Abstract
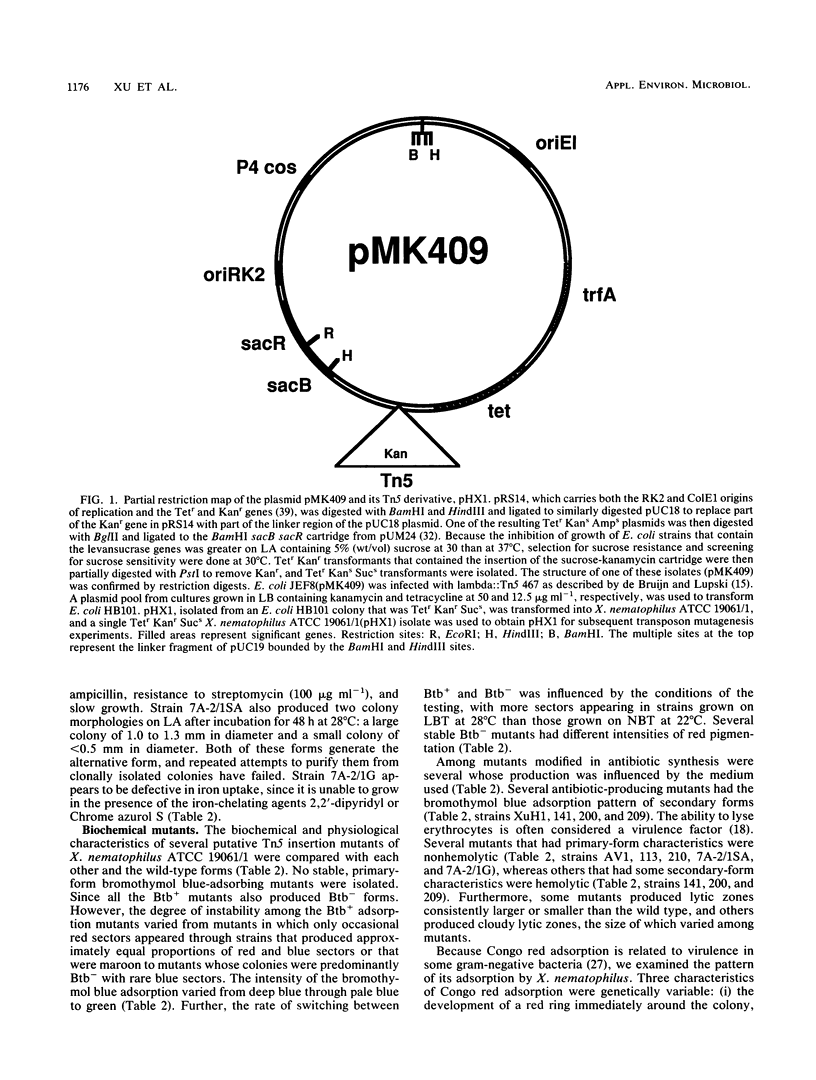
A negative-selection vector, pHX1, was constructed for use in transposon mutagenesis of Xenorhabdus nematophilus ATCC 19061. pHX1 contains the Bacillus subtilis levansucrase gene which confers sucrose sensitivity. In addition, various Tn5-containing plasmids with different replication origins were transferred by conjugation from Escherichia coli into X. nematophilus ATCC 19061, and one of these plasmids, pGS9, yields Tn5 insertion mutants of X. nematophilus ATCC 19061. By using these two delivery vehicles, more than 250 putative Tn5 insertion mutants of X. nematophilus ATCC 19061 were isolated and were then characterized. Mutants that were altered in bromothymol blue adsorption, ability to lyse sheep erythrocytes, production of antibiotics on a variety of media, and virulence for Galleria mellonella were found.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J. Neoaplectana species: specificity of association with bacteria of the genus Xenorhabdus. Exp Parasitol. 1983 Apr;55(2):258–263. doi: 10.1016/0014-4894(83)90020-6. [DOI] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Cloning the gene for Congo red binding in Shigella flexneri. Infect Immun. 1985 Apr;48(1):165–168. doi: 10.1128/iai.48.1.165-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C. I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985 Nov;164(2):918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O., Jr, Thomas G. M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 1966 May;56(2):385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57(2-3):239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Browne G. M., Rogers P. L. Antibacterial activity of different Zymomonas mobilis strains. Microbios. 1984;39(157-158):187–192. [PubMed] [Google Scholar]
- Somerville J. E., Shatters R. G., Kahn M. L. Isolation, characterization, and complementation of Rhizobium meliloti 104A14 mutants that lack glutamine synthetase II activity. J Bacteriol. 1989 Sep;171(9):5079–5086. doi: 10.1128/jb.171.9.5079-5086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Hurlbert R. E. Toxicity of Irradiated Media for Xenorhabdus spp. Appl Environ Microbiol. 1990 Mar;56(3):815–818. doi: 10.1128/aem.56.3.815-818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Lohrke S., Hurlbert I. M., Hurlbert R. E. Transformation of Xenorhabdus nematophilus. Appl Environ Microbiol. 1989 Apr;55(4):806–812. doi: 10.1128/aem.55.4.806-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Lohrke S., Hurlbert I. M., Hurlbert R. E. Transformation of Xenorhabdus nematophilus. Appl Environ Microbiol. 1989 Apr;55(4):806–812. doi: 10.1128/aem.55.4.806-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]