase and cytochrome P-450 in N-demethylation reactions. Arch Biochem Biophys. 1977;180:363–373. doi: 10.1016/0003-9861(77)90050-9. [DOI] [PubMed] [Google Scholar]
- Prough RA, Freeman PC, Hines RN. The oxidation of hydrazine derivatives catalyzed by the purified liver microsomal FAD-containing monooxygenase. J Biol Chem. 1981;256:4178–4184. [PubMed] [Google Scholar]
- Rauckman EJ, Rosen GM, Kitchell BB. Superoxide radical as an intermediate in the oxidation of hydroxylamines by mixed function amine oxidase. Mol Pharmacol. 1979;15:131–137. [PubMed] [Google Scholar]
- Rawden HC, Kokwaro GO, Ward SA, Edwards G. Relative contribution of cytochromes P-450 and flavin-containing monooxygenases to the metabolism of albendazole by human liver microsomes. Br J Clin Pharmacol. 2000;49:313–322. doi: 10.1046/j.1365-2125.2000.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhan M, Chalifa-Caspi V, Prilusky J, Lancet D. GeneCards: encyclopedia for genes, proteins and diseases. 1997 doi: 10.1016/s0168-9525(97)01103-7. Weizmann Institute of World Wide Web URL: http://bioinformatics.weizmann.ac.il/cards. [DOI] [PubMed]
- Rettie AE, Lang DH. Can caffeine metabolism be used an in vivo probe for human flavin-containing monooxygenase activity? Pharmacogenetics. 2000;10:275–277. doi: 10.1097/00008571-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Rettie AE, Bogucki BD, Lim I, Meier GP. Stereoselective sulfoxidation of a series of alkyl p-tolyl sulfides by microsomal and purified flavin-containing monooxygenase. Mol Pharmacol. 1990;37:643–651. [PubMed] [Google Scholar]
- Rettie AE, Lawton MP, Sadeque AJM, Meier GP, Philpot RM. Prochiral sulfoxidation as a probe for multiple forms of the microsomal flavin-containing monooxygenase: studies with rabbit FMO1, FMO2, FMO3, and FMO5 expressed in Escherichia coli. Arch Biochem Biophys. 1994;311:369–377. doi: 10.1006/abbi.1994.1250. [DOI] [PubMed] [Google Scholar]
- Rettie AE, Meier GP, Sadeque AJ. Prochiral sulfides as in vitro probes for multiple forms of the flavin-containing monooxygenase. Chem Biol Interact. 1995;96:3–15. doi: 10.1016/0009-2797(94)03579-w. [DOI] [PubMed] [Google Scholar]
- Ring BJ, Wrighton SA, Aldridge SLK, Hansen K, Haehner B, Shipley LA. Flavin-containing monooxygenase-mediated N-oxidation of the M1-muscarinic agonist xanomeline. Drug Metab Dispos. 1999;27:1099–1103. [PubMed] [Google Scholar]
- Ripp SL, Overby LH, Philpot RM, Elfarra AA. Oxidation of cysteine S-conjugates by rabbit liver microsomes and cDNA-expressed flavin-containing monooxygenases: studies with S-(1,2-dichlorovinyl)-l-cysteine, S-(1,2,2-trichlorovinyl)-l-cysteine, S-allyl-l-cysteine, and S-benzyl-l-cysteine. Mol Pharmacol. 1997;51:507–515. [PubMed] [Google Scholar]
- Ripp SL, Itagaki K, Philpot RM, Elfarra AA. Methione S-oxidation in human and rabbit liver microsomes: evidence for a high-affinity methionine S-oxidase activity that is distinct from flavin-containing monooxygenase 3. Arch Biochem Biophys. 1999;367:322–332. doi: 10.1006/abbi.1999.1247. [DOI] [PubMed] [Google Scholar]
- Rodrigues AD, Kukulka MJ, Ferrero JL, Cashman JR. In vitro hepatic metabolism of ABT-418 in chimpanzee (Pan troglodytes). A unique pattern of microsomal flavin-containing monooxygenase stereoselective N′-oxidation. Drug Metab Dispos. 1995;23:1143–1152. [PubMed] [Google Scholar]
- Rodriguez RJ, Acosta D., Jr Metabolism of ketoconazole and deacetylated ketoconzaole by rat hepatic microsomes and flavin-containing monooxygenases. Drug Metab Dispos. 1997a;25:772–777. [PubMed] [Google Scholar]
- Rodriguez RJ, Acosta D., Jr N-deacetyl ketoconazole-induced hepatotoxicity in a primary culture system of rat hepatocytes. Toxicology. 1997b;117:123–131. doi: 10.1016/s0300-483x(96)03560-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Buckholz CJ. Hepatotoxicity of ketoconazole in Sprague–Dawley rats: glutathione depletion, flavin-containing monooxygenases-mediated bioactivation and hepatic covalent binding. Xenobiotica. 2003;33:429–441. doi: 10.1080/0049825031000072243. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, Miranda CL. Isoform specificity of N-deacetyl ketoconazole by human and rabbit flavin-containing monooxygenases. Drug Metab Dispos. 2000;28:1083–1086. [PubMed] [Google Scholar]
- Rodriguez RJ, Proteau PJ, Marquez BL, Hetherington CL, Buckholz CJ, O’Connell KL. Flavin-containing monooxygenase-mediated metabolism of N-deacetyl ketoconazole by rat hepatic microsomes. Drug Metab Dispos. 1999;27:880–886. [PubMed] [Google Scholar]
- Rooseboom M, Commandeur JN, Floor GC, Rettie AE, Vermeulen NP. Selenoxidation by flavin-containing monooxygenases as a novel pathway for beta-elimination of selenocysteine Se-conjugates. Chem Res Toxicol. 2001;14:127–134. doi: 10.1021/tx0001326. [DOI] [PubMed] [Google Scholar]
- Rosvold EA, McGlynn KA, Lustbader ED, Buetow KH. Identification of an NAD(P)H:quinone oxidoreductase polymorphism and its association with lung cancer and smoking. Pharmacogenetics. 1995;5:199–206. doi: 10.1097/00008571-199508000-00003. [DOI] [PubMed] [Google Scholar]
- Rossmann MG, Moras D, Olsen KW. Chemical and biological evolution of a nucleotide-binding protein. Nature. 1974;250:194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Rouer E, Lemoine A, Cresteil T, Rouet P, Leroux JP. Effects of genetic or chemically induced diabetes on imipramine metabolism. Respective involvement of flavin monooxygenase and cytochrome P- 450-dependent monooxygenase. Drug Metab Dispos. 1987;15:524–528. [PubMed] [Google Scholar]
- Rouer E, Rouet P, Delpech M, Leroux J-P. Purification and comparison of liver microsomal flavin-containing monooxygenase from normal and streptozotocin-diabetic rats. Biochem Pharmacol. 1988;37:3455–3459. doi: 10.1016/0006-2952(88)90696-x. [DOI] [PubMed] [Google Scholar]
- Ruse MJ, Waring RH. The effect of methimazole on thioamides bioactivation and toxicity. Toxicol Lett. 1991;58:37–41. doi: 10.1016/0378-4274(91)90188-c. [DOI] [PubMed] [Google Scholar]
- Sabourin PJ, Hodgson E. Characterization of the purified microsomal FAD-containing monooxygenase from mouse and pig liver. Chem Biol Interact. 1984;51:125–139. doi: 10.1016/0009-2797(84)90025-5. [DOI] [PubMed] [Google Scholar]
- Sadeque AJ, Thummel KE, Rettie AE. Purification of macaque liver flavin-containing monooxygenase: a form of the enzyme related immunochemically to an isozyme expressed selectively in adult human liver. Biochim Biophys Acta. 1993;1162:127–134. doi: 10.1016/0167-4838(93)90138-h. [DOI] [PubMed] [Google Scholar]
- Sardas S, Akyol D, Green RL, Mellon T, Gokmen O, Cholerton S. Trimethylamine N-oxidation in Turkish women with bacterial vaginosis. Pharmacogenetics. 1996;6:459–463. doi: 10.1097/00008571-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Schupke H, Hempel R, Peter G, Hermann R, Wessel K, Engel J, et al. New metabolic pathways of α-lipoic acid. Drug Metab Dispos. 2001;29:855–862. [PubMed] [Google Scholar]
- Scott AM, Powell GM, Upshall DG, Curtis CG. Pulmonary toxicity of thioureas in the rat. Environ Health Perspect. 1990;85:43–50. doi: 10.1289/ehp.85-1568340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehin-Johnson S, Williams DE, Larsen-Su S, Stresser DM, Hines RN. Tissue-specific expression of flavin-containing monooxygenase (FMO) forms 1 and 2 in the rabbit. J Pharmacol Exp Ther. 1995;272:1293–1299. [PubMed] [Google Scholar]
- Smith PB, Crespi C. Thiourea toxicity in mouse C3H/10T1/2 cells expressing human flavin-dependent monooxygenase 3. Biochem Pharmacol. 2002;63:1941–1948. doi: 10.1016/s0006-2952(02)00978-4. [DOI] [PubMed] [Google Scholar]
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- Smyser BP, Hodgson E. Metabolism of phosphorus-containing compounds by pig liver microsomal FAD-containing monooxygenase. Biochem Pharmacol. 1985;34:1145–1150. doi: 10.1016/0006-2952(85)90487-3. [DOI] [PubMed] [Google Scholar]
- Smyser BP, Sabourin PJ, Hodgson E. Oxidation of pesticides by purified microsomal FAD-containing monooxygenase from mouse and pig liver. Pestic Biochem Physiol. 1985;24:368–374. [Google Scholar]
- Sofer SS, Ziegler DM. Microsomal mixed-function amine oxidase. Oxidation products of piperazine-substituted phenothiazine drugs. Drug Metab Dispos. 1978;6:232–239. [PubMed] [Google Scholar]
- Stehr M, Diekmann H, Smau L, Seth O, Ghisla S, Singh M, et al. A hydrophobic sequence motif common to N-hydroxylating enzymes. Trends Biochem Sci. 1998;23:56–57. doi: 10.1016/s0968-0004(97)01166-3. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Melton RJ, Zaya MJ, Engel LC. Expression and characterization of functional dog flavin-containing monooxygenase 1. Mol Pharmacol. 2003;63:271–275. doi: 10.1124/mol.63.2.271. [DOI] [PubMed] [Google Scholar]
- Stormer E, Roots I, Brockmoller J. Benzydamine N-oxidation as an index reaction reflecting FMO activity in human liver microsomes and impact of FMO3 polymorphisms on enzyme activity. Br J Clin Pharmacol. 2000;50:553–561. doi: 10.1046/j.1365-2125.2000.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JK, Robertus JD. Yeast flavin-containing monooxygenase is induced by the unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:121–126. doi: 10.1073/pnas.97.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JK, Robertus JD. Role of yeast flavin-containing monooxygenase in maintenance of thiol-disulfide redox potential. Methods Enzymol. 2002;348:113–121. doi: 10.1016/s0076-6879(02)48631-4. [DOI] [PubMed] [Google Scholar]
- Suh JK, Poulsen LL, Ziegler DM, Robertus JD. Yeast flavin-containing monooxygenase generates oxidizing equivalents that control protein folding in the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:2687–2691. doi: 10.1073/pnas.96.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JK, Poulsen LL, Ziegler DM, Robertus JD. Redox regulation of yeast flavin-containing monooxygenase. Arch Biochem Biophys. 2000;381:317–322. doi: 10.1006/abbi.2000.1965. [DOI] [PubMed] [Google Scholar]
- Taioli E, Ford J, Trachman J, Li Y, Demopoulos R, Garte S. Lung cancer risk & CYP1A1 genotype in African Americans. Carcinogenesis. 1998;19:813–817. doi: 10.1093/carcin/19.5.813. [DOI] [PubMed] [Google Scholar]
- Takamura T, Sakurai M, Ota T, Ando H, Honda M, Kaneko S. Genes for systemic vascular complications are differentially expressed in the livers of type 2 diabetic patients. Diabetologia. 2004;47:638–647. doi: 10.1007/s00125-004-1366-y. [DOI] [PubMed] [Google Scholar]
- Taylor KH, Ziegler DM. Studies on substrate specificity of the hog liver flavin-containing monooxygenase: anionic organic sulfur compounds. Biochem Pharmacol. 1987;36:141–146. doi: 10.1016/0006-2952(87)90391-1. [DOI] [PubMed] [Google Scholar]
- Thieme R, Pai E, Schrimer R, Schulz G. Three-dimensional structure of glutathione reductase at 2 Å resolution. J Mol Biol. 1981;152:763–782. doi: 10.1016/0022-2836(81)90126-1. [DOI] [PubMed] [Google Scholar]
- Treacy EP, Akerman BR, Chow LML, Youil R, Bibeau C, Lin J, et al. Mutations of the flavin-containing monooxygenase gene (FMO3) cause trimethylaminuria, a defect in detoxication. Hum Mol Genet. 1998;7:839–845. doi: 10.1093/hmg/7.5.839. [DOI] [PubMed] [Google Scholar]
- Tugnait M, Hawes EM, McKay G, Rettie AE, Haining RL, Midha KK. N-oxygenation of clozapine by flavin-containing monooxygenase. Drug Metab Dispos. 1997;25:524–527. [PubMed] [Google Scholar]
- Tynes RE, Hodgson E. Catalytic activity and substrate specificity of the flavin-containing monooxygenase in microsomal systems: characterization of the hepatic, pulmonary and renal enzymes of the mouse, rabbit and rat. Arch Biochem Biophys. 1985a;240:77–93. doi: 10.1016/0003-9861(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Tynes RE, Hodgson E. Magnitude of involvement of the mammalian flavin-containing monooxygenase in the microsomal oxidation of pesticides. J Agric Food Chem. 1985b;33:471–479. [Google Scholar]
- Tynes RE, Sabourin PJ, Hodgson E. Identification of distinct hepatic and pulmonary forms of microsomal flavin-containing monooxygenase in the mouse and rabbit. Biochem Biophys Res Commun. 1985;126:1069–1075. doi: 10.1016/0006-291x(85)90294-3. [DOI] [PubMed] [Google Scholar]
- Tynes RE, Sabourin PJ, Hodgson E, Philpot RM. Formation of hydrogen peroxide and N-hydroxylated amines catalyzed by pulmonary flavin-containing monooxygenase in the presence of primary alkylamines. Arch Biochem Biophys. 1986;251:654–664. doi: 10.1016/0003-9861(86)90375-9. [DOI] [PubMed] [Google Scholar]
- Usmani KA, Karoly ED, Hodgson E, Rose RL. In vitro sulfoxidation of thioether compounds by human cytochrome P450 and flavin-containing monooxygenase isoforms with particular reference to the CYP2C subfamily. Drug Metab Dispos. 2004;32:333–339. doi: 10.1124/dmd.32.3.333. [DOI] [PubMed] [Google Scholar]
- Vallon O. New sequence motifs in flavoproteins: evidence for common ancestry and tools to predict structure. Proteins. 2000;38:95–114. doi: 10.1002/(sici)1097-0134(20000101)38:1<95::aid-prot10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Vannelli TA, Dykman A, Ortiz de Montellano PR. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J Biol Chem. 2002;277:12824–12829. doi: 10.1074/jbc.M110751200. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Levi PE, Hodgson E. The effect of detergents on the purified flavin-containing monooxygenase of mouse liver, kidney and lungs. Gen Pharmacol. 1991a;22:549–552. doi: 10.1016/0306-3623(91)90022-x. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Levi PE, Hodgson E. The flavin-containing monooxygenase of mouse kidney. A comparison with the liver enzyme. Biochem Pharmacol. 1991b;42:1411–1420. doi: 10.1016/0006-2952(91)90453-c. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Blake B, Levi PE, Hodgson E. The flavin-containing monooxygenase in mouse lung: evidence for expression of multiple forms. J Biochem Toxicol. 1992;7:163–169. doi: 10.1002/jbt.2570070305. [DOI] [PubMed] [Google Scholar]
- Virkel G, Lifschitz A, Sallovitz J, Pis A, Lanusse C. Comparative hepatic and extrahepatic enantioselective sulfoxidation of albendazole and fenbendazole in sheep and cattle. Drug Metab Dispos. 2004;32:536–544. doi: 10.1124/dmd.32.5.536. [DOI] [PubMed] [Google Scholar]
- Wang T, Shankar K, Ronis MJ, Mehendale HM. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J Pharmacol Exp Ther. 2000;292:473–479. [PubMed] [Google Scholar]
- Washio T, Arisawa H, Kohsaka K, Yasuda H. Identification of human drug-metabolizing enzymes involved in the metabolism of SNI-2011. Biol Pharm Bull. 2001;24:1263–1266. doi: 10.1248/bpb.24.1263. [DOI] [PubMed] [Google Scholar]
- Weissman J, Trevor A, Chiba K, Peterson LA, Caldera P, Castagnoli N, Jr, et al. Metabolism of the nigrostriatal toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by liver homogenate fractions. J Med Chem. 1985;28:996–999. doi: 10.1021/jm00146a005. [DOI] [PubMed] [Google Scholar]
- Whestine JR, Yueh M-F, Hopp KA, McCarver DG, Williams DE, Park C-S, et al. Ethnic differences in human flavin-containing monooxygenase 2 (FMO2) polymorphisms: detection of expressed protein in African Americans. Toxicol Appl Pharmacol. 2000;168:216–224. doi: 10.1006/taap.2000.9050. [DOI] [PubMed] [Google Scholar]
- Wierenga RK, De Maeyer MCH, Hol WG. Interaction of pyrophosphate moieties with α-helices in dinucleotide binding proteins. Biochemistry. 1985;24:1346–1357. [Google Scholar]
- Williams DE. Factors regulating the activity of the rabbit lung flavin-containing monooxygenase. In: Hlavica P, Damani LA, editors. N-Oxidation of Drugs. Biochem Pharmacol Toxicol. NY: Chapman and Hall; 1991. –91.pp. 105 [Google Scholar]
- Williams DE, Ziegler DM, Nordin DJ, Hale SE, Masters BSS. Rabbit lung flavin-containing monooxygenase is immunochemically and catalytically distinct from the liver enzyme. Biochem Biophys Res Commun. 1984;125:116–122. doi: 10.1016/s0006-291x(84)80342-3. [DOI] [PubMed] [Google Scholar]
- Williams DE, Hale SE, Muerhoff AS, Masters BSS. Rabbit lung flavin-containing monooxygenase. Purification and induction during pregnancy. Mol Pharmacol. 1985;28:381–390. [PubMed] [Google Scholar]
- Williams DE, Shigenaga MK, Castagnoli N., Jr The role of cytochromes P-450 and flavin-containing monooxygenase in the metabolism of (S)-nicotine by rabbit lung. Drug Metab Dispos. 1990;18:418–428. [PubMed] [Google Scholar]
- Wu R-F, Ichikawa Y. Characteristic properties and kinetic analysis with neurotoxins of porcine FAD-containing monooxygenase. Biochim Biophys Acta. 1994;1208:204–210. doi: 10.1016/0167-4838(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Wu R-F, Ichikawa Y. Inhibition of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine metabolic activity of porcine FAD-containing monooxygenase by selective monoamine oxidase-B inhibitors. FEBS Lett. 1995;358:145–148. doi: 10.1016/0014-5793(94)01412-t. [DOI] [PubMed] [Google Scholar]
- Wu R-F, Miura S, Ichikawa Y. Neurotoxins: 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-6,7-dihydroxy-tetrahydroisoquinoline as substrates for FAD-containing monooxygenase of porcine liver microsomes. Biochem Pharmacol. 1992;44:2079–2081. doi: 10.1016/0006-2952(92)90111-u. [DOI] [PubMed] [Google Scholar]
- Wu X, Gwyn K, Amos CI, Makan N, Hong WK, Spitz MR. The association of microsomal epoxide hydrolase polymorphisms and lung cancer risk in African–Americans and Mexican–Americans. Carcinogenesis. 2001;22:923–928. doi: 10.1093/carcin/22.6.923. [DOI] [PubMed] [Google Scholar]
- Wu R-F, Liao CX, Tomita S, Ichikawa Y, Terada LS. Porcine FAD-containing monooxygenase metabolizes lidocaine, bupivacaine and propranolol in vitro. Life Sci. 2004;75:1011–1019. doi: 10.1016/j.lfs.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Wynalda MA, Hutzler JM, Koets MD, Podoll T, Wienkers LC. In vitro metabolism of clindamycin in human liver and intestinal microsomes. Drug Metab Dispos. 2003;31:878–887. doi: 10.1124/dmd.31.7.878. [DOI] [PubMed] [Google Scholar]
- Yamada H, Baba T, Hirata Y, Oguri K, Yoshimura H. Studies on the N-demethylation of methamphetamine by liver microsomes of guinea pigs and rats: the role of flavin-containing monooxygenase and cytochrome P-450 systems. Xenobiotica. 1984;14:861–866. doi: 10.3109/00498258409151484. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Fujieda M, Togashi M, Saito T, Preti G, Cashman JR, et al. Effects of the dietary supplements, activated charcoal and copper chlorophyllin, on urinary excretion of trimethylamine in Japanese trimethylaminuria patients. Life Sci. 2004;74:2739–2747. doi: 10.1016/j.lfs.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Yeung CK, Lang DH, Thummel KE, Rettie AE. Immunoquantitation of FMO1 in human liver, kidney, and intestine. Drug Metab Dispos. 2000;28:1107–1111. [PubMed] [Google Scholar]
- Yueh M-F, Krueger SK, Williams DE. Pulmonary flavin-containing monooxygenase (FMO) in Rhesus Macaque: expression of FMO2 protein and analysis of the cDNA. Biochim Biophys Acta. 1997;1350:267–271. doi: 10.1016/s0167-4781(97)00004-3. [DOI] [PubMed] [Google Scholar]
- Zhang AQ, Mitchell SC, Smith RL. Discontinuous distribution of N-oxidation of dietary derived trimethylamine in a British population. Xenobiotica. 1996;26:957–961. doi: 10.3109/00498259609052497. [DOI] [PubMed] [Google Scholar]
- Zhang J, Tran Q, Lattard V, Cashman JR. Deleterious mutations in the flavin-containing monooxygenase 3 (FMO3) gene causing trimethylaminuria. Pharmacogenetics. 2003;13:495–500. doi: 10.1097/00008571-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Zhou S, Kestell P, Paxton JW. 6-Methylhydroxylation of the anti-cancer agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) by flavin-containing monooxygenase 3. Eur J Drug Metab Pharmacokinet. 2002;27:179–183. doi: 10.1007/BF03190455. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. Microsomal flavin-containing monooxygenase: oxygenation of nucleophilic nitrogen and sulfur compounds. In: Jakoby WB, editor. Enzymatic basis of detoxication. Vol. 1. NY: Academic Press; 1980. –201.pp. 227 [Google Scholar]
- Ziegler DM. Functional groups bearing sulfur. In: Jakoby WB, Bend JR, Caldwell J, editors. Metabolism of functional groups. NY: Academic Press; 1982. –171.pp. 184 [Google Scholar]
- Ziegler DM. Metabolic oxygenation of organic nitrogen and sulfur compounds. In: Mitchell JR, Horning MG, editors. Drug metabolism and drug toxicity. New York: Raven Press; 1984. –33.pp. 53 [Google Scholar]
- Ziegler DM. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19:1–32. doi: 10.3109/03602538809049617. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. Mechanism, multiple forms and substrate specificities of flavin-containing monooxygenases. In: Hlavic P, Damani LA, editors. N-Oxidation of Drugs: Biochemistry, Pharmacology and Toxicology. London: Chapman and Hall; 1991. –59.pp. 68 [Google Scholar]
- Ziegler DM. Recent studies on the structure and function of multisubstrate flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 1993;33:179–199. doi: 10.1146/annurev.pa.33.040193.001143. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. An overview of the mechanism, substrate specificities and structure of FMOs. Drug Metab Rev. 2002;34:503–511. doi: 10.1081/dmr-120005650. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Mitchell CH. Microsomal oxidase IV: properties of mixed-function amine oxidase isolated from pig liver microsomes. Arch Biochem Biophys. 1972;150:116–125. doi: 10.1016/0003-9861(72)90017-3. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Pettit FH. Formation of an intermediate N-oxide in the oxidative demethylation of N,N-dimethylaniline catalyzed by liver microsomes. Biochem Biophys Res Commun. 1964;15:188–193. doi: 10.1016/0006-291x(64)90322-5. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Poulsen LL. Hepatic microsomal mixed-function amine oxidase. Methods Enzymol. 1978;52C:142–151. doi: 10.1016/s0076-6879(78)52016-8. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Poulsen LL. Catalytic mechanism of FMO-catalyzed N- and S-oxidations. In: Gooderham N, editor. Drug Metabolism. Towards the Next Millennium. Amsterdam: IOS Press; 1998. –30.pp. 38 [Google Scholar]
- Ziegler DM, Jollow D, Cook DE. Properties of a purified liver microsomal mixed function amine oxidase. In: Kamin H, editor. Flavins and Flavoproteins. 3rd International Symposium. 1971a. –507.pp. 522 [Google Scholar]
- Ziegler DM, Poulsen LL, McKee EM. Interaction of primary amines with a mixed-function amine oxidase isolated from pig liver microsomes. Xenobiotica. 1971b;1:523–531. doi: 10.3109/00498257109041521. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Duffel MW, Poulsen LL. Studies on the nature and regulation of the cellular thio:disulphide potential. Ciba Found Symp. 1979;72:191–204. doi: 10.1002/9780470720554.ch12. [DOI] [PubMed] [Google Scholar]
- Ziegler DM, Poulsen LL, Duffel MW. Microsomes, Drug oxidation, and chemical carcinogenesis. NY: Academic Press; 1980. Kinetic studies of mechanism and substrate specificity of the microsomal flavin-containing monooxygenase; –637.pp. 645 [Google Scholar]
- Ziegler DM, Graf P, Poulsen LL, Stahl W, Sies H. NADPH-dependent oxidation of reduced ebselen, 2-selenylbenzanilide, and of 2-(methylseleno)benzanilide catalyzed by pig liver flavin-containing monooxygenase. Chem Res Toxicol. 1992;5:163–166. doi: 10.1021/tx00026a004. [DOI] [PubMed] [Google Scholar]
- Ziegler-Skylakakis K, Nill S, Pan J-F, Andrae U. S-oxygenation of thiourea results in the formation of genotoxic products. Environ Mol Mutagen. 1998;31:362–373. [PubMed] [Google Scholar]
- Zschocke J, Kohlmueller D, Quak E, Meissner T, Hoffmann GF, Mayatepek E. Mild trimethylaminuria caused by common variants in FMO3 gene. Lancet. 1999;354:834–835. doi: 10.1016/s0140-6736(99)80019-1. [DOI] [PubMed] [Google Scholar]