Abstract
Edwardsiella tarda is pathogen of fish and other animals. The aim of this study was to investigate the viable but nonculturable (VBNC) state and virulence retention of this bacterium. Edwardsiella tarda CW7 was cultured in sterilized aged seawater at 4°C. Total cell counts remained constant throughout the 28-day period by acridine orange direct counting, while plate counts declined to undetectable levels (<0.1 CFU/ml) within 28 days by plate counting. The direct viable counts, on the other hand, declined to ca. 109 CFU/ml active cells and remained fairly constant at this level by direct viable counting. These results indicated that a large population of cells existed in a viable but nonculturable state. VBNC E. tarda CW7 could resuscitate in experimental chick embryos and in the presence of nutrition with a temperature upshift. The resuscitative times were 6 days and 8 days, respectively. The morphological changes of VBNC, normal, and resuscitative E. tarda CW7 cells were studied with a scanning electron microscope. The results showed that when the cells entered into the VBNC state, they gradually changed in shape from short rods to coccoid and decreased in size, but the resuscitative cells did not show any obvious differences from the normal cells. The VBNC and the resuscitative E. tarda CW7 cells were intraperitoneally inoculated into turbot separately, and the fish inoculated with the resuscitative cells died within 7 days, which suggested that VBNC E. tarda CW7 might retain pathogenicity.
Edwardsiella tarda, an enteric gram-negative bacterium of the Enterobacteriaceae, is the causative agent of the systemic disease edwardsiellosis in freshwater and marine fish worldwide. The bacterium causes septicemia with extensive skin lesions and affects internal organs, such as the liver, kidney, spleen, and muscle (33, 52). It has been isolated from a variety of animals, including fish, birds, mammals, and reptiles (12, 27, 31, 46, 47), and environmental water (32, 50). Several potential virulence properties have been suggested to contribute to the pathogenesis of E. tarda, namely, the production of dermatotoxin (45) and hemolysin (14) and the ability to resist phagocyte-mediated destruction and to invade epithelial cells (19, 22, 43). However, little is known about the pathogenic mechanism of E. tarda, and the causes of disease occurrence are still elusive.
Many bacteria have developed strategies for metamorphosis into more or less sophisticated survival forms in response to harsh environmental conditions, such as a temperature change, high salinity, or nutrient deprivation (4, 5). The formation of viable but nonculturable (VBNC) cells of bacteria has been proposed as a strategy to survive adverse conditions (10). The first clear evidence of the existence of the VBNC state in pathogens was provided by Xu et al. (51), who showed that enterotoxic Escherichia coli and Vibrio cholerae cells suspended in artificial seawater remained viable but quickly lost their ability to colonize culture media. The reality of the VBNC state has since been demonstrated with other human and fish bacterial pathogens, including Vibrio vulnificus (28), Salmonella enterica serovar Enteritidis (39), enterotoxigenic Escherichia coli (11, 25), Helicobacter pylori, Campylobacter jejuni (2, 38), Legionella pneumophila (17), Shigella dysenteriae (18), Aeromonas hydrophila (36), Vibrio parahaemolyticus (49), and Enterococcus spp. (9).
It has been demonstrated that VBNC cells are active in metabolism (16, 20, 53) and are able to recover from their dormant state, becoming metabolically active and fully culturable (26, 40, 48). Some pathogens in the VBNC state may retain their pathogenicity (8, 34, 35, 37, 44). Although edwardsiellosis occurs over a long period of time during a year, E. tarda focuses on high-temperature months and is seldom isolated when the temperature of the water is low. The suggestion that E. tarda can survive for a long period and may become nonculturable in an aquatic environment has been made (41).
The aim of present study was to investigate the VBNC state of E. tarda CW7 experimentally induced under starvation conditions at a low temperature and to verify the retention of the virulence of VBNC cells.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
E. tarda CW7 was isolated from diseased turbot (Scophthalmus maximus) in China and used in this study. The strain was stored in cultured broth with 10% (vol/vol) glycerol at −85°C and cultured in Luria-Bertani (LB) agar at 26°C.
Preparation of the microcosm.
The microcosm was prepared by filtering aged seawater (ASW) through a 0.22-μm Millipore filter and sterilized by autoclaving.
Exponentially growing bacterial cell cultures were washed with autoclaved saline, which was filtered through a 0.22-μm-pore-size membrane filter (Millipore). The washed cells were then resuspended in an oligotrophic microcosm at a final density of 1011 CFU/ml as described previously (23). The inoculated microcosm was maintained at 4°C, and the counts of culturable cells were determined on LB agar at intervals by the plate count method. The experiments were conducted in triplicate.
Culturability and viability assays.
To determine the number of culturable cells, dilutions of microcosm samples were plated on LB agar and incubated at 26°C for 48 h. When there was less than one platable cell in 10 ml of sample (determined by filtering 10-ml samples and placing the filter on LB agar), the bacteria in the microcosm were determined to be nonculturable. All culturability studies were performed three times.
To determine the total number of cells, dilutions of microcosm samples were withdrawn at timed intervals and processed according to the acridine orange direct counting (AODC) method described by Hobbie et al. (15). Samples fixed with formalin (final concentration, 2%) were stained with acridine orange (final concentration, 0.01%) for 2 min and filtered onto 0.22-μm-pore-size black polycarbonate filters (Millipore). The filters were examined under an Olympus epifluorescence microscope (BH2-RFL filter set, BP490 excitation filter, EY455 supplementary exciter filter, O515 barrier filter). The total number of bacteria was estimated by counting a minimum of 10 fields.
To determine the number of viable cells, dilutions of microcosm samples were withdrawn at timed intervals and performed according to the direct viable counting (DVC) method described by Kogure et al. (20), with incubation of the samples in yeast extract (final concentration, 0.025%) and nalidixic acid (final concentration, 0.002%) at 26°C for 16 h before acridine orange staining. Cells which were elongated to at least twice the length of AODC control cells were scored as viable. The number of viable bacteria was estimated by counting a minimum of 10 fields.
Resuscitation of the VBNC cells.
For resuscitation of the VBNC cells in animal experiments, 30 chick embryos were used and divided into three groups, each comprised of 10 chick embryos. Each chicken embryo in the first group was inoculated with 100 μl of VBNC cells (<0.1 CFU/ml). The second and the third groups were inoculated with the normal bacteria and autoclaved saline as positive and negative controls, respectively. Culturability of these samples was determined by extracting the content of chick embryos and plating it on LB agar every day. The test was considered positive when the organisms were isolated on the culture media.
For resuscitation of the VBNC cells with the temperature upshifted, 10 ml of the VBNC cells was removed from the microcosm and allowed to incubate at 26°C. The culturability of these samples was determined by plating the cells on LB agar each day. The test was considered positive when the organisms were isolated on the culture media.
To determine the effect of nutrition addition on the resuscitation of VBNC cells, 10 ml of the VBNC cells was removed from the microcosm, yeast extract was added to the samples (final concentration, 0.025%), and the samples were then incubated at 26°C. Culturability was subsequently determined as described above each day. All of the experiments were performed in triplicate.
Scanning electron microscopy.
The normal cells, VBNC cells, and resuscitative cells were harvested by centrifugation and were fixed with 3% (vol/vol) glutaraldehyde at room temperature for 4 h. The samples were washed with phosphate-buffered saline, filtered onto 0.22-μm-pore-size Nucleopore polycarbonate filters, dehydrated in a graded ethanol series (30%, 50%, 70%, 80%, and 100%), which was replaced by isoamyl acetate, critical point dried in CO2 in an XD-1 apparatus (Eiko), and coated with palladium-gold (AuPd) in an IB-3 vacuum evaporator coating apparatus (Eiko). The coated samples were then examined in a model JSM-840 scanning electron microscope (JEOL, Japan).
Virulence of the VBNC and the resuscitative cells.
Turbot were used to investigate the retention of virulence in the VBNC and the resuscitative cells. Twenty turbot weighting 10 to 15 g were divided into four groups. Each group, comprised of five fish, was kept in 200-liter tanks with aerated seawater at 16 to 20°C and fed with commercial pellets. The four groups were intraperitoneally inoculated with 100 μl of the VBNC cells, the resuscitative cells (108 CFU/ml), the normal cells (108 CFU/ml), or autoclaved saline. The injected fish were then kept at 16 to 20°C for 30 days, and mortality was recorded. The cause of death was confirmed by reisolating the bacteria from the ascites fluid, kidneys, and spleens of the dead fish using LB agar.
RESULTS
Studies of entry into the VBNC state of E. tarda CW7.
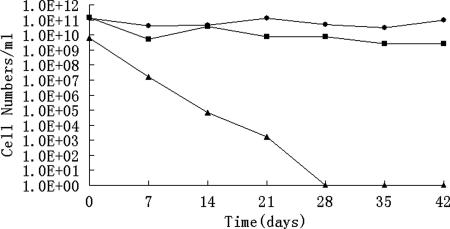
Figure 1 shows the response of E. tarda CW7 following its incubation in ASW at 4°C. E. tarda CW7 entered the VBNC state within about 28 days of incubation in the ASW microcosm at 4°C, as evaluated by plate counting of spreading cells on LB agar. Throughout this period, all total direct counts in the studies remained near the original inoculum level of ca. 1011 CFU/ml. The active cells, on the other hand, declined to ca. 109 CFU/ml and remained fairly constant at this level, as demonstrated by DVC staining. These results indicated that a large population of cells existed in the VBNC state.
FIG. 1.
Entry of E. tarda CW7 into the VBNC state in an ASW microcosm at 4°C, as determined by AODC (•), DVC (▪), and plate counting (▴) methods.
Morphological changes of the VBNC E. tarda CW7.
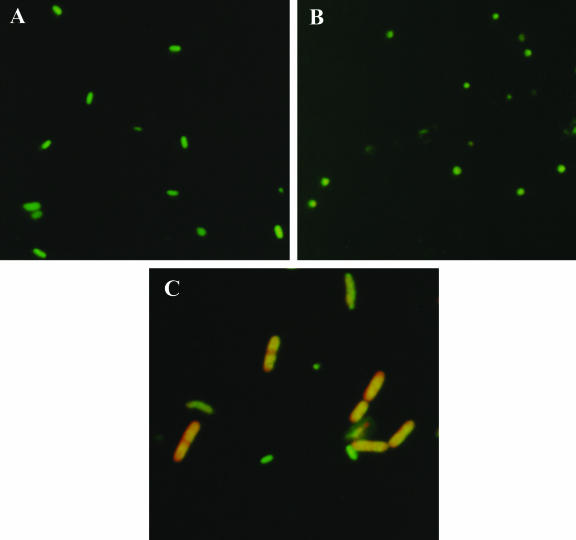
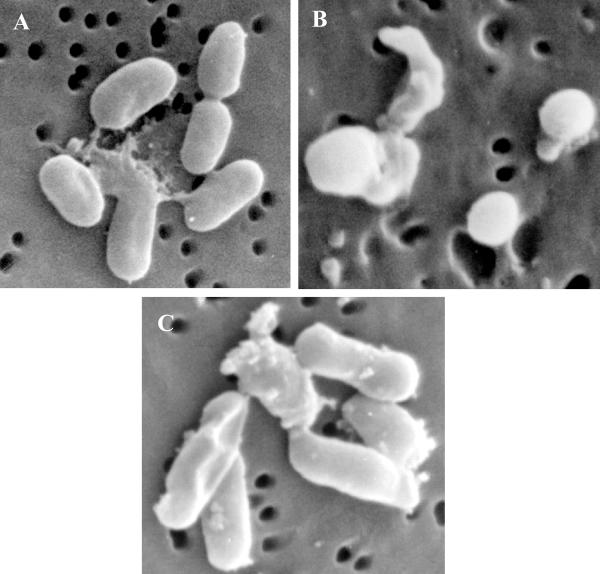
The VBNC cells of E. tarda CW7 exhibited several morphological changes under an epifluorescence microscope by the AODC method. Compared to normal cells (Fig. 2A), the VBNC cells gradually changed from short rods to coccoid cells and decreased in size (Fig. 2B). The red elongated cells were defined as viable by the DVC method (Fig. 2C). When observed with a scanning electron microscope, the VBNC E. tarda CW7 cells changed from short rods to coccoid cells and the cell size decreased (Fig. 3B). The average size of the normal cells (Fig. 3A) was 1.9 × 1.1 μm, but the average radius of the coccoid VBNC cells was 0.5 μm. The normal cells and the resuscitative cells (Fig. 3C) showed no morphological differences.
FIG. 2.
Morphological characteristics of E. tarda CW7 under an epifluorescence microscope (magnification, ×1,000). (A and B) Normal and VBNC cells analyzed by the AODC method. (C) VBNC cells analyzed by DVC.
FIG. 3.
Morphological characteristics of E. tarda CW7 analyzed with a scanning electron microscope (magnification, ×20,000). (A) Normal cells. (B) VBNC cells. (C) Resuscitative cells.
Resuscitation of the VBNC cells.
Chick embryos were used to resuscitate the VBNC cells of E. tarda CW7. The experimental chick embryos injected with the VBNC cells remained alive, and autopsy did not show any obvious damage, but the microorganisms were reisolated from the injected chick embryos on the sixth day. These cells were the resuscitated cells, which were used in the next experiment. The chick embryos inoculated with the normal cells died within 3 days, and the organisms were reisolated from all of the dead embryos. Confirmation of the identities of these isolates as E. tarda CW7 was made by PCR amplification of whole-cell lysates of the colonies that developed. No microorganism was isolated from the chick embryos that had been injected with the autoclaved saline.
When the VBNC cells were incubated in the presence of yeast extract at 26°C, culturable populations were observed on the medium on the eighth day, but when the cells were subjected to a temperature upshift (to ca. 26°C) in the absence of nutrition for 1 month, no culturable population was observed on the medium.
Virulence of the VBNC and the resuscitative cells.
Twenty turbot were divided into four groups for a virulence study. The group inoculated with normal E. tarda CW7 all died on the fifth day, and the group inoculated with the resuscitative E. tarda CW7 also died on the seventh day. All of the dead fish showed similar symptoms, such as serious ascites, hemorrhage of the liver, and enlarged kidneys. Culturable cells of E. tarda CW7 could be isolated from the ascites fluid and the organs and were identified by PCR amplification. The fish which were inoculated with the VBNC cells and autoclaved saline remained alive during the experimental time, and E. tarda CW7 was not isolated from these animals (Table 1).
TABLE 1.
Virulence in turbot of E. tarda CW7 in different statesa
| Inoculum | Dose (no. of CFU/fish)b | No. of dead fish on indicated day after inoculation
|
Total no. of dead fish | Mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 30 | ||||
| VBNC cells | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Resuscitative cells | 108 | 0 | 0 | 0 | 3 | 2 | 0 | 5 | 100 |
| Normal cells | 108 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 100 |
| Sterile saline (control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Five turbot were in each group tested.
Determined by DVC counting.
DISCUSSION
Several bacteria have been reported to enter the VBNC state. The times required to enter the VBNC state vary greatly for different bacteria. Escherichia coli K-12 strain W3110 could enter the VBNC state in nonsterile river water in less than 10 days (3). Escherichia coli O157:H− strain E 32511/HSC became nonculturable in sterilized distilled water microcosms at 4°C in about 21 days (24). Roszak et al. reported that Salmonella serovar Enteritidis 13-1BB entered the nonculturable state within 48 h when it was incubated in Potomac River water (39). The clinical isolate Vibrio cholerae ATCC 14035 was found to enter the VBNC state in 6 to 9 days in Chesapeake Bay water at 4 to 6°C (51). Vibrio vulnificus CVD713 and C7184 could enter the VBNC state in artificial seawater at 5°C within 7 days (30), while Linder and Oliver reported that Vibrio vulnificus entered the VBNC state after incubation for 24 days (21). Aeromonas hydrophila, a fish pathogen, was reported to enter the VBNC state when incubated in 0.35% NaCl for 50 days at 25°C (36). Wong et al. reported that 24 strains of Vibrio parahaemolyticus isolated from clinical and environmental samples entered the VBNC state within 14 to 49 days when they were incubated in artificial seawater at 4.5°C, and there was no difference in incubation time between the clinical and environmental isolates (49). In the present study, it was shown that E. tarda CW7 became nonculturable when it was inoculated at 4°C into ASW for 28 days (<0.1 CFU/ml). Throughout this period, all total direct counts remained near the original level of ca. 1011 CFU/ml. The number of active cells, on the other hand, declined to ca. 109 CFU/ml and remained fairly constant at this level, as demonstrated by the DVC staining method.
Morphological changes of the VBNC E. tarda CW7 cells were studied by the methods of epifluorescence microscopy and scanning electron microscopy. During entry into the VBNC state, E. tarda CW7 cells decreased in size and became coccoid, which was in agreement with the results reported by other authors (6).
Another important characteristic of VBNC cells is their ability to be resuscitated in vitro (26) and in a natural estuarine environment (29) as a consequence of environmental changes, e.g., an increase in temperature. E. tarda CW7 in the VBNC state could resuscitate in the presence of nutrition with temperature upshift. Some investigators believe that elevated nutrition might be toxic in some manner to cells in the VBNC state (48), but our experiments suggested that nutrition is very important to the resuscitation of E. tarda CW7; resuscitation could be achieved only with temperature upshift in the presence of nutrition. Studies of the virulence of bacteria in the VBNC state have been reported by many authors. VBNC Escherichia coli and Vibrio cholerae cells were recovered from rabbit ileal loops in which enterotoxigenicity was exhibited (7, 13). Pommepuy et al. also reported that Escherichia coli H10407 cells in the VBNC state produce enterotoxin when they are incubated in rabbit intestinal loops, as indicated by ganglioside enzyme-linked immunosorbent assay, and are thus of potential public health concern (34). Chlorine-stressed Yersinia enterocolitica displayed virulence characteristics similar to those of control cultures (42). VBNC Legionella pneumophila cells caused chick embryos to die when they were injected into the embryos (17). Resuscitation of E. tarda CW7 cells also occurred in chick embryos when they were injected into the embryos, but the injected embryos remained alive and did not show any obvious damage, which could be explained as a temporary inability to express the virulence characteristics of VBNC E. tarda CW7. The resuscitative E. tarda CW7 cells showed pathogenicity when they were intraperitoneally inoculated into turbot. The fish inoculated with the resuscitative cells all died within 7 days, showing similar symptoms, such as ascites, hemorrhagic livers, and enlarged kidneys. E. tarda CW7 was reisolated from these organs, which indicated that resuscitative E. tarda CW7 retained its pathogenic potential. These results were in agreement with those obtained by Baffone et al., who reported that the virulence characteristics of Vibrio parahaemolyticus and Vibrio alginolyticus strains, which seemed to disappear after resuscitation in the mouse, were subsequently reactivated by means of two consecutive passages of the strains in the rat ileal loop model (1).
In conclusion, Edwardsiella tarda CW7, like other pathogenic bacteria, could enter the VBNC state in adverse environments. VBNC E. tarda CW7 was able to resuscitate in the experimental chick embryos and in the presence of nutrition with a temperature upshift. VBNC cells might keep their pathogenic potential when they are resuscitated from the VBNC state, and Edwardsiella tarda CW7 cells retained their pathogenicity and caused disease.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (30371108) and National High-Tech R & D Program grant 2003AA622070.
Footnotes
Published ahead of print on 22 December 2006.
REFERENCES
- 1.Baffone, W., B. Citterio, E. Vittoria, A. Casaroli, R. Campana, L. Falzano, and G. Donelli. 2003. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int. J. Food Microbiol. 89:31-39. [DOI] [PubMed] [Google Scholar]
- 2.Baffone, W., A. Casaroli, B. Citterio, L. Pierfelici, R. Campana, E. Vittoria, E. Guaglianone, and G. Donelli. 2006. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int. J. Food Microbiol. 107:83-91. [DOI] [PubMed] [Google Scholar]
- 3.Bogosian, G., L. E. Sammons, P. J. Morris, J. P. O'Neil, M. A. Heitkamp, and D. B. Weber. 1996. Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl. Environ. Microbiol. 62:4114-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casida, L. E., Jr. 1965. Abundant microorganisms in soil. Appl. Microbiol. 13:327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casida, L. E., Jr. 1977. Small cells in pure cultures of Agromyces ramosus and in natural soil. Can. J. Microbiol. 23:214-216. [DOI] [PubMed] [Google Scholar]
- 6.Chaiyanan, S., S. Chaiyanan, A. Huq, T. Maugel, and R. R. Colwell. 2001. Viability of the nonculturable Vibrio cholerae O1 and O139. Syst. Appl. Microbiol. 24:331-341. [DOI] [PubMed] [Google Scholar]
- 7.Colwell, R. R., P. R. Brayton, D. J. Grimes, D. B. Roszak, S. A. Huq, and L. M. Palmer. 1985. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology 3:817-820. [Google Scholar]
- 8.Colwell, R. R., P. Brayton, D. Herrington, B. Tall, A. Huq, and M. M. Levine. 1996. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J. Microbiol. Biotechnol. 12:28-31. [DOI] [PubMed] [Google Scholar]
- 9.del Mar Lleo, M., B. Bonato, D. Benedetti, and P. Canepari. 2005. Survival of enterococcal species in aquatic environments. FEMS Microbiol. Ecol. 54:189-196. [DOI] [PubMed] [Google Scholar]
- 10.Effendi, I., and B. Austin. 1995. Dormant/unculturable cells of fish pathogen Aeromonas salmonicida. Microb. Ecol. 30:183-192. [DOI] [PubMed] [Google Scholar]
- 11.Flint, K. P. 1987. The long-term survival of Escherichia coli in river water. J. Appl. Bacteriol. 63:261-270. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein, E. J., E. O. Agyare, A. E. Vagvolgyi, and M. Halpern. 1981. Aerobic bacterial oral flora of garter snakes: development of normal flora and pathogenic potential for snakes and humans. J. Clin. Microbiol. 13:954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimes, D. J., and R. R. Colwell. 1986. Viability and virulence of Escherichia coli suspended by membrane chamber in semitropical ocean water. FEMS Microbiol. Lett. 34:161-165. [Google Scholar]
- 14.Hirono, I., N. Tange, and T. Aoki. 1997. Iron-regulated haemolysin gene from Edwardsiella tarda. Mol. Microbiol. 24:851-856. [DOI] [PubMed] [Google Scholar]
- 15.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppe, H. G. 1978. Relations between active bacteria and heterotrophic potential in the sea. Neth. J. Sea Res. 12:78-98. [Google Scholar]
- 17.Hussong, D., R. R. Colwell, M. O'Brien, E. Weiss, A. D. Pearson, R. M. Weiner, and W. D. Burge. 1987. Viable Legionella pneumophila not detectable by culture on agar medium. Bio/Technology 5:947-950. [Google Scholar]
- 18.Islam, M. S., M. K. Hasan, M. A. Miah, G. C. Sur, A. Felsenstein, M. Venkatesan, R. B. Sack, and M. J. Albert. 1993. Use of the polymerase chain reaction and fluorescent-antibody methods for detecting viable but nonculturable Shigella dysenteriae type 1 in laboratory microcosms. Appl. Environ. Microbiol. 59:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janda, J. M., S. L. Abbott, S. Kroske-Bystrom, W. K. Cheung, C. Powers, R. P. Kokka, and K. Tamura. 1991. Pathogenic properties of Edwardsiella species. J. Clin. Microbiol. 29:1997-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kogure, K., U. Simidu, and N. Taga. 1979. A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 25:415-420. [DOI] [PubMed] [Google Scholar]
- 21.Linder, K., and J. D. Oliver. 1989. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl. Environ. Microbiol. 55:2837-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling, S. H. M., X. H. Wang, L. Xie, T. M. Lim, and K. Y. Leung. 2000. Use of green fluorescent protein (GFP) to study the invasion pathways of Edwardsiella tarda in in vivo and in vitro fish models. Microbiology 146:7-19. [DOI] [PubMed] [Google Scholar]
- 23.Lleo, M. M., M. C. Tafi, and P. Canepari. 1998. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst. Appl. Microbiol. 21:333-339. [DOI] [PubMed] [Google Scholar]
- 24.Mizunoe, Y., S. N. Wai, A. Takade, and S. Yoshida. 1999. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157:H7 cells by using H2O2-degrading compounds. Arch. Microbiol. 172:63-67. [DOI] [PubMed] [Google Scholar]
- 25.Na, S. H., K. Miyanaga, H. Unno, and Y. Tanji. 2006. The survival response of Escherichia coli K12 in a natural environment. Appl. Microbiol. Biotechnol. 72:386-392. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson, L., J. D. Oliver, and S. Kjelleberg. 1991. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J. Bacteriol. 173:5054-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nucci, C., W. D. da Silveira, S. da Silva Correa, G. Nakazato, S. Y. Bando, M. A. Ribeiro, and A. F. Pestana de Castro. 2002. Microbiological comparative study of isolates of Edwardsiella tarda isolated in different countries from fish and humans. Vet. Microbiol. 89:29-39. [DOI] [PubMed] [Google Scholar]
- 28.Oliver, J. D., and D. Wanucha. 1989. Survival of Vibrio vulnificus at reduced temperatures and elevated nutrient. J. Food Saf. 10:79-86. [Google Scholar]
- 29.Oliver, J. D., F. Hite, D. McDougald, N. L. Andon, and L. M. Simpson. 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in a natural environment. Appl. Environ. Microbiol. 61:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver, J. D., and R. Bockian. 1995. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol. 61:2620-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens, D. R., S. L. Nelson, and J. B. Addison. 1974. Isolation of Edwardsiella tarda from swine. Appl. Environ. Microbiol. 27:703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitlik, S., S. A. Berger, and D. Huminer. 1987. Nonenteric infections acquired through contact with water. Rev. Infect. Dis. 9:54-63. [DOI] [PubMed] [Google Scholar]
- 33.Plumb, J. A. 1993. Edwardsiella septicemia, p. 61-79. In V. Inglis, R. J. Roberts, and N. R. Bromage (ed.), Bacterial diseases of fish. Blackwell, Oxford, United Kingdom.
- 34.Pommepuy, M., M. Butin, A. Derrien, M. Gourmelon, R. R. Colwell, and M. Cormier. 1996. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl. Environ. Microbiol. 62:4621-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman, I., M. Shahamat, P. A. Kirchman, E. Russek-Cohen, and R. R. Colwell. 1994. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl. Environ. Microbiol. 60:3573-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman, M. H., S. Suzuki, and K. Kawai. 2001. Formation of viable but non-culturable state (VBNC) of Aeromonas hydrophila and its virulence in goldfish, Carassius auratus. Microbiol. Res. 156:103-106. [DOI] [PubMed] [Google Scholar]
- 37.Ravel, J., I. T. Knight, C. E. Monahan, R. T. Hill, and R. R. Colwell. 1995. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology 141:377-383. [DOI] [PubMed] [Google Scholar]
- 38.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roszak, D. B., D. J. Grimes, and R. R. Colwell. 1984. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 30:334-338. [DOI] [PubMed] [Google Scholar]
- 40.Roth, W. G., M. P. Leckie, and D. N. Dietzler. 1988. Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl. Environ. Microbiol. 54:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai, M., S. Atsuta, and M. Kobayashi. 1994. Survival of fish pathogen Edwardsiella tarda in seawater and fresh. Bull. Eur. Assoc. Fish Pathol. 14:188-190. [Google Scholar]
- 42.Singh, A., and G. A. McFeters. 1987. Survival and virulence of copper- and chlorine-stressed Yersinia enterocolitica in experimentally infected mice. Appl. Environ. Microbiol. 53:1768-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasa Rao, P. S., T. M. Lim, and K. Y. Leung. 2001. Opsonized virulent Edwardsiella tarda strains are able to adhere to and survive and replicate within fish phagocytes but fail to stimulate reactive oxygen intermediates. Infect. Immun. 69:5689-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern, N. J., D. M. Jones, I. V. Wesley, and D. M. Rollins. 1994. Colonization of chicks by non-culturable Campylobacter spp. Lett. Appl. Microbiol. 18:333-336. [Google Scholar]
- 45.Ullah, M. A., and T. Arai. 1983. Pathological activities of the naturally occurring strains of Edwardsiella tarda. Fish Pathol. 18:65-70. [Google Scholar]
- 46.Van Damme, L. R., and J. Vandepitte. 1980. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl. Environ. Microbiol. 39:475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White, F. H., C. F. Simpson, and L. E. Williams, Jr. 1973. Isolation of Edwardsiella tarda from aquatic animal species and surface waters in Florida. J. Wildl. Dis. 9:204-208. [DOI] [PubMed] [Google Scholar]
- 48.Whitesides, M. D., and J. D. Oliver. 1997. Resuscitation of Vibrio vulnificus from the viable but nonculturable State. Appl. Environ. Microbiol. 63:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong, H. C., P. Wang, S. Y. Chen, and S. W. Chiu. 2004. Resuscitation of viable but non-culturable Vibrio parahaemolyticus in a minimum salt medium. FEMS Microbiol. Lett. 233:269-275. [DOI] [PubMed] [Google Scholar]
- 50.Wyatt, L. E., R. Nickelson, and C. Vanderzant. 1979. Edwardsiella tarda in freshwater catfish and their environment. Appl. Environ. Microbiol. 38:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu, H. S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 52.Yan, X. H., Y. Li, J. X. Chen, Y. G. Wang, and Q. F. Li. 2006. Studies on the characteristics of a pathogenic Edwardsiella tarda isolated from diseased Scophthalmus maximus. J. Ocean Univ. China 36:649-654. [Google Scholar]
- 53.Zimmermann, R., R. Iturriaga, and J. Becker-Birek. 1978. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl. Environ. Microbiol. 36:926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]