Abstract
Phosphorylation of tau protein is regulated by several kinases, especially glycogen synthase kinase 3β (GSK-3β), cyclin-dependent protein kinase 5 (cdk5) and cAMP-dependent protein kinase (PKA). Phosphorylation of tau by PKA primes it for phosphorylation by GSK-3β, but the site-specific modulation of GSK-3β–catalyzed tau phosphorylation by the prephosphorylation has not been well investigated. Here, we found that prephosphorylation by PKA promotes GSK-3β–catalyzed tau phosphorylation at Thr181, Ser199, Ser202, Thr205, Thr217, Thr231, Ser396 and Ser422, but inhibits its phosphorylation at Thr212 and Ser404. In contrast, the prephosphorylation had no significant effect on its subsequent phosphorylation by cdk5 at Thr181, Ser199, Thr205, Thr231 and Ser422; inhibited it at Ser202, Thr212, Thr217 and Ser404; and slightly promoted it at Ser396. These studies reveal the nature of the inter-regulation of tau phosphorylation by the three major tau kinases.
Keywords: Tau, Glycogen synthase kinase 3β, Cyclin-dependent protein kinase 5, cAMP-dependent protein kinase, Neurofibrillary degeneration, Alzheimer disease
1. Introduction
Neurofibrillary degeneration is characterized by the abnormal intracellular deposition of hyperphosphorylated microtubule-associated protein tau in the form of paired helical filaments (PHF) and straight filaments [1–3]. It is a common feature of Alzheimer disease (AD) and several related neurodegenerative disorders [4] and correlates with the severity of dementia in AD [5–7]. Many studies have demonstrated that abnormal hyperphosphorylation of tau is crucial to neurofibrillary degeneration [4,8]. However, the molecular mechanisms leading to abnormal tau hyperphosphorylation in AD and other neurodegenerative diseases are not yet fully understood.
Efforts aiming to elucidate the mechanisms of abnormal tau hyperphosphorylation have led to identification of several protein kinases that may catalyze tau phosphorylation in the brain. Among these tau kinase candidates, glycogen synthase kinase 3β (GSK-3β), cyclin-dependent protein kinase 5 (cdk5) and cAMP-dependent protein kinase (PKA) have been most implicated. The former two were actually described as tau kinase I and tau kinase II, respectively [9,10]. These two kinases can phosphorylate tau at multiple sites (for a summary of the phosphorylation sites, see [11]). It has been observed that prephosphorylation at certain sites primes tau to be a better substrate in vitro [12–16] as well as in vivo [17] for GSK-3β. These studies indicate the complexity of the regulation of tau phosphorylation. Tau is hyperphosphorylated at as many as 37 sites in AD brain (see [11] for the list of phosphorylation sites), but the effect of priming on the phosphorylation of tau by GSK-3β at most of the phosphorylation sites has not been reported.
In this study, we investigated the effects of prephosphorylation of tau by PKA on its subsequent phosphorylation by GSK-3β or cdk5 at individual phosphorylation sites. We found that PKA-induced tau phosphorylation promotes its subsequent phosphorylation at most sites catalyzed by GSK-3β, whereas it differentially affects its subsequent phosphorylation by cdk5.
2. Materials and methods
2.1. Materials
The catalytic subunit of PKA and GSK-3β were purchased from Sigma (St. Louis, MO, USA) and CalBiochem (San Diego, CA, USA), respectively. Recombinant cdk5 and p25 (an activator of cdk5) were expressed, purified and reconstituted into an active holoenzyme, as described previously [18]. The largest isoform of recombinant human tau, tau441, was expressed and purified from E. coli as described previously [19]. The tau polyclonal antibody R134d against tau in a phosphorylation-independent manner was raised in rabbits, as reported previously [20]. Phosphorylation-dependent and site-specific tau antibodies pT181, pS199, pS202, pT205, pT212, pS214, pT217, pT231, pS262, pS396, pS404, pS409 and pS422 were purchased from Biosource International (Camarillo, CA, USA). Monoclonal antibody PHF-1 that recognizes tau phosphorylated at Ser396/Ser404 was kindly provided by Dr. P. Davies of Albert Einstein College of Medicine, Bronx, NY, USA. Peroxidase-conjugated anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA); ECL Kit was from Amersham Pharmacia (Costa Mesa, CA, USA); and [γ-32P]ATP was from ICN Biomedicals (Costa Mesa, CA, USA).
2.2. Phosphorylation of tau in vitro
The in vitro phosphorylation was carried out by incubating tau441 (0.2 mg/ml) at 30 oC in a phosphorylation reaction mixture. For PKA-catalyzed phosphorylation, the reaction mixture contained 40 mM HEPES, pH 6.8, 10 mM β-mercaptoethanol, 10 mM MgCl2, 1.0 mM EGTA, and 0.2 mM ATP or [γ-32P]ATP (~500 cpm/pmol), 10 μg/ml PKA, and protease inhibitors (2 μg/ml aprotinin, 2 μg/ml pepstatin, 5 μg/ml leupeptin, and 1.0 mM PMSF). For cdk5-catalyzed phosphorylation, the reaction mixture contained 40 mM HEPES (pH 7.4), 10 mM β-mercaptoethanol, 10 mM MgCl2, 0.2 mM [γ-32P]ATP (~500 cpm/pmol), 6.4 μg/ml cdk5/p25 and protease inhibitors. For GSK-3β-catalyzed phosphorylation, the mixture contained 40 mM HEPES (pH 7.4), 10 mM MgCl2, 10 mM β-mercaptoethanol, 0.2 mM [γ-32P]ATP (~500 cpm/pmol), 0.4 μg/ml GSK-3β and protease inhibitors. After incubation for various periods of time, the reaction was stopped, the 32P-labeled tau was separated from free [γ-32P]ATP by paper chromatography, and the radioactivity of tau was determined by Cerenkov counting, as described previously [21].
For prephosphorylation of tau with PKA, tau441 was phosphorylated, as described above, with non-radioactive ATP at 30 oC for 90 min. Then, the reaction mixture was heated in boiling water for 5 min to inactivate PKA. The heat-treated mixture was briefly centrifuged, and the resultant supernatant containing heat-stable tau441was passed through a Sephadex G-50 minicolumn to exchange its buffer into 40 mM HEPES (pH 7.4). Fractions containing P-tau were pooled and stored at −20 oC for further phosphorylation reaction with GSK-3β or cdk5. Under this condition, approximately 2–2.5 moles of phosphate were added to each mole of tau441. The non-prephosphorylated control tau441 was treated the same way except PKA was omitted from the reaction mixture.
2.3. Determination of site-specific phosphorylation of tau
For detecting site-specific phosphorylation of tau, the phosphorylation reactions were carried out with non-radioactive ATP, and the reactions were stopped by adding 1/3 volume of four-fold concentrated SDS-polyacrymide gel electrophoresis (PAGE) sample buffer (240 mM Tris-HCl, pH 6.8, 8.0% SDS, 20% β-mercaptoethanol, 40% glycerol and 0.08% bromophenol blue), followed by heating in boiling water for 5 min. The phosphorylation of tau at each specific site was detected by Western blots with various phosphorylation-dependent and site-specific tau antibodies (at a dilution of 1:1,000), as described previously [22]. A phosphorylation-independent tau antibody, R134d (1:5,000), was also used to detect total tau. For time kinetic studies of tau phosphorylation, aliquots of reaction mixture were removed, and the reaction was terminated by heating in a boiling water bath for 5 min. After the mixture was cooled down, the phosphorylation of tau at each specific site was measured by using an immuno-dot-blot assay, as described [23].
3. Results
To learn the overall effect of prephosphorylation by PKA on the subsequent phosphorylation of tau by GSK-3β or cdk5, we first phosphorylated tau with PKA and non-radioactive ATP to a stoichiometry of 2–2.5, and then used this prephosphorylated tau (P-tau) and the control-treated tau in parallel as substrates for subsequent phosphorylation by GSK-3β or cdk5. We found that, under the conditions used, prephosphorylation of tau with PKA increased its phosphorylation by GSK-3β dramatically, but had no significant effect on cdk5-catalyzed tau phosphorylation (Fig. 1). GSK-3β and cdk5 each phosphorylated tau to a stoichiometry of nearly 1 after 90 min incubation. In contrast, GSK-3β phosphorylated P-tau to approximately 3 mol P/mol of tau in 90 min.
Fig. 1.
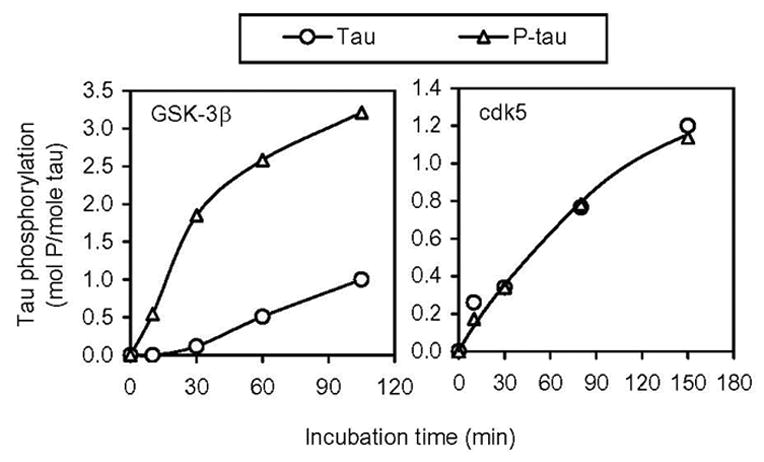
Effect of prephosphorylation of tau by PKA on the subsequent phosphorylation by GSK-3β and cdk5. Recombinant tau441 with (P-tau, Δ) or without (○) prephosphorylation by PKA was incubated with GSK-3β (left panel) or cdk5/p25 (right panel) for various periods of time, followed by determination of tau phosphorylation by counting the radioactivity after removing free [γ-32P]ATP by ascending paper chromatography.
PKA, GSK-3β and cdk5 each can phosphorylate tau at multiple sites [11]. To examine the phosphorylation sites that are modulated by prephosphorylation, we used Western blots developed with phosphorylation-dependent and site-specific tau antibodies to determine tau phosphorylation at various sites after incubation of tau and P-tau with GSK-3β or cdk5. We observed that pre-incubation of tau with PKA resulted in strong immunostaining of tau with antibodies pS214, pS262 and pS409 (Fig. 2, compare lane 4 with lane 3), indicating phosphorylation of tau at Ser214, Ser262 and Ser409 by PKA. The increased immunostaining with these phosphorylation-dependent tau antibodies was specifically due to tau phosphorylation at these sites, because the same amount of tau was loaded in each lane, as seen in R134d blot that detects total tau protein. PKA-phosphorylated tau was not stained with antibodies pT181, pS199, pS202, pT205, pT217, pT231, pS396, pS404, pS422 or PHF-1 (Fig. 2). These results are consistent with previous studies showing that Ser214, Ser262 and Ser409 are major phosphorylation sites of tau by PKA [15,24,25].
Fig. 2.
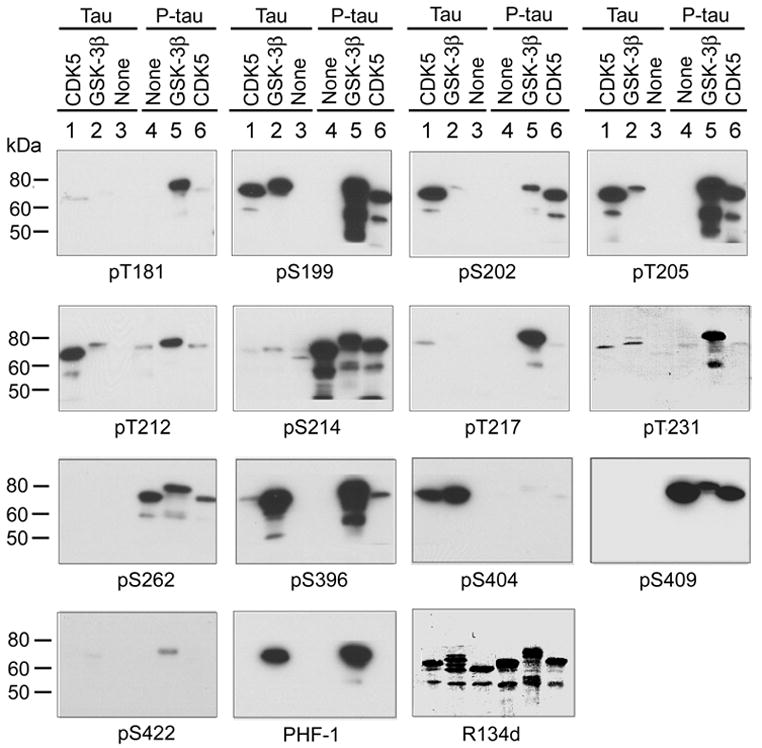
Site-specific effects of prephosphorylation of tau by PKA on the subsequent phosphorylation by GSK-3β and cdk5. Recombinant tau441 was first phosphorylated by PKA, and then the phosphorylated tau (P-tau) and the control-treated tau (Tau) were further incubated with GSK-3β or cdk5/p25 for 150 min. The phosphorylation of tau and P-tau at various phosphorylation sites was examined by Western blots developed with phosphorylation-dependent and site-specific tau antibodies, as indicated under each blot.
Analysis of the blots shown in Fig. 2 indicated that without prephosphorylation, tau441 was phosphorylated by GSK-3β at Ser199, Thr205, Thr212, Thr231, Ser396, Ser404 and Ser396/Ser404 (PHF-1 sites; compare lane 2 with lane 3), and by cdk5 at Ser199, Ser202, Thr205, Thr212, Thr217, Thr231, Ser396 and Ser404 (compare lane 1 with lane 3). The site-specific phosphorylation of tau by these two kinases in the present study is consistent with previous studies (summarized in [11]). A remarkable upward shift of the apparent molecular weight of tau after phosphorylation with these kinases was also seen in the R134d (to total tau) blot, which further confirmed tau phosphorylation. When phosphorylation of tau and of P-tau was compared, we found that prephosphorylation by PKA dramatically promoted tau phosphorylation with GSK-3β at Thr181, Ser199, Ser202, Thr205, Thr217, Thr231, Ser396, and Ser422, but inhibited it at Ser404 (compare lane 5 with lane 2). In contrast, the prephosphorylation had no detectable effect on tau phosphorylation by cdk5 at Thr181, Ser199, Thr205, Thr231 and Ser422; inhibited it at Ser202, Thr212, Thr217 and Ser404; and slightly promoted it at Ser396 (compare lane 6 with lane 1). The strong immunostaining of lanes 5 and 6 in pS214, pS262 and pS409 blots resulted from tau phosphorylation with PKA, because prephosphorylated tau without further treatment with GSK-3β or cdk5 had the same or even stronger staining with these antibodies (compare lanes 5 and 6 with lane 4). These results suggest that prephosphorylation of tau with PKA modulates its subsequent phosphorylation in both site-specific and kinase-specific manners.
To quantitate the site-specific effects of PKA-induced prephosphorylation on the subsequent phosphorylation of tau with GSK-3β and cdk5, we investigated the phosphorylation-time kinetics of the prephosphorylated and control-treated tau. Tau phosphorylation at each individual phosphorylation site at various time points was determined by an immuno-dot-blot assay using the phosphorylation-dependent and site-specific tau antibodies. We found that prephosphorylation of tau with PKA dramatically increased GSK-3β-catalyzed phosphorylation at Thr181, Ser199, Ser202, Thr205, Thr217, Thr231, Ser396 and Ser422, but inhibited the phosphorylation at Thr212 and Ser404 (Fig. 3), which is consistent with the Western blots shown in Fig. 2, except at Thr212. Because Ser214, Ser262 and Ser409 of tau are not phosphorylated by GSK-3β or cdk5 [11], the time kinetics of phosphorylation at these three sites were not studied by the immuno-dot-blot assay. It is interesting to note that the prephosphorylation had an opposite effect on GSK-3β–catalyzed tau phosphorylation at the adjacent sites Ser396 (stimulating) and Ser404 (inhibiting) (Figs. 2 and 3). The immunoreactivity of tau with PHF-1, a monoclonal antibody against the phosphorylated epitopes of both Ser396 and ser404 of tau [26], was also increased slightly after phosphorylation with PKA followed with GSK-3β (Fig. 3), suggesting that this increase in PHF-1 immunoreactivity was due to tau phosphorylation at Ser396. This phenomenon is consistent with the higher affinity of PHF-1 to phosphorylated Ser396 than to phosphorylated Ser404 of tau [26].
Fig. 3.
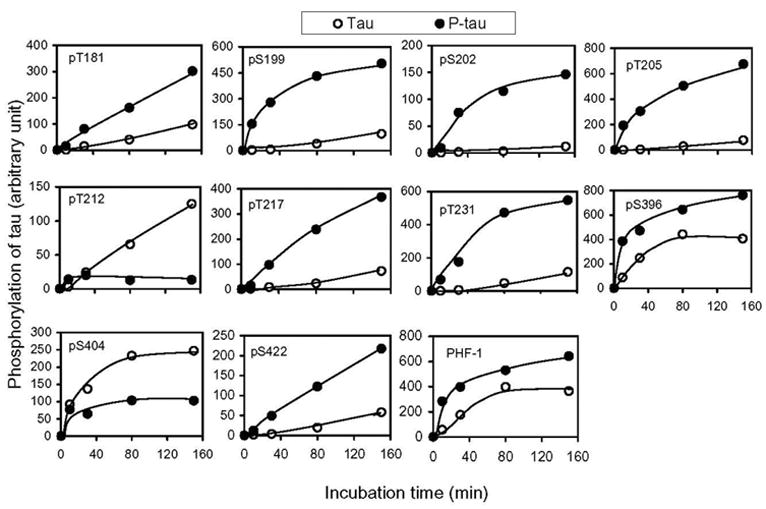
Quantitation of the effects of prephosphorylation by PKA on the subsequent phosphorylation at individual sites of tau by GSK-3β. Tau441 (Tau) and PKA-phosphorylated tau441 (P-tau) (0.2 mg/ml) were incubated with GSK-3β for various periods of time, and the phosphorylation level of tau at each individual site was determined by using an immuno-dot-blot assay developed with phosphorylation-dependent and site-specific tau antibodies, as indicated in each panel. All the data had been normalized with the amounts of tau protein, as determined by using phosphorylation-independent tau antibody R134d.
Time kinetic analysis of cdk5-catalyzed tau phosphorylation revealed that PKA-induced prephosphorylation had no significant effect on cdk5-catalyzed phosphorylation at Thr181, Ser199, Thr205, Thr231 or Ser422, but had an inhibitory effect on the phosphorylation at Ser202, Thr212, Thr217 and Ser404 (Fig. 4). The most marked inhibitory effect was seen at Thr212 and Ser404. The only exception was Ser396 of tau, the phosphorylation of which was slightly promoted by PKA-catalyzed prephosphorylation.
Fig. 4.
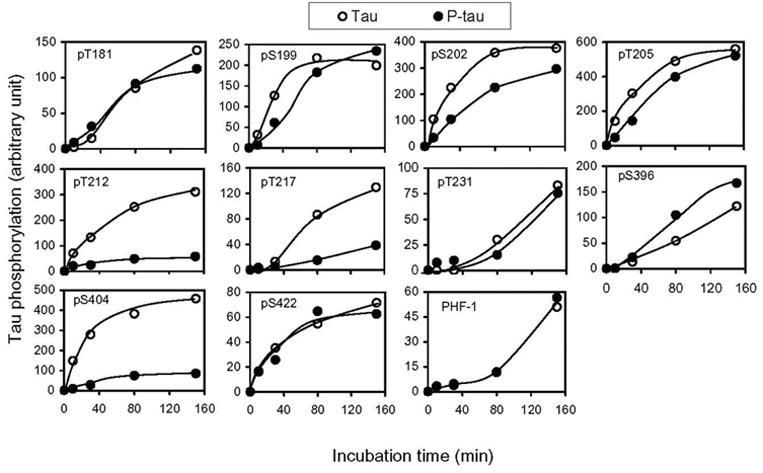
Quantitation of the effects of prephosphorylation of tau by PKA on the subsequent phosphorylation at individual sites by cdk5/p25. Tau441 (Tau) and PKA-phosphorylated tau441 (P-tau) (0.2 mg/ml) were incubated with cdk5/p25 for various periods of time, and the phosphorylation level of tau at each individual site was determined by using an immuno-dot-blot assay developed with phosphorylation-dependent and site-specific tau antibodies, as indicated in each panel. All the data had been normalized with the amounts of tau protein, as determined by using phosphorylation-independent tau antibody R134d.
4. Discussion
The largest isoform of tau (441 amino acids) in human brain contains 80 serine and threonine residues, and nearly half of them are phosphorylated to certain extents in AD brain. Because the substrate specificities of protein kinases are commonly highly selective toward the primary sequences around the acceptor serine and threonine residues, no single kinase can phosphorylate all the abnormal hyperphosphorylation sites of tau. Thus, more than one protein kinase must be involved in the regulation of tau phosphorylation and in the abnormal hyperphosphorylation of tau in AD brain. In addition, modifications of tau itself as a substrate protein, such as glycosylation [22,23,27], also affect tau phosphorylation. Regulation of tau phosphorylation appears to be more complex because phosphorylation of tau by one kinase modulates the subsequent phosphorylation by others [12–17], although the site specificity of such modulations has not been well studied. In the present study, we investigated such modulations by the three most likely tau kinase candidates, GSK-3β, cdk5 and PKA. To our knowledge, this is the first thorough study on the site-specific effects of PKA-catalyzed phosphorylation on the subsequent tau phosphorylation at more than ten individual phosphorylation sites catalyzed by GSK-3β and cdk5.
GSK-3β and cdk5 largely phosphorylate the same serine and threonine residues of tau (for comparison, see [11]), but we found in this study that its regulation by PKA-catalyzed prephosphorylation is very different. Prephosphorylation of tau by PKA primes further phosphorylation by GSK-3β at most of the phosphorylation sites, whereas it inhibits or has no effect on its subsequent phosphorylation by cdk5 at most of the phosphorylation sites. It appears that the priming effect is a characteristic of GSK-3β–catalyzed phosphorylation, because such an effect has been observed with other GSK-3β substrates such as glycogen synthase, the G-subunit and inhibitor-2 of protein phosphatase 1, β-catenin, and transcription factors CREB, eIF and NFAT [16,28,29]. However, the priming effect on GSK-3β–catalyzed tau phosphorylation does not follow the pattern with other substrates, in which the primed phosphorylation sites are four amino acids N-terminal to the prephosphorylation sites (Ser/Thr-X-X-X-pSer/pThr) [30,31]. The primed phosphorylation sites we observed in this study are not located at the residues four amino acids N-terminal to the PKA-induced tau phosphorylation sites (Ser214, Ser262 and Ser409). Because antibody against phosphoserine at positions 210 and 258 of tau, which are four amino acids N-terminal to Ser214 and Ser262, is not available, we do not know whether these two serine residues are phosphorylated by GSK-3β after prephosphorylation with PKA. Position 405 of tau is a proline and thus cannot be phosphorylated. Nevertheless, our results showing the primed tau phosphorylation at several phosphorylation sites outside the Ser/Thr-X-X-X-pSer/pThr motif suggest that PKA-catalyzed phosphorylation could induce a conformation change of tau that facilitates its further phosphorylation by GSK-3β at multiple sites. Our results indicate that the modulation of GSK-3β–catalyzed tau phosphorylation by prephosphorylation is site-specific. Actually, GSK-3β–catalyzed phosphorylation of tau at two of ten sites studied (Thr212 and Ser404) is inhibited rather than promoted by PKA-induced prephosphorylation.
The effects of prephosphorylation on cdk5-catalyzed phosphorylation of tau had not been investigated previously. We found that in contrast to GSK-3β, cdk5-catalyzed tau phosphorylation is either unaffected or inhibited (with the exception of Ser396) by prephosphorylation with PKA. Therefore, the modulations of tau phosphorylation by prephosphorylation are not only site-specific, but also kinase-specific. These results also suggest that despite the fact that these two kinases largely catalyze phosphorylation of the same residues of tau protein, they may have some different catalyzing mechanisms and play some different roles in the regulation of tau phosphorylation.
Our findings provide detailed insight into the regulation of GSK-3β- and cdk5-catalyzed phosphorylation of tau by its prephosphorylation with PKA. In our previous study, we found that injection of forskolin (a PKA activator) into the lateral ventricle of rat brain not only induced tau phosphorylation at PKA sites, but also at Tau-1 (Ser198/Ser199/Ser202) and PHF-1 (Ser396/Ser404) epitopes by the basal level of GSK-3β activity [17]. We further confirmed the involvement of GSK-3β in tau phosphorylation at the Tau-1 and PHF-1 epitopes, because co-injection of LiCl (a GSK-3β inhibitor) blocked forskolin-induced tau phosphorylation at these epitopes, whereas co-injection of ONU 112455A (an inhibitor of cdc2 and cdk5) failed to block it [17]. Although this previous study did not investigate individual GSK-3β-catalyzed phosphorylation sites of tau, it suggests that the regulation of GSK-3β- and cdk5-catalyzed phosphorylation of tau by its prephosphorylation with PKA, as we observed here in vitro, occurs in vivo. Such regulation may also have important physiological and pathological significance, because the primed phosphorylation by GSK-3β inhibits tau’s microtubule binding activity to a much larger extent than the non-primed phosphorylation [12,15] and, more importantly, impairs spatial memory of the rats [17].
In conclusion, we have investigated quantitatively the modulation of GSK-3β– and cdk5-catalyzed tau phosphorylation at individual phosphorylation sites by prephosphorylation with PKA. We found that such modulations are both site-specific and kinase-specific. Our studies provide novel insight into the mechanism of the complex regulation of tau phosphorylation by PKA, GSK-3β and cdk5.
Acknowledgments
We thank Dr. Jerry Wang of the Hong Kong University of Science and Technology, Hong Kong, China, for providing plasmids of cdk5 and p25, Ms. Maureen Marlow for editorial suggestions, and Ms. Janet Murphy and Ms. Sonia Warren for secretarial assistance. This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and Nantong University, and by grants from the National Institutes of Health (AG016760, AG019158, AG027429), the U.S. Alzheimer’s Association (NIRG-03-4721, IIRG-05-13095), the Natural Science Foundation of Jiangsu (BK2004047) and the Li Foundation, Inc.
Abbreviations
- AD
Alzheimer disease
- PKA
cAMP-dependent protein kinase
- cdk5
cyclin-dependent protein kinase 5
- GSK-3β
glycogen synthase kinase-3β
- NFT
neurofibrillary tangles
- PAGE
polyacrymide gel electrophoresis
- PHF
paired helical filaments
- P-tau
tau prephosphorylated by PKA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montejo de Garcini E, Serrano L, Avila J. Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun. 1986;141:790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K, Alonso A. del C., Chen S, Chohan MO, El-Akkad E, Gong C-X, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta. 2005;1739:198–210. doi: 10.1016/j.bbadis.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol (Berl) 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 6.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 7.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 8.Gong C-X, Liu F, Grundke-Iqbal I, Iqbal K. Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer disease: a therapeutic target. J Biomed Biotech. 2006 doi: 10.1155/JBB/2006/31825. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro K, Kobayashi S, Omori A, Takamatsu M, Yonekura S, Anzai K, Imahori K, Uchida T. Identification of the 23 kDa subunit of tau protein kinase II as a putative activator of cdk5 in bovine brain. FEBS Lett. 1994;342:203–208. doi: 10.1016/0014-5793(94)80501-6. [DOI] [PubMed] [Google Scholar]
- 11.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer’s disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 12.Cho JH, Johnson GV. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem. 2003;278:187–193. doi: 10.1074/jbc.M206236200. [DOI] [PubMed] [Google Scholar]
- 13.Sengupta A, Wu Q, Grundke-Iqbal I, Iqbal K, Singh TJ. Potentiation of GSK-3-catalyzed Alzheimer-like phosphorylation of human tau by cdk5. Mol Cell Biochem. 1997;167:99–105. doi: 10.1023/a:1006883924775. [DOI] [PubMed] [Google Scholar]
- 14.Singh TJ, Zaidi T, Grundke-Iqbal I, Iqbal K. Modulation of GSK-3-catalyzed phosphorylation of microtubule-associated protein tau by non-proline-dependent protein kinases. FEBS Lett. 1995;358:4–8. doi: 10.1016/0014-5793(94)01383-c. [DOI] [PubMed] [Google Scholar]
- 15.Wang JZ, Wu Q, Smith A, Grundke-Iqbal I, Iqbal K. Tau is phosphorylated by GSK-3 at several sites found in Alzheimer disease and its biological activity markedly inhibited only after it is prephosphorylated by A-kinase. FEBS Lett. 1998;436:28–34. doi: 10.1016/s0014-5793(98)01090-4. [DOI] [PubMed] [Google Scholar]
- 16.Welsh GI, Wilson C, Proud CG. GSK3: a SHAGGY frog story. Trends Cell Biol. 1996;6:274–279. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu SJ, Zhang JY, Li HL, Fang ZY, Wang Q, Deng HM, Gong C-X, Grundke-Iqbal I, Iqbal K, Wang JZ. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–50088. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- 18.Qi Z, Huang QQ, Lee KY, Lew J, Wang JH. Reconstitution of neuronal cdc2-like kinase from bacteria-expressed cdk5 and an active fragment of the brain-specific activator. J Biol Chem. 1995;270:10847–10854. doi: 10.1074/jbc.270.18.10847. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A. del C., Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer's disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem. 2001;276:37967–37973. doi: 10.1074/jbc.M105365200. [DOI] [PubMed] [Google Scholar]
- 20.Tatebayashi Y, Iqbal K, Grundke-Iqbal I. Dynamic regulation of expression and phosphorylation of tau by fibroblast growth factor-2 in neural progenitor cells from adult rat hippocampus. J Neurosci. 1999;19:5245–5254. doi: 10.1523/JNEUROSCI.19-13-05245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong CX, Shaikh S, Grundke-Iqbal I, Iqbal K. Inhibition of protein phosphatase-2B (calcineurin) activity towards Alzheimer abnormally phosphorylated tau by neuroleptics. Brain Res. 1996;741:95–102. doi: 10.1016/s0006-8993(96)00904-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Iqbal K, Grandke-Iqbal I, Gong CX. Involvement of aberrant glycosylation in phosphorylation of tau by cdk5 and GSK-3beta. FEBS Lett. 2002;530:209–214. doi: 10.1016/s0014-5793(02)03487-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Zaidi T, Grandke-Iqbal I, Iqbal K, Gong CX. Aberrant glycosylation modulates phosphorylation of tau by protein kinase A and dephosphorylation of tau by protein phosphatase 2A and 5. Neuroscience. 2002;115:829–837. doi: 10.1016/s0306-4522(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 24.Litersky JM, Johnson GV, Jakes R, Goedert M, Lee M, Seubert P. Tau protein is phosphorylated by cyclic AMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II within its microtubule-binding domains at Ser-262 and Ser-356. Biochem J. 1996;316:655–660. doi: 10.1042/bj3160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott CW, Spreen RC, Herman JL, Chow FP, Davison MD, Young J, Caputo C. Phosphorylation of recombinant tau by cAMP-dependent protein kinase: identification of phosphorylation sites and effect on microtubule assembly. J Biol Chem. 1993;268:1168–1173. [PubMed] [Google Scholar]
- 26.Otvos L, Jr, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;15:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW, Gong CX. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:10804–10809. doi: 10.1073/pnas.0400348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 29.Hagen T, Di Daniel E, Culbert AA, Reith AD. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J Biol Chem. 2002;277:23330–23335. doi: 10.1074/jbc.M201364200. [DOI] [PubMed] [Google Scholar]
- 30.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 31.Harwood AJ. Regulation of GSK-3: a cellular multiprocessor. Cell. 2001;105:821–824. doi: 10.1016/s0092-8674(01)00412-3. [DOI] [PubMed] [Google Scholar]


