Abstract
Weight loss diets for humans that are based on a high intake of protein but low intake of fermentable carbohydrate may alter microbial activity and bacterial populations in the large intestine and thus impact on gut health. In this study, 19 healthy, obese (body mass index range, 30 to 42) volunteers were given in succession three different diets: maintenance (M) for 3 days (399 g carbohydrate/day) and then high protein/medium (164 g/day) carbohydrate (HPMC) and high protein/low (24 g/day) carbohydrate (HPLC) each for 4 weeks. Stool samples were collected at the end of each dietary regimen. Total fecal short-chain fatty acids were 114 mM, 74 mM, and 56 mM (P < 0.001) for M, HPMC, and HPLC diets, respectively, and there was a disproportionate reduction in fecal butyrate (18 mM, 9 mM, and 4 mM, respectively; P < 0.001) with decreasing carbohydrate. Major groups of fecal bacteria were monitored using nine 16S rRNA-targeted fluorescence in situ hybridization probes, relative to counts obtained with the broad probe Eub338. No significant change was seen in the relative counts of the bacteroides (Bac303) (mean, 29.6%) or the clostridial cluster XIVa (Erec482, 23.3%), cluster IX (Prop853, 9.3%), or cluster IV (Fprau645, 11.6%; Rbro730 plus Rfla729, 9.3%) groups. In contrast, the Roseburia spp. and Eubacterium rectale subgroup of cluster XIVa (11%, 8%, and 3% for M, HPMC, and HPLC, respectively; P < 0.001) and bifidobacteria (4%, 2.1%, and 1.9%, respectively; P = 0.026) decreased as carbohydrate intake decreased. The abundance of butyrate-producing bacteria related to Roseburia spp. and E. rectale correlated well with the decline in fecal butyrate.
Low-carbohydrate diets in which carbohydrates are largely replaced by an increased proportion of dietary protein and/or fat have proved a popular weight loss strategy for humans (1, 11, 36). The potential health impacts associated with increased protein (34) or fat (27) intake have been controversial among nutritionists, but less attention has been paid to the consequences of low carbohydrate supply. It has been argued that a lower carbohydrate supply may be advantageous in ameliorating insulin insensitivity (9), although this may not occur with low-glycemic-index foods (21). Furthermore, in the context of overall dietary advice consideration also needs to be given to the role of carbohydrates in maintenance of gut health and function.
Dietary carbohydrates include structural polysaccharides and oligosaccharides of plant origin plus resistant starch (14, 40) that are not digested in the small intestine and, instead, enter the colon. Here they can be fermented by the microbiota of the large intestine and normally provide the main energy supply to support microbial growth in the colon. Microbial fermentation may release as much as 10% of the dietary energy, mainly in the form of short-chain fatty acids (SCFA) that also act as energy sources for host cells (46). For example, butyrate is the preferred energy source for the epithelial cells of the colon (29, 50). Furthermore, butyrate has been implicated in the prevention of colitis and colorectal cancer (16, 35, 44, 53, 61).
Reduced intake of fermentable dietary carbohydrate might be expected to impact on both the activity and the abundance of the different bacterial groups that populate the large intestine (28, 37). The aim of the present study was therefore to investigate the effect of reduced carbohydrate intake upon bacterial populations and metabolites detected in fecal samples. Quantification of bacterial groups involved in particular metabolic roles is now feasible following development of specific 16S rRNA-targeted probes for many human colonic bacterial groups (25, 39, 48, 60). A panel of probes including those targeted to the most abundant groups of butyrate-producing bacteria found in human fecal samples (3, 6, 33, 60) was used to monitor the effect of a dietary shift from normal intakes of carbohydrate (399 g/day) to either moderate (164 g/day) or low (24 g/day) intakes as part of weight loss strategies in obese men. Significant relationships were established between dietary carbohydrate intake, the composition of the fecal microbiota, and fecal SCFA concentrations.
MATERIALS AND METHODS
Volunteer recruitment.
Obese, but otherwise healthy, male volunteers (n = 20) were recruited for a 9-week dietary intervention study (A. M. Johnstone, G. Horgan, S. Murison, D. M. Bremner, and G. E. Lobley, submitted for publication). One subject left the study early for reasons not associated with the protocol. All collected samples were analyzed. The volunteers were aged 36.7 ± 2.3 years (mean ± standard error of the mean; range, 20 to 57 years) with a mean body mass index (kg/m2) of 35.4 ± 0.9 (range, 30 to 42). Volunteers were selected based on absence of indices of metabolic syndrome and had no history of gastrointestinal problems. No antibiotics or drugs known to influence the fecal microbiota were taken during the course of the study. Ethical approval was granted by the Grampian Research Ethics Committee, and all volunteers provided informed, signed consent.
Experimental design.
This study, conducted over a 9-week period, included three 3-day intakes at energy maintenance with two intervening main diet periods, at either low or moderate carbohydrate intake, each for 28 days with the order randomized between subjects. Fecal samples were collected on three occasions, after 3 days on the first maintenance period and during the last 2 days on each of the main diets. Food intakes were quantified by weight, with any refusals also weighed.
Experimental dietary regimen.
The volunteers were weight stable (less than 2-kg change in recent months) on entry to the trial and were then offered an energy maintenance (M) diet (based on 1.6× resting metabolic rate) for 3 days. This diet comprised 13% protein, 52% carbohydrate, and 35% fat as calories. Subjects were then offered ad libitum two diets, which were either a high-protein, low-carbohydrate (HPLC; 30% protein, 4% carbohydrate, 66% fat as calories) diet or a high-protein, moderate-carbohydrate (HPMC; 30% protein, 35% carbohydrate, 35% fat) diet, each supplied for 4 weeks in a randomized crossover design. Between the two main diet periods and at the end of the study the subjects were given the maintenance diet for 3 days. All meals were of the same energy density (5.5 MJ/kg), and daily intakes were recorded by weight. Daily macronutrient intakes were calculated using the Windiet software program (Robert Gordon University, Aberdeen, United Kingdom), based on the type and quantity of each ingredient consumed and published food composition tables (24). Diet intake was analyzed (Johnstone et al., submitted) for maintenance, HPMC, and HPLC diets (Table 1).
TABLE 1.
Dietary intake (g/day) indicating mean values for 7 days preceding fecal sample (3 days for maintenance diet)
| Diet | Fat | Protein | Carbohydrate | Nonstarch polysaccharides | Starch |
|---|---|---|---|---|---|
| Maintenance | 122.9 | 94.3 | 398.8 | 27.9 | 187.3 |
| HPMC | 74.3 | 127.2 | 163.6 | 11.7 | 95.3 |
| HPLC | 126.0 | 119.5 | 23.9 | 6.1 | 2.7 |
| P value | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
Collection and preparation of fecal samples for analyses.
The fresh fecal samples provided from each volunteer were maintained at 4°C prior to processing on the same day as collection. Each sample was mixed, and four 0.5-g samples were taken for further analyses, which were performed blind. These were stored at −20°C for metabolite analyses or directly processed for fluorescence in situ hybridization (FISH) analysis.
Enumeration of bacteria in fecal samples by FISH analysis.
The fecal sample was mixed, and a subsample (0.5 g) was thoroughly mixed with 4.5 ml phosphate-buffered saline and fixed by mixing 1:3 in 4% (wt/vol) paraformaldehyde at 4°C for 16 h. Subsamples (0.8 ml) were stored at −20°C. FISH analysis was performed on the liquid phase (20, 31). Briefly, diluted cell suspensions were applied to gelatin-coated slides. Slides were then hybridized with 10 μl of the respective oligonucleotide probe (50 ng/μl stock solution) and washed. Cells were counted automatically using a DMRXA epifluorescence microscope (Leica) and image analysis software, except when the number of fluorescent cells was less than 10 per field of view, when the cells were counted manually. Total bacterial numbers were estimated with the Eub338 probe (2) while the other bacterial groups were assessed using a panel of nine probes (Table 2).
TABLE 2.
Bacterial groups recognized by the 16S rRNA-targeted fluorescence in situ hybridization probes
| Probe | Probe target | Reference for probe |
|---|---|---|
| Eub338 | Domain Bacteria | 2 |
| Erec482 | Clostridial clusters XIVa and XIVb | 25 |
| Bac303 | Bacteroides-Prevotella group | 42 |
| Fprau645 | F. prausnitzii group (subgroup of cluster IV) | 57 |
| Bif164 | Bifidobacterium genus | 38 |
| Rbro730/Rfla729 | Ruminococcus bromii and Ruminococcus flavefaciens (subgroup of cluster IV) | 31 |
| Rrec584 | Roseburia and Eubacterium group (subgroup of cluster XIVa) | 60 |
| Lab158 | Lactobacillus-Enterococcus group | 30 |
| Prop853 | Clostridial cluster IX | 60 |
| Dsv698 | Sulfate-reducing bacteria | 43 |
Fermentation product analysis.
The SCFA content of the samples was determined by capillary gas chromatography following conversion to t-butyldimethylsilyl derivatives (52). The lower limit for reliable detection of each product was taken as 0.2 mM. Ammonia concentrations were analyzed in samples diluted (1:3) with sterile water and then reacted with sodium phenate and sodium hypochlorite, with the optical density of the indophenol blue produced determined at 625 nm (62).
Statistical analysis.
The data analyzed were obtained from 19 volunteers, 14 of whom provided fecal samples for all three dietary periods. The remaining five volunteers provided samples for two periods only. As a result, data consisted of 18 samples each for the maintenance and HPLC diets and 16 samples for the HPMC diet. Data from the maintenance diet were from the 3-day period at the start of the study. SCFA concentrations and bacterial data, both as % and as log(bacterial count), were analyzed as one-way analysis of variance (ANOVA) with the subject as a random effect and diet as a fixed effect. The effect of order was initially included but was found to be not significant and so was excluded from subsequent analyses. When the effect of diet was significant, Tukey's test was used to compare diet means. Several of the samples had undetectable levels of the fermentation products lactate, formate, and succinate and numbers of lactobacilli. Consequently, these data did not meet the ANOVA requirements of normality and equality of variance and were therefore analyzed by Friedman's nonparametric ANOVA. The effects of carbohydrate intake and bacterial counts on fecal butyrate concentration were investigated using restricted maximum likelihood (REML), with subject as a random effect and carbohydrate intake or bacterial count as fixed effect. All statistical analyses were performed by using Genstat 8th Edition Release 8.1 (VSN International Ltd., Hemel Hempstead, Herts, United Kingdom). Significance was set at P < 5%.
RESULTS
Fecal samples were analyzed from subjects consuming a maintenance (also referred to here as high-carbohydrate) diet and from the same subjects towards the end of 4-week periods on an HPMC or HPLC diet (see Materials and Methods).
Changes in fecal metabolites.
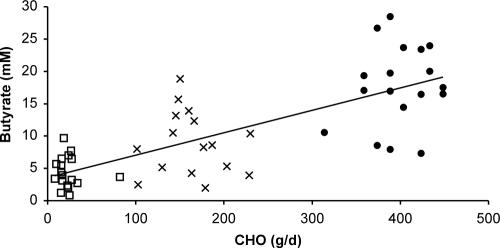
Total SCFA concentrations were lower during consumption of the HPMC and HPLC diets than during the maintenance period (74 and 56 versus 114 mM, respectively; P < 0.001). This was also the case for acetate, propionate, and valerate concentrations (P < 0.004) but not for formate or isobutyrate (Table 3). Butyrate concentrations were also lower for the HPLC than for the HPMC diet (P = 0.003). For the predominant SCFA, while concentrations decreased by approximately 50% between maintenance and low-carbohydrate diets, a greater proportional decrease (75%) was observed for butyrate (Table 3). This resulted in changes in the proportions of individual to total SCFA. Thus, acetate proportion increased (0.57, 0.60, and 0.64, P = 0.002) as carbohydrate intake decreased while propionate proportion was unaltered (0.18 to 0.19, P = 0.97). In contrast, butyrate proportion decreased as carbohydrate supply was lowered (0.16, 0.11, and 0.07, P < 0.001). The relationship between carbohydrate intake and butyrate concentration was linear (r = 0.76, P < 0.001, REML analysis) (Fig. 1). Fecal ammonia also declined with decreased carbohydrate intake (Table 3).
TABLE 3.
SCFA, lactate, and ammonia concentrations (mM)
| Dieta | Formate | Acetate | Propionate | Isobutyrate | Butyrate | Isovalerate | Valerate | Lactate | Succinate | Total SCFA | NH3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 0.56 | 65.09A | 20.28A | 2.27 | 17.67A | 2.13A | 3.14A | 1.23 | 1.27 | 113.6A | 51.67A |
| HPMC | 0.13 | 43.54B | 13.84B | 2.45 | 8.90B | 1.88AB | 2.04B | 0.13 | 0.79 | 73.7B | 42.98AB |
| HPLC | 0.27 | 35.50B | 10.81B | 1.91 | 4.36C | 1.34B | 1.38B | 0.00 | 0.63 | 56.2B | 33.03B |
| SEDb | 0.44 | 4.11 | 1.86 | 0.29 | 1.40 | 0.27 | 0.35 | 0.53 | 0.50 | 7.17 | 5.34 |
| Pb | 0.387c | <0.001 | <0.001 | 0.105 | <0.001 | 0.008 | <0.001 | 0.009c | 0.572c | <0.001 | 0.003 |
n = 18 for M and HPLC diets; n = 16 for HPMC diet.
Analyzed as one-way ANOVA with subject as random effect and diet as fixed effect. Standard error of the difference (SED) is based on 16 versus 18 observations. Diet means were compared by Tukey's test; different superscript capital letters within columns indicate P < 0.05.
Analyzed with Friedman nonparametric ANOVA (no post hoc comparisons performed).
FIG. 1.
Relationship between carbohydrate intake (average over 7 days preceding donation of stool sample for moderate- and low-carbohydrate diets; average of 3 days preceding stool sample for maintenance) and butyrate concentration in feces. •, maintenance diet; ×, HPMC diet; □, HPLC diet. Correlation, 0.76 (P < 0.001, REML).
Changes in fecal microbiota.
Based on the broad bacterial probe (Eub338), bacterial numbers (log count per g feces) were greater on the maintenance diet than on the other two diets (10.71, 10.55, and 10.56, P < 0.001). The most abundant bacterial groups (Table 4) detected by the 16S rRNA-targeted FISH probes were the gram-negative Cytophaga-Flavobacterium-Bacteroides group (Bacteroides spp., detected by Bac303 probe; 29 to 30% of total bacteria detected with Eub338) and gram-positive bacteria belonging to the clostridial cluster XIVa (detected by Erec482; 21 to 24%) or the clostridial cluster IV (combined subgroups detected with the Fprau645 and Rum729/730 probes; 16 to 24%). Two other groups that made significant contributions were clostridial cluster IX bacteria (9 to 11%) and bifidobacteria (2 to 4%). Overall, the group probes used accounted for a large proportion of the total bacteria in the stool samples, 87.3%, 88.8%, and 82.1% from the maintenance, HPMC, and HPLC diets, respectively. The proportions observed for the maintenance diet are similar to those obtained previously in FISH surveys of fecal samples from healthy volunteers (25), although only one study has previously reported the abundance of the cluster IX group (60). Lower estimates were obtained for Bacteroides spp. by fluorescence-activated cell sorting (48), but the values here are comparable to those obtained by automated microscopy (25). Overall bacterial numbers obtained here with the Eub338 probe were slightly lower than reported by some previous studies that also employed DAPI (4′,6′-diamidino-2-phenylindole) detection (e.g., see references 25 and 31) where counts exceeded 1011/g.
TABLE 4.
Abundance of bacterial groups (counts expressed as percentages of Eub338 counts) and total bacteria (log Eub338 count/g feces)
| Dieta | Bac303 | Fprau645 | Rfla729 plus Rbro730 | Bif164 | Erec482 | Prop853 | Lab158 | Dsv698 | Rrec584 | Erec-Rrec | Total log count/g feces |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 28.84 | 13.48 | 10.84 | 4.01A | 21.14 | 8.51 | 0.41 | 0.11 | 11.40A | 9.74A | 10.71A |
| HPMC | 29.96 | 11.39 | 10.00 | 2.09B | 24.40 | 10.76 | 0.11 | 0.13 | 7.79B | 16.61B | 10.55B |
| HPLC | 29.85 | 9.98 | 7.02 | 1.87B | 24.48 | 8.59 | 0.15 | 0.18 | 3.32C | 21.16C | 10.56B |
| SEDb | 2.32 | 1.72 | 1.99 | 0.88 | 2.03 | 1.45 | 0.20 | 0.04 | 1.11 | 2.16 | 0.05 |
| Pb | 0.774 | 0.089 | 0.102 | 0.026 | 0.173 | 0.254 | 0.353c | 0.238 | <0.001 | <0.001 | <0.001 |
n = 18 observations for M and HPLC diets and 16 observations for HPMC diet.
Analyzed as one-way ANOVA with subject as random effect and diet as fixed effect. Standard error of the difference (SED) is based on 16 versus 18 observations. Diet means were compared by Tukey's test; different superscript capital letters within columns indicate P < 0.05.
Analyzed with Friedman nonparametric ANOVA (no post hoc comparisons were performed).
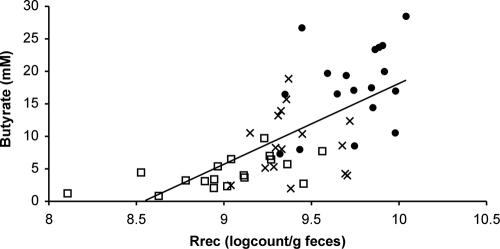
On the maintenance diet, bacteria detected with the Rrec584 probe, a subgroup of clostridial cluster XIVa, accounted, on average, for 11% (range, 6 to 21%) of the total Eub338 count (Table 4). This is slightly higher than reported recently for nonobese individuals with the same probe (3, 60). Bacteria targeted by the Rrec584 probe showed substantial decreases (P < 0.001) both in absolute numbers and as a proportion of total bacteria (P < 0.003; Table 4) as carbohydrate intake was lowered. This group of bacteria includes close relatives of Roseburia intestinalis and Eubacterium rectale, and all cultured representatives have butyrate as the main fermentation product from soluble sugars in pure culture (6, 18, 50). The decrease in numbers of bacteria within this group per gram feces paralleled the lowered fecal butyrate concentration (Fig. 2). This relationship was also observed within every individual (data not shown). Interestingly, relatives of Faecalibacterium prausnitzii, which have also been identified as a potentially important group of butyrate-producing bacteria (6), showed less of a response to reduced dietary carbohydrate and a weaker relationship (r = 0.36, P = 0.005 based on REML) with fecal butyrate concentrations.
FIG. 2.
Relationship between abundance of the Roseburia spp. and E. rectale group (detected using the Rrec584 probe) and butyrate concentration in feces. •, maintenance diet; ×, HPMC diet; □, HPLC diet. Correlation, 0.68 (P < 0.001, REML).
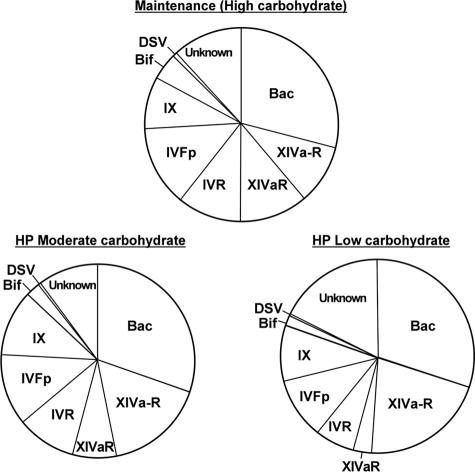
All bacteria recognized by the Rrec584 probe (Roseburia spp. and E. rectale group) are also recognized by the Erec482 probe, because this group is part of the larger clostridial XIVa cluster. There was, however, no concomitant decrease in the clostridial cluster XIVa group (estimated with the Erec482 probe) on the lower-carbohydrate diets (P = 0.17), implying that other groups within the XIVa cluster increased (P < 0.001) as carbohydrate intake was reduced (Erec-Rrec, Table 4; XIVa-R, Fig. 3).
FIG. 3.
Mean proportions of different bacterial groups in feces of human volunteers consuming maintenance, HPMC, or HPLC diets (assessed by FISH; also Table 4). The bacterial groups are represented as follows: Bac, Bacteroides spp. detected by Bac303; XIVaR, Roseburia spp. and E. rectale, detected by Rrec584; XIVa-R, clostridial cluster XIVa, detected by Erec482, minus those detected by Rrec584; IVR, cluster IV ruminococci detected by Rfla729 and Rbro730; IVFp, F. prausnitzii detected by Fprau645; IX, clostridial cluster IX bacteria detected by Prop853; Bif, Bifidobacterium spp. detected by Bif164; DSV, sulfate-reducing bacteria detected by Dsv698; unknown, bacteria detected by the broad Eub338 probe that were unaccounted for by the group probes used.
DISCUSSION
Total carbohydrate intake for the volunteers in this study decreased markedly from 399 g/day (maintenance) to 164 g/day (HPMC) and 24 g/day (HPLC). The predicted nonstarch polysaccharide (Englyst method) intakes were 28, 12, and 6 g/day (P < 0.001), respectively, while protein intake was lower for maintenance than for the other two diets (94, 127, and 120 g/day, P = 0.003) (Table 1). Carbohydrate content (26) and nonstarch polysaccharide intake were therefore lower than currently recommended in the United Kingdom (15) and in the United States (58).
In order to examine the impact of these dietary interventions upon the intestinal microbial community, a panel of targeted probes was employed that accounted for 82 to 89% of the total fecal bacteria detected, suggesting that most of the dominant species were covered. Previous studies with pure cultures provide an indication of the main substrates and metabolic products for different phylogenetic groups of human colonic bacteria (6, 23, 54, 55), although it should be noted that metabolic cross-feeding is an important feature of the colonic microbial ecosystem (7, 19). The available evidence suggests substantial similarity in species composition between feces and colonic samples (22, 32). It should be stressed, however, that most of the SCFA produced within the colon are absorbed across the mucosa and more than 85% of butyrate formed by bacterial fermentation is metabolized by the colonic epithelial cells (8). Nevertheless, fecal concentrations can provide an important indicator of conditions within the distal colon, where the risk of colorectal cancer is highest. Invasive techniques have shown that butyrate flows in the cecal or portal veins simulate the pattern of production within the colon of pigs and humans (5).
The two bacterial groups previously reported to be most abundant in human fecal samples by FISH analysis (25), the bacteroides (Cytophaga-Flavobacterium-Bacteroides) group and the clostridial cluster XIVa (Clostridium coccoides) group, constituted approximately 29% and 22% of total bacteria, respectively, in these volunteers. Neither group changed significantly with a reduction in dietary carbohydrate. This study also demonstrated the abundance of the clostridial cluster IX group, detected with the Prop852 probe, at approximately 9% of total fecal bacteria, but again no significant effect of dietary carbohydrate was seen. On the other hand, relatives of Roseburia spp. and E. rectale, a subgroup of clostridial cluster XIVa, showed a significant and marked progressive decrease as a fraction of total bacterial cells with decreasing carbohydrate intake. It has been shown recently that this group includes many strains that are able to utilize dietary carbohydrates such as starch, xylan, and inulin for growth (18, 20, 51). An interesting corollary is that the remainder of the cluster XIVa responded positively to decreasing dietary carbohydrate. While it appears that bacteria of the Roseburia spp. and E. rectale group may be particularly dependent upon dietary carbohydrate supply in order to maintain their populations in the colon, there must be other groups within cluster XIVa that are relatively more successful at low carbohydrate intakes. Roseburia spp. and E. rectale comprised 11% of the total bacteria, which compares with a mean of 7% for 10 nonobese subjects studied previously using the same group probe (3). This difference between studies may reflect either interindividual variation (the range was 6 to 21% for the current subjects) or, in light of the finding here, differences in the carbohydrate contents of the diets of free-living volunteers.
Two further groups were monitored that have been reported to include polysaccharide-degrading species: bifidobacteria (7) and cluster IV ruminococci (23). Bifidobacteria showed a significant reduction with decreased carbohydrate intake, consistent with the previously reported impact of certain prebiotics on this group (28). In general, the findings discussed above in relation to the cluster XIVa bacterial group suggest that such diet-related population changes may become more apparent as probes become available that target smaller and functionally more coherent groups of bacteria. This might apply within the Bacteroides genus, for example, where species are known to differ with respect to carbohydrate-utilizing abilities (54).
The close correlation between the population densities of Roseburia and E. rectale species and fecal butyrate concentrations in response to altered carbohydrate supply supports a dominant role for these bacteria in butyrate production. Another group of butyrate producers, related to F. prausnitzii, was present in numbers approximately equal to those of Roseburia spp. and E. rectale at maintenance intake (13.5 versus 11.4% of total bacteria) but showed a weak correlation with fecal butyrate concentrations. This would indicate that this bacterial group makes a smaller contribution to fecal butyrate formation from carbohydrates in vivo, consistent with lower rates of butyrate production by F. prausnitzii compared with Roseburia strains in pure culture (17).
The observed changes in the populations of the Roseburia spp. and E. rectale group and in Bifidobacterium spp. may be a direct consequence of insufficient substrate to support growth, but this may not be the only cause. Studies in vitro using continuous flow fermentors inoculated with human fecal bacteria and supplied with a mixed polysaccharide energy source (mainly starch) produced substantial quantities of butyrate at pH 5.5 (60). Under these conditions the Roseburia spp. and E. rectale group, again monitored with the Rrec584 probe, represented approximately 20% of total bacteria. When the pH was increased to 6.5, however, this caused a fourfold drop in butyrate concentration, coupled with the virtual elimination of Roseburia spp. over a 9-day period (60), despite the continued supply of polysaccharides. In vivo, low pH (<6) is thought to accompany active carbohydrate fermentation in the proximal colon (10, 41), and this may allow Roseburia spp. to compete for carbohydrate substrates against other bacteria, such as Bacteroides spp., that are inhibited at low pH (60) and do not produce butyrate. Strategies to increase butyrate production within the large intestine therefore may depend, in part, on supplying sufficient fermentable carbohydrates from the diet (13) to maintain a mildly acidic pH in the lumen of the proximal colon.
The changes in fecal butyrate in the present study represent the largest reported in a human dietary trial and provide the strongest evidence to date that butyrate production is largely determined by the content of fermentable carbohydrate in the diet. Furthermore, this study has provided clear evidence that the proportions of certain groups of colonic bacteria, as monitored in fecal samples, respond to dietary carbohydrate intake. Whether or not the observed changes involving reduced SCFA formation, particularly that of butyrate, and altered microbial community profiles impact on colonic health cannot be assessed from this study. Nonetheless, accumulated evidence indicates that butyrate may promote apoptosis in colorectal cancer cells and help prevent colorectal cancer (4, 45, 47, 49, 59). In addition, an increased butyrate supply has been proposed to prevent colitis (35, 56). The optimal supply of butyrate required in the large intestine to maintain intestinal health is unclear, particularly as the effects on colonocyte cell biology in vivo are complex (12). The present study was of limited duration, and it is unknown whether the relatively short period of reduced butyrate and SCFA supply to the colonic mucosa would have long-term consequences for gut health. Such considerations may become important if low-carbohydrate diets are consumed for longer periods without ensuring that adequate forms of appropriate fermentable substrates comprise part of the diet.
Acknowledgments
The Rowett Research Institute and Biomathematics and Statistics Scotland receive funding from the Scottish Executive Environment and Rural Affairs Department. A. Belenguer received financial support from Comisión Mixta Caja Inmaculada-Consejo Superior de Investigación y Desarrollo de la D.G.A. and Spanish Ministry of Education and Science.
Thanks are due to David Bremner and Marketa Hrdinova for skilled assistance.
Footnotes
Published ahead of print on 22 December 2006.
REFERENCES
- 1.Adam-Perrot, A., P. Clifton, and F. Brouns. 2006. Low-carbohydrate diets: nutritional and physiological aspects. Obes. Rev. 7:49-58. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chishom, A. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminov, R. I., A. W. Walker, S. H. Duncan, H. J. M. Harmsen, G. W. Welling, and H. J. Flint. 2006. Molecular diversity, cultivation, and improved detection by fluorescent in situ hybridization of a dominant group of human gut bacteria related to Roseburia spp. or Eubacterium rectale. Appl. Environ. Microbiol. 72:6371-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avivi-Green, C., S. Polak-Charcon, Z. Madar, and B. Schwartz. 2000. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol. Res. 12:83-95. [DOI] [PubMed] [Google Scholar]
- 5.Bach Knudsen, K. E., A. Serena, N. Canibe, and K. S. Juntunen. 2003. New insight into butyrate metabolism. Proc. Nutr. Soc. 62:81-86. [DOI] [PubMed] [Google Scholar]
- 6.Barcenilla, A., S. E. Pryde, J. C. Martin, S. H. Duncan, C. S. Stewart, C. Henderson, and H. J. Flint. 2000. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 66:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belenguer, A., S. H. Duncan, A. G. Calder, G. Holtrop, P. Louis, G. E. Lobley, and H. J. Flint. 2006. Two routes of metabolic cross-feeding between Bifodobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 72:3593-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman, E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567-590. [DOI] [PubMed] [Google Scholar]
- 9.Boden, G., K. Sargrad, C. Homko, M. Mozzoli, and T. P. Stein. 2005. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 142:403-411. [DOI] [PubMed] [Google Scholar]
- 10.Bown, R. L., J. A. Gibson, G. E. Sladen, B. Hicks, and A. M. Dawson. 1974. Effects of lactulose and other laxatives on ileal and colonic pH as measured by radiotelemetry device. Gut 1:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe, T. C. 2005. Safety of low-carbohydrate diets. Obes. Rev. 6:235-245. [DOI] [PubMed] [Google Scholar]
- 12.Csordas, A. 1996. Butyrate, aspirin and colorectal cancer. Eur. J. Cancer Prev. 5:221-231. [DOI] [PubMed] [Google Scholar]
- 13.Cummings, J. H., and H. J. Englyst. 1987. Fermentation in the human large intestine and the available substrates. Am. J. Clin. Nutr. 45:1243-1255. [DOI] [PubMed] [Google Scholar]
- 14.Cummings, J. H., and G. T. Macfarlane. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443-459. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health (United Kingdom). 2004. Choosing health, choosing a better diet: a consultation on priorities for a food and health action plan—rationale for nutritional priorities. Department of Health (United Kingdom), London, United Kingdom.
- 16.Di Sabatino, A., R. Morera, R. Ciccocioppo, P. Cazzola, S. Gott, F. P. Tinozzi, S. Tinnozi, and G. R. Corazza. 2005. Oral butyrate for mildly to moderately active Crohn's disease. Aliment. Pharmacol. Ther. 22:789-794. [DOI] [PubMed] [Google Scholar]
- 17.Duncan, S. H., A. Barcenilla, C. S. Stewart, S. E. Pryde, and H. J. Flint. 2002. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 68:5186-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan, S. H., G. L. Hold, A. Barcenilla, C. S. Stewart, and H. J. Flint. 2002. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 52:1-6. [DOI] [PubMed] [Google Scholar]
- 19.Duncan, S. H., G. Holtrop, G. E. Lobley, G. Calder, C. S. Stewart, and H. J. Flint. 2004. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 91:915-923. [DOI] [PubMed] [Google Scholar]
- 20.Duncan, S. H., K. P. Scott, A. G. Ramsay, H. J. M. Harmsen, G. W. Welling, C. S. Stewart, and H. J. Flint. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebbeling, C. B., M. M. Leidig, K. B. Sinclair, L. G. Seger-Shippee, H. A. Feldman, and D. S. Ludwig. 2005. Effects of an ad libitum low-glycemic load diet on cardiovascular disease risk factors in obese young adults. Am. J. Clin. Nutr. 81:976-982. [DOI] [PubMed] [Google Scholar]
- 22.Eckburg, P. B., C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flint, H. J. 2006. The significance of prokaryote diversity in the human gastrointestinal tract, p. 65-90. In N. A. Logan, H. M. Lappin-Scott, and P. C. F. Oyston (ed.), Prokaryotic diversity: mechanisms and significance. SGM Symposium, vol. 66. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 24.Food Standards Agency. 2002. McCance and Widdowson's the composition of foods, 6th summary ed. Royal Society of Chemistry, Cambridge, United Kingdom.
- 25.Franks, A. H., H. J. M. Harmsen, G. C. Raangs, G. J. Jansen, F. Schut, and G. W. Welling. 1998. Variations of bacterial populations in human feces quantified by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman, M. R., J. King, and E. Kennedy. 2001. Popular diets: a scientific review. Obes. Res. 9:1S-40S. [DOI] [PubMed] [Google Scholar]
- 27.German, J. B., and C. J. Dillard. 2004. Saturated fats: what dietary intake? Am. J. Clin. Nutr. 80:550-559. [DOI] [PubMed] [Google Scholar]
- 28.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 29.Gill, C. I. R., and I. R. Rowland. 2002. Diet and cancer: assessing the risk. Br. J. Nutr. 88(Suppl. 1):S73-S87. [DOI] [PubMed] [Google Scholar]
- 30.Harmsen, H. J. M., P. Elfferich, F. Schut, and G. W. Welling. 1999. A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 11:3-12. [Google Scholar]
- 31.Harmsen, H. J. M., G. C. Raangs, T. He, J. E. Degener, and G. W. Welling. 2002. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 68:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hold, G. L., S. E. Pryde, V. J. Russell, E. Furrie, and H. J. Flint. 2002. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol. Ecol. 39:33-39. [DOI] [PubMed] [Google Scholar]
- 33.Hold, G. L., A. Schwiertz, R. I. Aminov, M. Blaut, and H. J. Flint. 2003. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 69:4320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu, F. B. 2005. Protein, body weight, and cardiovascular health. Am. J. Clin. Nutr. 82:242S-247S. [DOI] [PubMed] [Google Scholar]
- 35.Jacobasch, G., M. Schiedl, M. Kruschewski, and K. Schmel. 1999. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Colorectal Dis. 14:201-211. [DOI] [PubMed] [Google Scholar]
- 36.Johnston, C. S., S. Tjonn, P. D. Swan, A. White, H. Hutchins, and B. Sears. 2006. Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets. Am. J. Clin. Nutr. 83:1055-1061. [DOI] [PubMed] [Google Scholar]
- 37.Kleesen, B., L. Hartmann, and M. Blaut. 2001. Oligofructose and long chain inulin: influence on the gut microbial ecology of rats associated with a human fecal flora. Br. J. Nutr. 86:375-382. [DOI] [PubMed] [Google Scholar]
- 38.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lay, C., M. Sutren, V. Rochet, K. Saunier, J. Dore, and L. Rigottier-Gois. 2005. Design and validation of 16S rRNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ. Microbiol. 7:933-946. [DOI] [PubMed] [Google Scholar]
- 40.Macfarlane, G. T., and G. R. Gibson. 1997. Carbohydrate fermentation, energy transduction and gas metabolism in the human large intestine, p. 269-318. In R. I. Mackie and B. A. White (ed.), Gastrointestinal microbiology, vol. 1. Chapman and Hall, New York, NY. [Google Scholar]
- 41.Macfarlane, G. T., G. R. Gibson, and J. H. Cummings. 1992. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 72:57-64. [DOI] [PubMed] [Google Scholar]
- 42.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 43.Manz, W., M. Eisenbrecher, T. R. Neu, and U. Szewzyk. 1998. Abundance and spatial organization of Gram-negative sulfate-reducing bacteria in activated sludge investigated by in situ probing with specific 16S rRNA targeted oligonucleotides. FEMS Microbiol. Ecol. 25:43-61. [Google Scholar]
- 44.Mariadason, J. M., G. A. Corner, and L. H. Augenlicht. 2000. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 60:4561-4572. [PubMed] [Google Scholar]
- 45.McIntyre, A., P. Gibson, and G. P. Young. 1993. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeil, N. I. 1984. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39:338-342. [DOI] [PubMed] [Google Scholar]
- 47.Mortensen, P. B., and M. R. Clausen. 1996. Short chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand. J. Gastroenterol. 31(Suppl. 216):132-148. [DOI] [PubMed] [Google Scholar]
- 48.Mueller, S., K. Saunier, C. Hanisch, E. Norin, L. Alm, T. Midtvedt, A. Cresci, S. Silvi, C. Orpianesi, M. C. Verdenelli, T. Clavel, C. Koebnick, H.-J. F. Zunft, J. Doré, and M. Blaut. 2006. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72:1027-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrin, P., F. Pierre, Y. Patry, M. Champ, M. Berreur, G. Pradal, F. Bornet, K. Meflah, and J. Menanteau. 2001. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 48:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pryde, S. E., S. H. Duncan, G. L. Hold, C. S. Stewart, and H. J. Flint. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217:133-139. [DOI] [PubMed] [Google Scholar]
- 51.Ramsay, A. G., K. P. Scott, J. C. Martin, M. T. Rincon, and H. J. Flint. 2006. Cell-associated α-amylases of butyrate-producing Firmicute bacteria from the human colon. Microbiology 152:3281-3290. [DOI] [PubMed] [Google Scholar]
- 52.Richardson, A. J., G. C. Calder, C. S. Stewart, and A. Smith. 1989. Simultaneous determination of volatile and non-volatile fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 9:5-8. [Google Scholar]
- 53.Rombeau, J. L. 2004. Investigations of short-chain fatty acids in humans. Clin. Nutr. 2004(Suppl. 1):19-23. [Google Scholar]
- 54.Salyers, A. A., J. R. Vercelotti, S. E. H. West, and T. D. Wilkins. 1977. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 33:319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salyers, A. A., S. E. H. West, J. R. Vercellotti, and T. D. Wilkins. 1977. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. Appl. Environ. Microbiol. 34:529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segain, J. P., D. R. de la Bletiere, A. Boureille, V. Leray, N. Gervois, C. Rosales, L. Ferrier, C. Bonnet, H. M. Blottiere, and J. P. Glamiche. 2000. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn's disease. Gut 47:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suau, A., V. Rochet, A. Sghir, S. Grammet, S. Breways, M. Sutern, L. Rigottier-Gois, and J. Dore. 2001. Fusobacterium prausnitzii and related species represent a dominant group within the human faecal flora. Syst. Appl. Microbiol. 24:139-145. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services. 2005. Dietary guidelines for Americans. U.S. Department of Health and Human Services, Washington, DC.
- 59.Wachtershauser, A., and J. Stein. 2000. Rationale for the luminal provision of butyrate in intestinal disease. Eur. J. Nutr. 39:164-171. [DOI] [PubMed] [Google Scholar]
- 60.Walker, A. W., S. H. Duncan, E. C. McWilliam Leitch, M. W. Child, and H. J. Flint. 2005. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 71:3692-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson, A. J. M. 2006. An overview of apoptosis and the prevention of colorectal cancer. Crit. Rev. Oncol. Hematol. 57:107-121. [DOI] [PubMed] [Google Scholar]
- 62.Whitehead, R., G. H. Cooke, and B. T. Chapman. 1967. Problems associated with the continuous monitoring of ammoniacal nitrogen in a river water. Anal. Chem. 11:337. [Google Scholar]