Abstract
The amygdalar basolateral nuclear complex (BLC) has very high levels of the type 3 serotonin receptor (5-HT3R). Previous studies have reported that 5-HT3R protein in the BLC is expressed in interneurons and that 5-HT3R mRNA is coexpressed with GABA and certain neuropeptides or calcium-binding proteins in these cells. However, there have been no detailed descriptions of the distribution of 5-HT3R+ neurons in the amygdala, and no quantitative studies of overlap of neurons expressing 5-HT3R protein with distinct interneuronal subpopulations in the BLC. The present investigation employed dual-labeling immunohistochemistry using antibodies to the 5-HT-3A receptor subunit (5-HT3AR) and specific interneuronal markers to address these questions. These studies revealed that there was a moderate density of nonpyramidal 5-HT3AR+ neurons in the BLC at all levels of the amygdala. In addition, immunostained cells were also seen in anterior portions of the cortical and medial nuclei. Although virtually all 5-HT3AR+ neurons in the BLC were GABA+, very few expressed neuropeptide or calcium-binding protein markers for individual subpopulations. The main interneuronal marker expressed by 5-HT3AR+ neurons was cholecystokinin (CCK), but only 8–16% of 5-HT3R+ neurons in the BLC, depending on the nucleus, were CCK+. Most of these CCK+/5-HT3AR+ double-labeled neurons appeared to belong to the subpopulation of large type L CCK+ interneurons. Very few 5-HT3AR+ neurons expressed calretinin, vasoactive intestinal peptide, or parvalbumin, and none expressed somatostatin or calbindin. Thus, the great majority of neurons expressing 5-HT3AR protein appear to constitute a previously unrecognized subpopulation of GABAergic interneurons in the BLC.
Keywords: serotonin, GABA, peptides, calcium-binding proteins
The amygdala receives a robust serotonergic innervation from the dorsal raphe nucleus that targets all amygdalar nuclei, especially the basolateral nuclear complex (Moore et al., 1978; Steinbusch, 1981; Fallon and Ciofi, 1992; Sadikot and Parent, 1990; Abrams et al., 2005). Microdialysis studies indicate that there is increased serotonin (5-HT) release in the amygdala during behavioral arousal and stress (Kawahara et al., 1993; Rueter and Jacobs, 1996). In addition, certain genetic variations in human serotonin transporter and tryptophan hydroxylase genes, which presumably result in altered extracellular serotonin levels, are associated with increased activation of the amygdala by emotional stimuli, as well as anxiety and depression in these individuals (Canli et al., 2005; Hariri and Holmes, 2006).
Animal studies also suggest that serotonergic neurotransmission in the basolateral nuclear complex of the amygdala (BLC) plays a critical role in stress, anxiety, and depression, but the exact effects depend on the type of serotonin receptor activated (Zangrossi and Graeff, 1994; Graeff et al., 1996; Zangrossi et al., 1999; De Mello Cruz et al., 2005; Abrams et al., 2005; Inoue et al., 2004). The type 3 serotonin receptor (5-HT3R) is unique in that it is the only serotonin receptor that is a ligand-gated ion channel (Barns and Sharp, 1999; Chameau and van Hooft, 2006). Thus, unlike all other 5-HT receptors, which are G-protein-coupled receptors, 5-HT3Rs are associated with fast excitatory synaptic potentials in the BLC and other brain regions (Sugita et al., 1992; Chameau and van Hooft, 2006). Although several distinct 5-HT3R subunits have been described, only the 3A subunit is found in significant concentrations in the rodent CNS (Morales and Wang, 2002; Chameau and van Hooft, 2006). Receptor autoradiographic studies performed in both rat (Waeber et al., 1990; Barnes et al., 1990; Gehlert et al., 1991; Laporte et al., 1992; Steward et al., 1993) and human brains (Bufton et al., 1993; Abi-Darghem, 1993) demonstrate very high levels of 5-HT3R binding in the BLC. Injections of 5-HT3R antagonists into the rat amygdala are anxiolytic in most experimental tests of anxiety (Costall et al., 1989; Higgens et al., 1991; Nevins and Anthony, 1994; Gargiulo et al., 1996).
Since different subpopulations of BLC neurons exhibit specific connections (Muller et al., 2003, 2005, 2006a, 2006b), understanding the effects of serotonergic neurotransmission on information processing by the BLC will require knowledge of the expression of 5-HT3R, and other serotonin receptors, by distinct cell types. Previous studies have shown that there are two major cell classes in the BLC, pyramidal neurons and nonpyramidal neurons. Although these cells do not exhibit a laminar organization, their morphology, synaptology, electrophysiology, and pharmacology are remarkably similar to their counterparts in the cerebral cortex (Carlsen and Heimer, 1988; McDonald, 1992a; Washburn and Moises, 1992; Rainnie et al., 1993; Paré et al., 2003). Thus, pyramidal neurons in the BLC are projection neurons with spiny dendrites that utilize glutamate as an excitatory neurotransmitter, whereas most nonpyramidal neurons are spine-sparse interneurons that utilize GABA as an inhibitory neurotransmitter. Recent dual-labeling immunohistochemical studies suggest that the BLC contains at least four distinct subpopulations of GABAergic interneurons that can be distinguished on the basis of their content of calcium-binding proteins and peptides. These subpopulations are: 1) parvalbumin+/calbindin+ neurons, 2) somatostatin+/calbindin+ neurons, 3) small bipolar and bitufted interneurons that exhibit extensive colocalization of vasoactive intestinal peptide, calretinin, and cholecystokinin, and 4) large multipolar cholecystokinin+ neurons that are often calbindin+ (McDonald and Betette, 2001; McDonald and Mascagni, 2001a, 2002, Mascagni and McDonald, 2003; Kemppainen and Pitkänen, 2000).
The neuronal localization of 5-HT3R in the rat forebrain has been studied using in situ hydridization techniques (Tecott et al., 1993; Morales and Bloom, 1997). These studies have mainly focused on the neocortex and hippocampus, but have also commented on the amygdala. In both the cortex and BLC, mRNA for 5-HT3R is mainly expressed in a subpopulation of GABAergic interneurons that are immunoreactive for CCK (Morales and Bloom, 1997). However, the exact extent of overlap of 5-HT3R mRNA expressing neurons with GABA+ and CCK+ neurons in the BLC was not determined. It is also not clear to what extent BLC neurons that express 5-HT3R mRNA also express 5-HT3R protein.
The results of immunohistochemical studies of 5-HT3R protein localization are largely congruent with in situ hybridization studies of 5-HT3R mRNA (e.g., 5-HT3R+ neurons are nonpyramidal cells in cortex and BLC), although there are some discrepancies (Morales and Bloom, 1997; Morales et al., 1998). It has been suggested that the expression of 5-HT3R mRNA, but not 5-HT3R protein, in some neurons may be due to differences in rates of gene transcription, mRNA translation, or protein transport (Morales et al., 1998). Although the identification of neurons expressing 5-HT3R protein in the BLC is of obvious importance in understanding electrophysiological and behavioral aspects of serotonergic neurotransmission in this region, there is no detailed information regarding the exact distribution of 5-HT3R-immunoreactive neurons in the amygdala, or their overlap with interneuronal subpopulations identified using immunohistochemical techniques (Mascagni and McDonald, 2003). The present investigation is the first study to use dual-labeling immunohistochemistry to address these questions.
EXPERIMENTAL PROCEDURES
Tissue Preparation
A total of 13 male Sprague-Dawley rats (250–350g; Harlan, Indianapolis, IN) were used in this study. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of South Carolina. All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Rats were anesthetized with chloral hydrate (350 mg/kg) and perfused intracardially with phosphate buffered saline (PBS; pH 7.4) containing 0.5% sodium nitrite (50 ml) followed by 4.0% paraformaldehyde (or a 4.0% paraformaldehyde-0.2% glutaraldehyde mixture in two rats used for dual localization of 5-HT3R and GABA) in 0.1 M phosphate buffer at pH 7.4 (500 ml). Following perfusion, all brains were removed and postfixed for 3.5 hours in 4.0% paraformaldehyde. Brains were sectioned on a vibratome at a thickness of 50 μm in the coronal plane. Sections were processed for immunohistochemistry in wells of tissue culture plates. All antibodies were diluted in 0.1M PBS containing Triton X-100 (0.5%) and 1% normal goat serum.
Immunoperoxidase experiments
Localization of the 5-HT3A receptor subunit (5-HT3AR) was performed in 8 rats using the avidin-biotin immunoperoxidase (ABC) technique. A rabbit polyclonal antibody to the 5-HT3AR was raised against amino acids 444–457 of the large putative intracellular loop of the receptor (1:4000; antiserum 0165; generously donated by Dr. Marisela Morales, National Institute on Drug Abuse, Baltimore, Maryland). Sections were incubated in primary antibody overnight at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a rabbit Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Nickel-enhanced DAB (3, 3′-diaminobenzidine-4HCl, Sigma Chemical Co., St. Louis, MO) was used as a chromogen to generate a black reaction product (Hancock, 1986). Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in ethanols, cleared in xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA). In some animals adjacent sections were counterstained with cresyl violet to identify nuclear borders. Sections were analyzed using an Olympus BHA and Nikon Eclipse E600 microscopes. Digital light micrographs were taken with a QImaging MicroPublisher 5.0 CCD camera (QImaging Corp., Burnaby, British Columbia). Brightness and contrast were adjusted using Photoshop 6.0 software.
Immunofluorescence experiments
Dual localization of 5-HT3AR with GABA or protein/peptide interneuronal markers (parvalbumin [PV], calbindin [CB], somatostatin [SOM], calretinin [CR], cholecystokinin [CCK], and vasoactive intestinal peptide [VIP]) was investigated in 6 rats. In the two rats fixed with the paraformaldehyde/glutaraldehyde mixture, only dual localization of 5-HT3AR and GABA was investigated. In the remaining four rats, which were fixed with paraformaldehyde, localization of 5-HT3AR with 4–5 different protein/peptide interneuronal markers were investigated in parallel series of equally-spaced sections at 200–250 μm intervals. Sections were incubated in a cocktail of the rabbit 5-HT3AR antibody (1:1000) and one of the following monoclonal interneuronal marker antibodies overnight at 4° C: mouse anti-PV (1:2000, Sigma Chemical Co., St. Louis, MO), rat anti-SOM (1:400; Chemicon International, Temecula, CA), mouse anti-CR (1:3000, Chemicon), mouse anti-CCK (1:500, antibody 93903 generously donated by Dr. John H. Walsh, UCLA), mouse anti-VIP antibody (1:6000; antibody CURE-V55 generously donated by Dr. John H. Walsh, UCLA), mouse anti-CB antibody (1:8000, Chemicon), or mouse anti-GABA antibody (1:50; antibody 115-AD5-A9, generously donated by Dr. Ismo Virtanen, Univ. of Helsinki). After incubation in the primary antibody cocktail, sections were then rinsed in 3 changes of PBS (10 min each), and then incubated in a cocktail of species-appropriate Alexa-568 and Alexa-488 labeled secondary antibodies (1:400; Molecular Probes, Eugene, OR), for 3 hrs at room temperature. All secondary antibodies were highly cross-adsorbed by the manufacturer to ensure specificity for primary antibodies raised in particular species. In each of the confocal immunofluorescence cases some control sections were processed with one of the two primary antibodies omitted. In all cases only the color of the corresponding secondary fluorescent antibody was observed, and only on the appropriate channel. These results indicated that the secondary antibodies were specific for rabbit or mouse IgGs and that there was no “crosstalk” between the red and green channels (Wouterlood et al., 1998).
Sections were then rinsed in 3 changes of PBS (10 min each), mounted on glass slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and examined with a Bio-Rad MRC-1024 confocal laser scanning system equipped with an argon-krypton laser attached to a Nikon Optiphot fluorescence microscope. Fluorescence of Alexa 488 (green) and Alexa 568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm and 568 nm channels. Since GABA-like immunoreactivity was only seen on the surface of the section, analysis of the colocalization of GABA with the 5-HT3A receptor subunit was accomplished by focusing on the upper and lower surfaces of the section using the lowest iris aperture of the confocal microscope (which results in the thinnest “optical section”). This step was not necessary for the other experiments since in those cases immunostaining extended throughout the entire thickness of the section. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software.
The rat BLC consists of the lateral, basolateral, and basomedial nuclei. Each nucleus can be divided into two or more subdivisions. We studied distinct subdivisions of two of the main nuclei of the BLC, the anterior subdivision of the basolateral nucleus (BLa) and the ventromedial subdivision of the lateral nucleus (Lvm) (Paxinos and Watson, 1997), to determine if the phenotypic organization of 5-HT receptor-expressing neurons might differ in different regions of the BLC. Bilateral counts of single-labeled and double-labeled neurons in these nuclei were pooled from 2 animals for each colocalization combination studied (approximately 5–7 sections for each subdivision in each animal). At 200X magnification, cell counts were made from the image of a 400 μm X 400 μm field displayed for merged (red/green) channels on the computer screen (double-labeled cells appear yellow). Images of the non-merged red and green channels were also displayed. Depending on the size of the nuclear subdivision at different levels of the amygdala, counts were made from either one such field positioned in the center of the subdivision (and involving about 80–90% of its cross-sectional area), or two adjacent non-overlapping fields. Only somata of nonpyramidal neurons were counted. Although some pyramidal cells contain low levels of CR or CB, they were easily distinguished from the intensely-stained nonpyramidal neurons at the antibody dilutions used in this study (McDonald and Mascagni, 2001a). Since dendrites of BLC nonpyramidal neurons are less than 3 μm wide, it was not difficult to distinguish somata from dendrites.
Antibody Specificity
The polyclonal antibody to the 5-HT3A receptor (antibody 0165 of Morales et al., 1996) is an affinity purified antibody raised in rabbit against a synthetic peptide containing amino acids 444–457 of the receptor, coupled to bovine serum albumin using glutaraldehyde. This amino acid sequence is located in the large intracellular loop between transmembrane regions M3 and M4. This antibody immunolabeled both recombinant and neuronal 5-HT3AR protein at a band of 50 kDa in western blots. Studies on tissue sections combining immunohistochemistry and in situ hybridization revealed extensive colocalization of 5-HT3AR immunolabeling and 5-HT3AR transcripts (Morales et al., 1996).
The interneuronal marker antibodies used in this investigation have been characterized and used extensively in previous studies of the cerebral cortex and basolateral amygdala (Szabat et al., 1992; Conde et al., 1994; Kawaguchi and Kubota, 1996; McDonald, 1996; Wong et al., 1996; Sloviter et al., 2001; McDonald and Betette, 2001; McDonald and Mascagni, 2001a, 2002, 2004; McDonald et al. 2004; Mascagni and McDonald, 2003). Each antibody produced the characteristic pattern of labeling for each of the interneuronal subpopulations seen in previous studies.
RESULTS
5-HT3AR staining in the forebrain at the level of the amygdala appeared to be identical to that obtained in previous studies using this antibody (Morales et al., 1998). Thus, there was a moderate density of nonpyramidal 5-HT3AR+ neurons in the neocortex, hippocampus, and striatum, as well as in the basolateral nuclear complex (BLC) of the amygdala (Fig. 1). In addition, immunostained cells were also seen in anterior portions of the cortical and medial amygdalar nuclei. 5-HT3AR+ neurons were of similar morphology in all of these regions. Most immunostained neurons in the BLC had medium-sized somata (14–20 μm long and 8–12 μm wide) that were ovoid or fusiform in shape (Fig. 2). Perikaryal immunostaining varied from intense to very light. Some 5-HT3AR+ nonpyramidal neurons in the BLC exhibited 1–4 lightly stained proximal dendrites radiating from the soma, but in most immunostained neurons no staining was detectable in dendrites. There was also very light staining in many pyramidal neurons (Fig. 2), but it was not clear if this staining was specific or represented non-specific background.
Fig. 1.
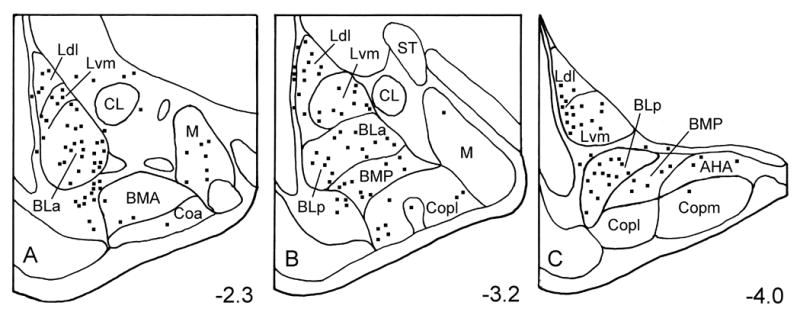
Distribution of 5-HT3AR+ neurons in the rat amygdala. Neurons were plotted from two 50 μm thick sections at each level of the amygdala (Bregma −2.3, −3.2, and −4.0 of Paxinos and Watson, 1997). A third section located between the two sections was stained with cresyl violet to identify nuclear borders. Abbreviations: AHA, amygdalohippocampal area; BLa, anterior subdivision of the basolateral nucleus; BLp, posterior subdivision of the basolateral nucleus; BMA, anterior basomedial nucleus; BMP, posterior basomedial nucleus; CL, lateral subdivision of the central nucleus; Coa, anterior cortical nucleus; Copl, posterolateral cortical nucleus; Copm, posteromedial cortical nucleus; Ldl, dorsolateral subdivision of the lateral nucleus; Lvm, ventromedial subdivision of the lateral nucleus; M, medial nucleus; ST, stria terminalis.
Fig. 2.
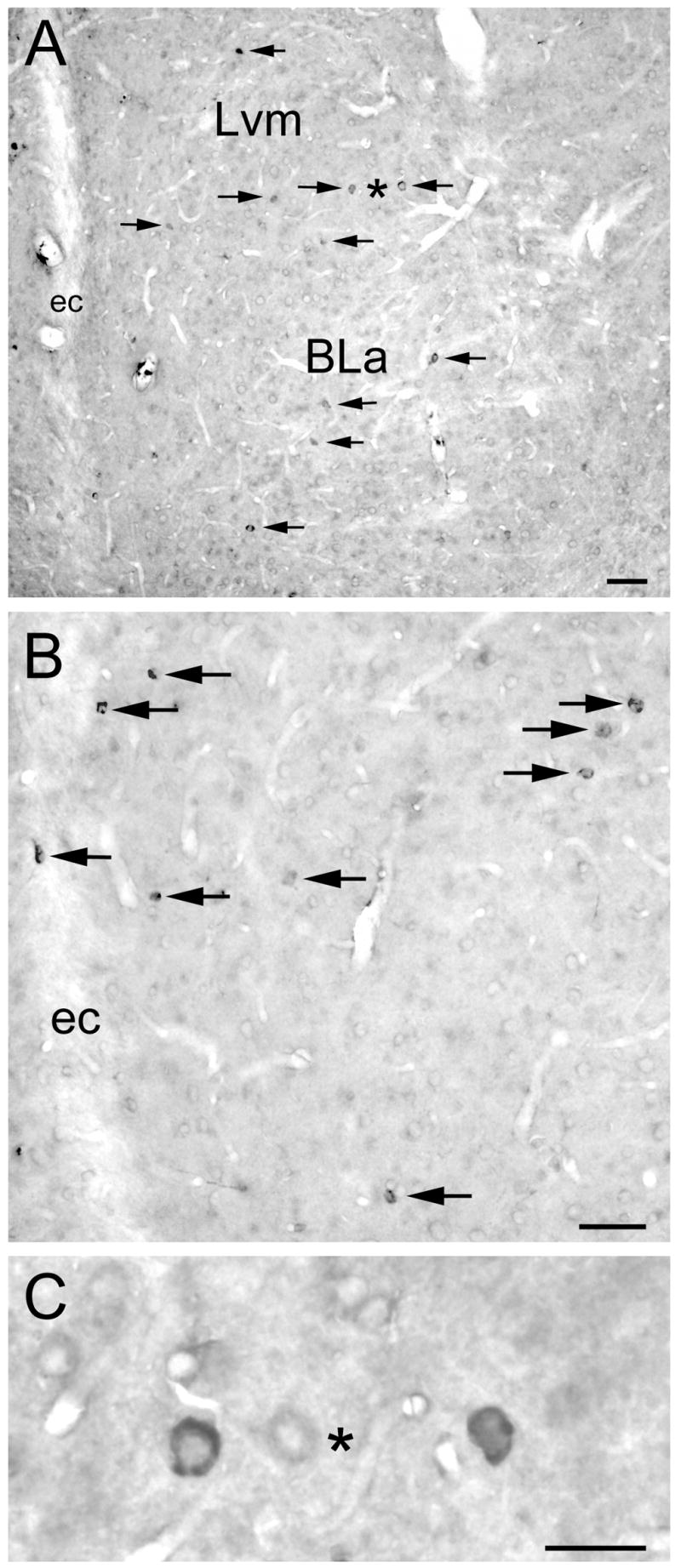
5-HT3AR immunoreactivity in the BLC. Arrows indicate immunostained cells, all of which appear to be nonpyramidal. A) Low power photomicrograph of the anterior subdivision of the basolateral nucleus (BLa) and the ventromedial subdivision of the lateral nucleus (Lvm) at the bregma −2.6 level (Paxinos and Watson, 1997). Asterisk is located between two immunostained neurons shown at higher magnification in C. ec, external capsule. Scale bar = 50 μm. B) Higher power photomicrograph of the Lvm at the bregma −3.3 level. Scale bar = 50 μm. C) High power photomicrograph of the two immunostained neurons indicated by an asterisk in A, but mediolateral orientation is flipped. Scale bar = 25 μm.
As in previous studies (Morales and Bloom, 1997; Morales et al., 1998), the morphology of 5-HT3AR+ neurons in the BLC suggested that these neurons were GABAergic interneurons. Dual-labeling confocal immunofluorescence studies were conducted to determine if this was the case, and to identify which interneuronal subpopulations were represented. Although virtually all 5-HT3AR+ neurons in the BLa and Lvm were GABA+, very few expressed neuropeptide or calcium-binding protein markers for individual subpopulations (Fig. 3; Table 1). The main subpopulation-specific interneuronal marker expressed by 5-HT3AR+ neurons was CCK, but only 16% of 5-HT3R+ neurons in the BLa, and 8% of 5-HT3AR+ neurons in the Lvm, were CCK+. Most of these CCK+/5-HT3AR+ double-labeled neurons appeared to belong to the subpopulation of larger type L CCK+ interneurons, rather than the subpopulation of small type S CCK+ subpopulation (Mascagni and McDonald, 2003; Fig. 3E, F). Very few 5-HT3R+ neurons expressed CR, VIP or PV, and none expressed SOM or CB.
Fig. 3.
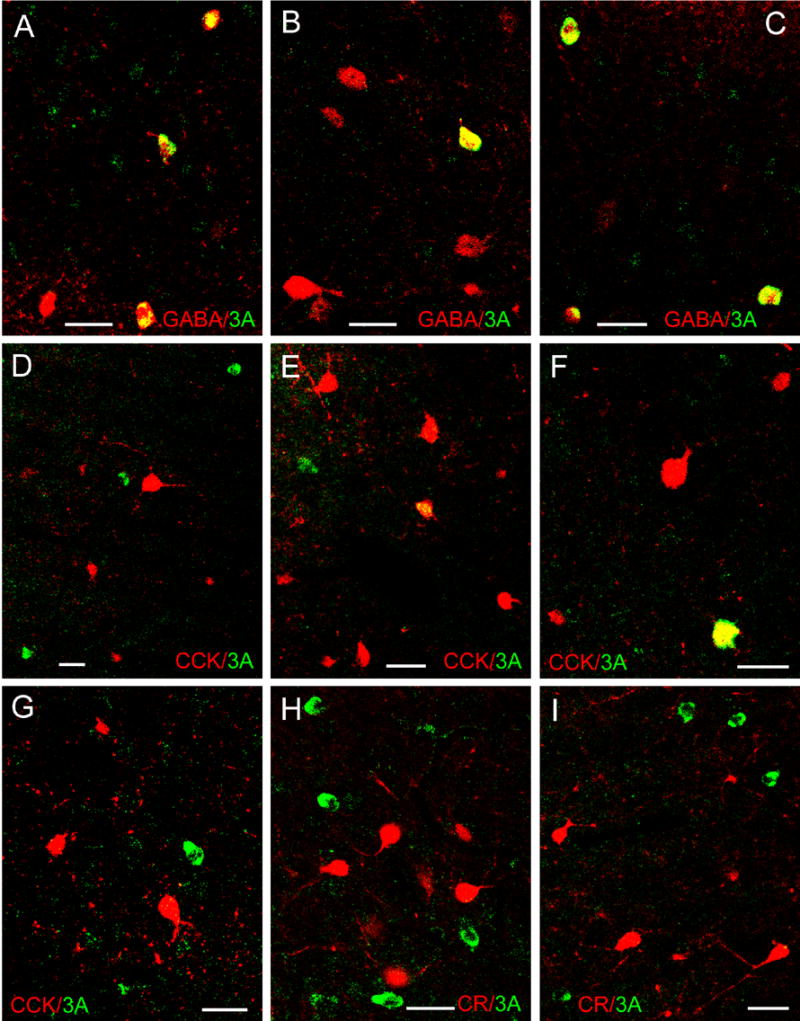
Dual localization of 5-HT3AR immunoreactivity (green) and interneuronal marker immunoreactivity (red) in the BLC using immunofluorescence confocal laser scanning microscopy. All images are merged images. A–C) Dual localization of 5-HT3AR (green) and GABA (red) immunoreactivity in the Lvm (A, C) and BLa (B). Note that all 5-HT3AR+ neurons in these fields are double-labeled (yellow). D–G) Dual localization of 5-HT3AR (green) and CCK (red) immunoreactivity in the Lvm (D, E) and BLa (F, G). Note that most CCK+ neurons are either large (type L neurons) or small (type S neurons). Most single-labeled 5-HT3AR+ neurons (green) are medium-sized or small, whereas double-labeled 5-HT3AR+/CCK+ neurons (yellow) are large. (H–I) Dual localization of 5-HT3AR (green) and CR (red) immunoreactivity in the BLa (H) and Lvm (I). Although both 5-HT3AR+ neurons and CR+ neurons are robustly labeled, there are no double-labeled 5-HT3AR+/CR+ neurons in these fields. All scale bars = 25 μm.
Table 1.
Colocalization of 5-HT3AR and interneuronal markers in the basolateral amygdala.
| Marker | Nucleus | 5-HT3AR+Single-labeled Neurons | 5-HT3AR+/Marker+Double-labeled Neurons | Percentage of 5-HT3AR+ Neurons Double-labeled |
|---|---|---|---|---|
| GABA | BLa
Lvm |
0
1 |
32
31 |
100% (32/32)
96.9% (31/32) |
| CCK | BLa
Lvm |
47
57 |
9
5 |
16.1% (9/56)
8.1% (5/62) |
| CR | BLa
Lvm |
49
48 |
1
2 |
2.0% (1/50)
4.0% (2/50) |
| PV | BLa
Lvm |
50
30 |
4*
0 |
7.4% (4/54)
0.0% (0/30) |
| SOM | BLa
Lvm |
30
30 |
0
0 |
0.0% (0/30)
0.0% (0/30) |
| CB | BLa
Lvm |
30
30 |
0
0 |
0.0% (0/30)
0.0% (0/30) |
| VIP | BLa
Lvm |
30
57 |
0
1 |
0.0% (0/30)
1.7% (1/58) |
All four neurons were very lightly stained for 5-HT3AR
Additional cell counts were performed in the two 5-HT3AR/GABA preparations and in two 5-HT3AR/CCK preparations to determine the percentage of GABA+ and CCK+ interneurons that were 5-HT3AR+. These analyses revealed that 5-HT3AR+ neurons constituted 6.7% (18/269) of GABA+ neurons in the BLa, and 12.2% (32/262) of GABA+ neurons in the Lvm. 5-HT3AR+ neurons constituted 10.7% (9/84) of CCK+ neurons in the BLa, and 6.3% (5/80) of CCK+ neurons in the Lvm.
DISCUSSION
This is the first study to use double-labeling immunohistochemistry to study the phenotypes of 5-HT3AR+ neurons in the BLC. As suggested in a previous immunohistochemical study (Morales et al., 1998), 5-HT3AR+ neurons in the amygdala were nonpyramidal interneurons that were mainly located in the BLC. The present investigation demonstrated that virtually all neurons in the BLC that expressed 5-HT3AR protein were GABA-immunoreactive, consistent with a previous report that 70–95% of telencephalic neurons expressing mRNA for 5-HT3AR were GABAergic (Morales and Bloom, 1997). Surprisingly, however, the present study found that there was little overlap of 5-HT3AR+ neurons with the four main subpopulations of immunohistochemically-identifed GABAergic interneurons in the BLC (Mascagni and McDonald, 2003). These results contrast with recent studies of GluR1, GABAA, GABAB, substance P, and cannabinoid receptors in the BLC, which demonstrated differential expression of these receptors among one or more of the four main interneuronal subpopulations (McDonald, 1996; McDonald and Mascagni, 2001b, 2004; McDonald et al., 2004; Levita et al., 2003). Thus, the great majority of neurons expressing 5-HT3AR protein appear to constitute a previously unrecognized subpopulation of medium-sized interneurons in the BLC.
The only BLC interneuronal subpopulation to exhibit significant 5-HT3AR protein expression was the subpopulation of large CCK+ interneurons (Type L CCK+ neurons, Mascagni and McDonald, 2003); 16% of 5-HT3AR+ neurons in the basolateral nucleus and 8% of 5-HT3AR+ neurons in the lateral nucleus were CCK+. This is consistent with a previous study which reported colocalization of CCK peptide and 5-HT3AR mRNA, but the extent of colocalization was not quantitated in the BLC (Morales and Bloom, 1997). It is of interest that no calbindin-positive (CB+) neurons in the BLC were 5-HT3AR+, since approximately one-third of type L CCK+ interneurons are CB+. Likewise, Morales and Bloom (1997) found little colocalization of 5-HT3AR mRNA and CB in the BLC, with the exception of an occasional neuron in the basomedial nucleus. There is colocalization of CCK with calretinin (CR) and VIP in a subpopulation of small CCK+ cells in the BLC (type S CCK+ cells); the finding in the present study of little CR/5-HT3AR or VIP/5-HT3AR colocalization provides further evidence that it is the type L CCK+ neurons that express 5-HT3AR protein. Dual-labeling immunohistochemical studies have shown that many of the large type L CCK+ interneurons, but none of the small type S CCK+ cells, exhibit CB1 cannabinoid receptor immunoreactivity (McDonald and Mascagni, 2001b; Katona et al., 2001). Consistent with these immunohistochemical findings, there is extensive colocalization of 5-HT3AR and CB1 receptor mRNA in neurons of the BLC (Hermann et al., 2002; Morales et al., 2004). However, when plots of neurons expressing 5-HT3A and CB1 receptor mRNA in the BLC (see Fig. 1 of Morales et al., 2004) are compared with plots of neurons expressing 5-HT3A and CB1 receptor protein (see Fig. 1 of the present study; Fig. 3 of McDonald and Mascagni, 2001b), it is clear that the density of neurons expressing mRNA for these proteins is much higher than that of neurons expressing the actual proteins. This discrepancy may be due to a variety of factors including low rates of mRNA translation or rapid transport of these proteins from the cell body in some neurons.
Since immunohistochemical studies in the rat neocortex have demonstrated interneuronal subpopulations that are very similar to those in the BLC (Kubota et al., 1994; Kubota and Kawaguchi, 1997), it is of interest to compare patterns of the 5-HT3AR distribution in these two regions. As in the BLC, there is colocalization of 5-HT3AR mRNA with CCK immunoreactivity in the cortex (Morales and Bloom, 1997). However, unlike the BLC, the degree of colocalization has been quantitated for the cortex. Although the extent of colocalization varies in different cortical areas, on average about 50% of neurons expressing 5-HT3AR mRNA are CCK-immunoreactive. This greater degree of expression of 5-HT3AR mRNA expression in CCK+ cortical neurons, compared with the extent of 5-HT3AR protein expression in CCK+ BLC neurons, may in part be related to the overall greater density of neurons expressing 5-HT3AR mRNA versus protein (see above). One major difference in the neocortex was the extensive colocalization of 5-HT3AR mRNA with calbindin (CB) immunoreactivity; between 33 and 63% of CB+ cortical neurons expressed 5-HT3AR mRNA, depending on the cortical area (Morales and Bloom, 1997). This was not seen in the BLC (see above). A dual-labeling study of the primate neocortex using the same 5-HT3AR antibody used in the present study demonstrated 5-HT3AR immunoreactivity in subpopulations of small interneurons exhibiting GABA, CR, CB and substance P receptor immunoreactivity, although no quantitative data was collected and colocalization with CCK was not attempted (Jakab and Goldman-Rakic, 2000); the findings of colocalization of 5-HT3AR with GABA, CR, and CB is consistent with the 5-HT3AR mRNA colocalization findings in rat neocortex (Morales and Bloom, 1997).
The 5-HT3AR antibody used in the present study primarily stains cell bodies, where immunoreactivity is mainly confined to the nuclear envelope and granular endoplasmic reticulum (Morales and Bloom 1996). This pattern of staining has suggested that the antibody mainly labels unassembled subunits of the receptor rather than the assembled functional receptor in the plasma membrane (Morales et al., 1998). Thus, while the antibody provides a useful marker for identifying neurons that express the 5-HT3AR protein, it cannot be used to precisely localize the site of the functional receptor. Using another 5-HT3AR antibody that does appear to localize the assembled receptor, Miquel et al. (2002) found that 5-HT3AR immunoreactivity was distributed in both dendritic and axonal processes in the amygdala. This localization pattern is consistent with electrophysiological studies showing both postsynaptic and presynaptic actions of 5-HT at 5-HT3A receptors in the BLC (see below).
Although previous immunohistochemical and in situ hydridization investigations suggested that only GABAergic interneurons in the BLC express 5-HT3ARs (Morales and Bloom 1996, Morales and Bloom 1997, Morales et al., 1998) electrophysiological studies indicate that pyramidal cells in the BLC may also possess these receptors (but see Koyama et al., 2000). Thus, it has been reported that 40% of neurons in the lateral nucleus exhibited postsynaptic excitation mediated by 5-HT3Rs (Sugita et al., 1992), whereas GABAergic interneurons are thought to constitute less than one-quarter of BLC neurons (McDonald, 1985, 1992b). Likewise, in another study 53% of neurons in the BLC and cortical nuclei were excited by a putative 5-HT3R mechanism, suggesting that pyramidal neurons as well as interneurons in the BLC might express 5-HT3Rs (Stein et al., 2000). In the present study, and also in a previous study using the same antibody (see Fig. 10 of Morales et al., 1998), there was light staining of pyramidal cells. However, it remains to be determined whether this represents light immunostaining or non-specific background.
To date there have been no studies of 5-HT3R-mediated postsynaptic responses in identified interneurons in the BLC. However, there is abundant evidence in the neocortex (Ferezou et al., 2002; Xiang and Prince, 2003; Puig et al., 2004) and hippocampus (Ropert and Guy, 1991; Kawa, 1994; McMahon and Kauer, 1997; Sudweeks et al., 2002) that 5-HT can indirectly inhibit pyramidal cells by activating postsynaptic interneuronal 5-HT3Rs. Since electron microscopic studies in the BLC indicate that PV+, VIP+, and somatostatin+ interneurons in the BLC provide a robust inhibitory innervation of pyramidal cells (Muller et al., 2003a, 2003b, 2006), it seems likely that the CCK+ and non-CCK+ interneurons in the BLC that express 5-HT3ARs (present study) have similar connections and will inhibit BLC pyramidal cells subsequent to 5-HT3AR-mediated postsynaptic excitation of these interneurons. There is also evidence that 5-HT can increase GABA release from BLC interneurons via a 5-HT3R-mediated presynaptic action on interneuronal axon terminals synapsing with pyramidal cells (Koyama et al., 2000). Thus, much of the 5-HT3AR protein found in the cell bodies of BLC interneurons may ultimately be transported to the axon terminals of these neurons, consistent with electron microscopic studies (Miquel et al., 2002). For the type L CCK+ neurons in the BLC that exhibit 5-HT3AR and CB1 receptor immunoreactivity, this is just one of several possible receptor-mediated mechanisms modulating release from these terminals, since activation of CB1 cannabinoid receptors on these terminals decreases GABA release (Katona et al., 2001). Moreover, it is possible that 5-HT3Rs on these terminals also increase CCK release, since this effect is seen in the neocortex (Paudice and Raiteri, 1991; Raiteri et al., 1993).
In summary, some of the neurons expressing 5-HT3AR subunit protein in the BLC belong to a subpopulation of large GABAergic interneurons that are CCK+ (type L CCK+ interneurons), but most appear to constitute a previously unrecognized subpopulation of medium-sized GABAergic interneurons. In the awake state typical serotonergic neurons in the raphe fire in a clock-like manner at about 1–2 Hz, depending on the state of behavioral arousal (Jacobs and Azmitia, 1992). Thus, 5-HT3AR+ interneurons in the BLC would presumably be rhythmically excited at these frequencies and ultimately inhibit neighboring pyramidal neurons. Although inhibition of BLC pyramidal neurons is usually associated with reduction in fear and anxiety (Davis et al., 1994), previous behavioral studies suggest that activation of 5-HT3Rs has the opposite effect in most experimental tests of anxiety (Costall et al., 1989; Higgens et al., 1991; Nevins and Anthony, 1994; Gargiulo et al., 1996). This suggests that interneurons expressing 5-HT3ARs may have inhibitory connections with other GABAergic interneurons, similar to VIP and PV subpopulations in the BLC (Muller et al., 2003, 2005). Activation of these interneuronal networks via a 5-HT3AR-mediated mechanism could then result in disinhibition of pyramidal cells. Further study of the basic electrophysiological properties of 5-HT3R+ interneurons, and how these characteristics, as well as the network properties of the BLC (e.g., Rainnie, 1999) are altered by 5-HT will be required to understand the role of amygdalar 5-HT3 receptors in emotion.
Fig. 4.
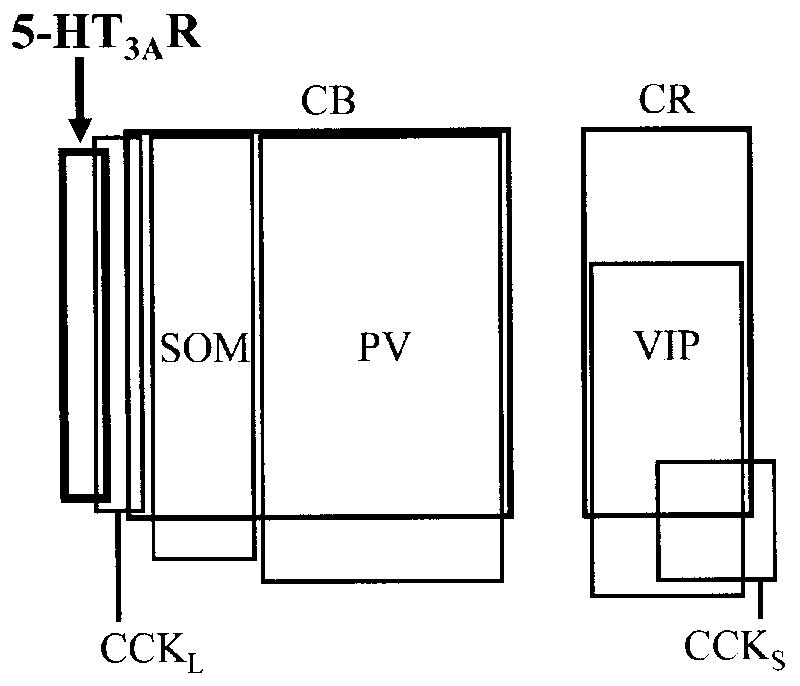
Diagram showing the overlap and relative proportions of neuronal populations containing calcium-binding proteins, neuropeptides, and 5-HT3AR in the anterior subdivision of the basolateral nucleus (BLa). The relationship of the 5-HT3AR+ subpopulation to the other interneuronal subpopulations is similar in the lateral nucleus. Thick lines indicate the 5-HT3AR + subpopulation. Lines of intermediate thickness indicate two populations of calcium-binding proteins (CB+ and CR+ neurons), each of which is comprised of several subpopulations. Data on the relationships among SOM+, PV+, CB+, CR+, VIP, and CCK+ neurons is taken from previous quantitative studies (for a review see Mascagni and McDonald, 2003); the relative sizes of the rectangles representing these subpopulations are depicted in relation to the total population of GABA+ neurons in each nucleus (see Fig. 3 of McDonald and Mascagni, 2002). Note that the 5-HT3AR+ subpopulation is relatively small and slightly overlaps the subpopulation of large type L CCK+ neurons (CCKL) that do not contain CB. In the present study it appeared that none of the 5-HT3AR+ neurons corresponded to the subpopulation of small type S CCK+ neurons (CCKS).
Acknowledgments
We are very grateful for the generous donation of the antibody to the 5-HT3AR subunit (antiserum 0165) by Dr. Marisela Morales (National Institute on Drug Abuse, Baltimore, Maryland), the mouse anti-CCK antibody (antibody #9303) and mouse anti-VIP antibody (antibody CURE-V55) by Dr. John Walsh (CURE/Digestive Diseases Research Center, Antibody/RIA Core, NIH Grant #DK41301, Los Angeles, CA), and the mouse anti-GABA antibody (antibody 115-AD5-A9) by Dr. Ismo Virtanen (University of Helsinki, Finland). The authors would also like to thank Dr. Marisela Morales (National Institute on Drug Abuse), Dr. Donald Rainnie (Emory University School of Medicine), and Dr. Jay Muller (University of South Carolina School of Medicine) for their critical reading of an earlier draft of this manuscript. This work was supported by NIH Grant R01-NS38998.
Abbreviations
- 5-HT
serotonin
- 5-HT3R
serotonin type 3 receptor
- 5-HT3AR
serotonin type 3A receptor subunit
- AHA
amygdalohippocampal area
- BLa
anterior subdivision of the basolateral amygdalar nucleus
- BLp
posterior subdivision of the basolateral amygdalar nucleus
- BLC
basolateral nuclear complex of the amygdala
- BMA
anterior basomedial nucleus
- BMP
posterior basomedial nucleus
- CB
calbindin
- CCK
cholecystokinin
- CL
lateral subdivision of the central nucleus
- Coa
anterior cortical nucleus
- Copl
posterolateral cortical nucleus
- Copm
posteromedial cortical nucleus
- CR
calretinin
- GABA
gamma aminobutyric acid
- Ldl
dorsolateral subdivision of the lateral amygdalar nucleus
- Lvm
ventromedial subdivision of the lateral amygdalar nucleus
- M
medial nucleus
- PV
parvalbumin
- SOM
somatostatin
- ST
stria terminalis
- VIP
vasoactive intestinal polypeptide
Footnotes
Section Editor: Charles Gerfen (Neuroanatomy)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H] LY278584 binding sites in human brain. J Neurochem. 1993;60:730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Champaneria S, Costall B, Naylor RJ. Characterisation and autoradiographic localisation of 5-HT3 receptor recognition sites identified with [3H]-(S)-zacopride in the forebrain of the rat. Neuropharmacology. 1990;29:1037–1045. doi: 10.1016/0028-3908(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bufton KE, Steward LJ, Barber PC, Barnes NM. Distribution and characterization of the [3H]granisetron-labelled 5-HT3 receptor in the human forebrain. Neuropharmacology. 1993;32:1325–1331. doi: 10.1016/0028-3908(93)90027-z. [DOI] [PubMed] [Google Scholar]
- Canli T, Congdon E, Gutknecht L, Constable RT, Lesch KP. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J Neural Transm. 2005;112:1479–1485. doi: 10.1007/s00702-005-0391-4. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Chameau P, van Hooft JA. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res. 2006 doi: 10.1007/s00441-006-0255-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Neuroanatomical sites of action of 5-HT3 receptor agonist and antagonists for alteration of aversive behaviour in the mouse. Br J Pharmacol. 1989;96:325–332. doi: 10.1111/j.1476-5381.1989.tb11821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- de Mello Cruz AP, Pinheiro G, Alves SH, Ferreira G, Mendes M, Faria L, Macedo CE, Motta V, Landeira-Fernandez J. Behavioral effects of systemically administered MK-212 are prevented by ritanserin microinfusion into the basolateral amygdala of rats exposed to the elevated plus-maze. Psychopharmacology (Berl) 2005;182:345–354. doi: 10.1007/s00213-005-0108-2. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Ciofi P. Distribution of monoamines within the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992. pp. 97–114. [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo PA, Viana MB, Graeff FG, Silva MA, Tomaz C. Effects of anxiety and memory of systemic and intra-amygdala injection of 5-HT3 receptor antagonist BRL 46470A. Neuropsychobiology. 1996;33:189–195. doi: 10.1159/000119276. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Wong DT, Robertson DW. Localization of 5-HT3 receptors in the rat brain using [3H] LY278584. Brain Res. 1991;553:149–154. doi: 10.1016/0006-8993(91)90242-n. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;75:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Jones BJ, Oakley NR, Tyers MB. Evidence that the amygdala is involved in the disinhibitory effects of 5-HT3 receptor antagonists. Psychopharmacology (Berl) 1991;104:545–551. doi: 10.1007/BF02245664. [DOI] [PubMed] [Google Scholar]
- Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S, Koyama T. Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol. 2004;497:311–316. doi: 10.1016/j.ejphar.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: a patch-clamp study. J Neurophysiol. 1994;71:1935–1947. doi: 10.1152/jn.1994.71.5.1935. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol. 2000;529:373–383. doi: 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Two distinct subgroups of cholecystokinin-immunoreactive cortical interneurons. Brain Res. 1997;752:175–183. doi: 10.1016/s0006-8993(96)01446-1. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H. Quantitative autoradiographic mapping of 5-HT3 receptors in the rat CNS using [125I]iodo-zacopride and [3H]zacopride as radioligands. Synapse. 1992;10:271–281. doi: 10.1002/syn.890100402. [DOI] [PubMed] [Google Scholar]
- Levita L, Mania I, Rainnie DG. Subtypes of substance P receptor immunoreactive interneurons in the rat basolateral amygdala. Brain Res. 2003;981:41–51. doi: 10.1016/s0006-8993(03)02870-1. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Immunohistochemical identification of gamma-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. New York: Wiley-Liss; 1992a. pp. 67–96. [Google Scholar]
- McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull. 1992b;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett. 1996;208:175–178. doi: 10.1016/0304-3940(96)12585-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001a;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001b;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Parvalbumin-containing interneurons in the basolateral amygdala express high levels of the alpha1 subunit of the GABAA receptor. J Comp Neurol. 2004;473:137–146. doi: 10.1002/cne.20101. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Muller JF. Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdala. Brain Res. 2004;1018:147–158. doi: 10.1016/j.brainres.2004.05.053. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Emerit MB, Nosjean A, Simon A, Rumajogee P, Brisorgueil MJ, Doucet E, Hamon M, Verge D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur J Neurosci. 2002;15:449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [DOI] [PubMed] [Google Scholar]
- Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180:417–438. doi: 10.1002/cne.901800302. [DOI] [PubMed] [Google Scholar]
- Morales M, Battenberg E, de Lecea L, Sanna PP, Bloom FE. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Brain Res Mol Brain Res. 1996;36:251–60. doi: 10.1016/0169-328x(96)88406-3. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402:385–401. [PubMed] [Google Scholar]
- Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, Jho DH. Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. J Comp Neurol. 2004;468:205–216. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25:7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006a;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2006b doi: 10.1002/cne.21185. In Press. [DOI] [PubMed] [Google Scholar]
- Nevins ME, Anthony EW. Antagonists at the serotonin-3 receptor can reduce the fear-potentiated startle response in the rat: evidence for different types of anxiolytic activity? J Pharmacol Exp Ther. 1994;268:248–54. [PubMed] [Google Scholar]
- Paré D, Royer S, Smith Y, Lang EJ. Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci. 2003;985:78–91. doi: 10.1111/j.1749-6632.2003.tb07073.x. [DOI] [PubMed] [Google Scholar]
- Paudice P, Raiteri M. Cholecystokinin release mediated by 5-HT3 receptors in rat cerebral cortex and nucleus accumbens. Br J Pharmacol. 1991;103:1790–1794. doi: 10.1111/j.1476-5381.1991.tb09864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Puig MV, Santana N, Celada P, Mengod G, Artigas F. In vivo excitation of GABA interneurons in the medial prefrontal cortex through 5-HT3 receptors. Cereb Cortex. 2004;14:1365–1375. doi: 10.1093/cercor/bhh097. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Intracellular recordings from morphologically identified neurons of the basolateral amygdala. J Neurophysiol. 1993;69:1350–1361. doi: 10.1152/jn.1993.69.4.1350. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Paudice P, Vallebuona F. Inhibition by 5-HT3 receptor antagonists of release of cholecystokinin-like immunoreactivity from the frontal cortex of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:111–114. doi: 10.1007/BF00168781. [DOI] [PubMed] [Google Scholar]
- Ropert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J Physiol. 1991;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–447. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABA(B) (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Stein C, Davidowa H, Albrecht D. 5-HT(1A) receptor-mediated inhibition and 5-HT(2) as well as 5-HT(3) receptor-mediated excitation in different subdivisions of the rat amygdala. Synapse. 2000;38:328–337. doi: 10.1002/1098-2396(20001201)38:3<328::AID-SYN12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steward LJ, West KE, Kilpatrick GJ, Barnes NM. Labelling of 5-HT3 receptor recognition sites in the rat brain using the agonist radioligand [3H]meta-chlorophenylbiguanide. Eur J Pharmacol. 1993;243:13–18. doi: 10.1016/0014-2999(93)90161-a. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Hooft JA, Yakel JL. Serotonin 5-HT(3) receptors in rat CA1 hippocampal interneurons: functional and molecular characterization. J Physiol. 2002;544:715–726. doi: 10.1113/jphysiol.2002.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Shen KZ, North RA. 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992;8:199–203. doi: 10.1016/0896-6273(92)90121-s. [DOI] [PubMed] [Google Scholar]
- Szabat E, Soinila S, Happola O, Linnala A, Virtanen I. A new monoclonal antibody against the GABA-protein conjugate shows immunoreactivity in sensory neurons of the rat. Neuroscience. 1992;47:409–420. doi: 10.1016/0306-4522(92)90255-z. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci U S A. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Pinkus LM, Palacios JM. The (S)-isomer of [3H]zacopride labels 5-HT3 receptors with high affinity in rat brain. Eur J Pharmacol. 1990;181:283–287. doi: 10.1016/0014-2999(90)90090-s. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Sternini C, Lloyd K, De Giorgio R, Walsh JH. Monoclonal antibody to VIP: production, characterization, immunoneutralizing activity, and usefulness in cytochemical staining. Hybridoma. 1996;15:133–139. doi: 10.1089/hyb.1996.15.133. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Van Denderen JC, Blijleven N, Van Minnen J, Hartig W. Two-laser dual-immunofluorescence confocal laser scanning microscopy using Cy2- and Cy5-conjugated secondary antibodies: unequivocal detection of co-localization of neuronal markers. Brain Res Brain Res Protoc. 1998;2:149–159. doi: 10.1016/s1385-299x(97)00038-x. [DOI] [PubMed] [Google Scholar]
- Zangrossi H, Jr, Graeff FG. Behavioral effects of intra-amygdala injections of GABA and 5-HT in the elevated plus-maze. Braz J Med Biol Res. 1994;27:2453–2456. [PubMed] [Google Scholar]
- Zangrossi H, Jr, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino)tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369:267–270. doi: 10.1016/s0014-2999(99)00075-8. [DOI] [PubMed] [Google Scholar]


