Abstract
The impact of host nutrition on symbiont regulation in the pea aphid Acyrthosiphon pisum was investigated. The population density of the obligate symbiont Buchnera aphidicola positively correlated with dietary nitrogen levels. In contrast, the population density of the facultative symbiont Serratia symbiotica increased in aphids reared on low-nitrogen diets, indicating distinct regulatory mechanisms in the same insect host.
The endosymbiotic bacterial partners of aphids (Insecta: Sternorrhyncha) fall into two categories: the obligate “primary” symbiont Buchnera sp. found in almost all aphids and the facultative “secondary” bacteria whose presence is not universal (3, 6, 16). The association between aphids and Buchnera sp. is well documented: the bacteria are housed in specialized host cells, the bacteriocytes or mycetocytes, and they supplement the insects' diet through the provision of essential amino acids (see reference 6 for a full review). In contrast, the association between aphids and their secondary symbionts is less well understood, although the presence of secondary bacteria in symbiosis alongside Buchnera sp. has been known for many years (3, 13). The secondary bacteria are transmitted vertically between host generations (3, 4), but their distribution patterns within and between aphid populations suggest that occasional horizontal transmission must have occurred (5, 19).
The infection density of endosymbionts is one of the most important factors for understanding their biological effects. A reduction in infection density may result in imperfect vertical transmission and attenuated phenotypic effects, which could lead to loss of the infection in host populations. Excessive infection density may lead to enhanced negative or positive phenotypic effects on the host that could significantly influence host fitness and, at their extreme, could cause pathological damage. The proliferation of endosymbionts relies on the consumption of resources from the insect body; consequently, infection densities may be significantly influenced by the nutritional condition of the host. To date, however, no studies have investigated the impact of nutrition on the population density of insect endosymbionts.
In this study, the impact of nutrition on the population density of the obligate symbiont Buchnera aphidicola (17) and the facultative symbiont Serratia symbiotica (16) was investigated using the pea aphid Acyrthosiphon pisum. Two clonal lineages of pea aphid were used: (i) clone IS, a naturally S. symbiotica-infected line (9), and (ii) clone AISTIS, in which the S. symbiotica infection was generated artificially by hemolymph injection (10). Both of these aphid clones also contain B. aphidicola. Continuous infection with S. symbiotica for all aphid generations and the absence of any other facultative symbionts were confirmed by PCR assay and observation by light microscopy. The nutritional condition of the insects was altered by rearing cohorts of genetically identical aphids for the first 7 days of nymphal development on chemically defined artificial diets containing final total nitrogen concentrations of 25, 50, 100, and 150 mM. Diet preparation and composition were identical to those described previously (21), with 50 mol percent essential amino acid content and 500 mM sucrose.
Insect performance.
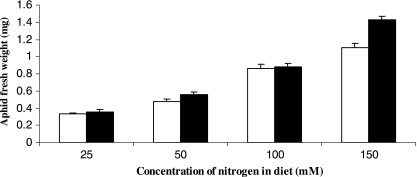
Both aphid clones IS and AISTIS settled and fed on the diets, as indicated by the excretion of honeydew and regular production of exuviae. After 7 days feeding on the diets a small number of aphids on the 150 mM nitrogen diet had molted to the adult stadium. The other aphids feeding on the 150 mM nitrogen diet and all aphids reared on the 100 mM nitrogen diet were in the fourth nymphal stadium. Aphids on the 25 mM and 50 mM nitrogen diets were noticeably smaller and had not molted beyond the third nymphal stadium. The weight gain of the insects positively correlated with the total nitrogen concentration in the diet (Fig. 1) such that aphids reared on the 150 mM diet were approximately threefold larger than aphids reared on the 25 mM diet. In addition, clone AISTIS was slightly larger than clone IS at all nitrogen concentrations (see “clone” data in the legend to Fig. 1). These data highlight the importance of nitrogen as a limiting factor in the nutrition of aphids and other phytophagous insects (2, 12, 18). The remainder of this paper examines how the nutritional condition of the host, as influenced by dietary nitrogen, impacts populations of obligate and facultative endosymbiotic bacteria.
FIG. 1.
The impact of total dietary nitrogen on the body weight of fresh aphids reared on chemically defined diets for 7 days. Open bars, clone IS; closed bars, clone AISTIS; all data represent means ± standard errors (n = 5). ANOVA results: clone, F(1,32) = 7.8 (0.01 > P > 0.001); diet, F(3,32) = 175.8 (P < 0.001); interaction, F(3,32) = 1.4 (P > 0.05).
Symbiont populations in the aphids.
The bacteria in the aphids were quantified by quantitative real-time PCR using a TaqMan PCR core reagent kit and an ABI 7700 system (Applied Biosystems) as previously described (10), and the results are reported in Table 1. This approach quantifies the number of bacterial genomes within an insect rather than the absolute number of bacteria: the B. aphidicola genome (but not the S. symbiotica genome) is thought to be remarkably polyploid (11). The microbiota were quantified from aphids of the same chronological age (7 days) to limit such confounding effects of ploidy and developmental time on the different diets (see above), and individual aphid fresh weight was used to convert absolute numbers of bacterial genomes to genome densities (preliminary results indicated that insect cell number, as quantified in terms of elongation factor 1α, was not a reliable indicator of aphid size, probably due to differences in the rate of ovariole development on the different diets).
TABLE 1.
Bacterial populations in 7-day-old pea aphids reared on chemically-defined diets of different total nitrogen concentrationsa
| Clone and concentration of nitrogen in diet (mM) | Buchnera titer (no. of groEL gene copies per insect × 107)b | Serratia titer (no. of groEL gene copies per insect × 107)c | Contribution of Serratia to total bacterial load (% gene copies) | Buchnera density (no. of groEL gene copies per mg aphid wt × 107)d | Serratia density (no. of groEL gene copies per mg aphid wt × 107)e |
|---|---|---|---|---|---|
| IS | |||||
| 25 | 4.96 ± 0.228 | 0.22 ± 0.066 | 4.2 | 15.1 ± 0.70 | 0.7 ± 0.19 |
| 50 | 9.80 ± 0.732 | 0.39 ± 0.070 | 4.0 | 19.6 ± 1.47 | 0.8 ± 0.14 |
| 100 | 27.76 ± 2.482 | 0.30 ± 0.080 | 1.1 | 28.5 ± 2.85 | 0.3 ± 0.09 |
| 150 | 19.01 ± 1.948 | 0.26 ± 0.079 | 1.3 | 17.1 ± 1.76 | 0.2 ± 0.07 |
| AISTIS | |||||
| 25 | 4.54 ± 0.371 | 0.44 ± 0.170 | 8.2 | 12.6 ± 1.03 | 1.2 ± 0.47 |
| 50 | 8.65 ± 0.794 | 0.79 ± 0.156 | 8.3 | 15.5 ± 1.42 | 1.4 ± 0.28 |
| 100 | 18.56 ± 1.608 | 0.21 ± 0.054 | 1.1 | 21.1 ± 1.83 | 0.2 ± 0.06 |
| 150 | 46.74 ± 3.592 | 0.70 ± 0.107 | 1.5 | 32.9 ± 2.53 | 0.5 ± 0.07 |
All data, except nitrogen concentration and percent contribution, represent means ± standard errors (n = 5).
ANOVA results: F(1,32) for clone, 2.7 (P not significant); F(3,32) for diet, 187.8 (P < 0.001); F(3,32) for interaction, 20.8 (P < 0.001). (All ANOVA variables were ln transformed to obtain normality and homogenous variance prior to analysis.)
ANOVA results: F(1,32) for clone, 7.9 (0.01 > P > 0.001); F(3,32) for diet, 4.2 (0.05 > P > 0.01); F(3,32) for interaction, 2.5 (P not significant).
ANOVA results: F(1,32) for clone, 0.1 (P not significant); F(3,32) for diet, 19.9 (P < 0.001); F(3,32) for interaction, 14.3 (P < 0.001).
ANOVA results: F(1,32) for clone, 4.9 (0.05 > P > 0.01); F(3,32) for diet, 11.4 (P < 0.001); F(3,32) for interaction, 1.8 (P not significant).
B. aphidicola genome number was quantified in terms of groEL gene copies by use of primers BuchGroEL-AF1 (5′-CAGCAACATTATTAGCACAATCTATAGTAAAT-3′) and BuchGroEL-AR1 (5′-TGATAACAGCTTTATCAATTCCACGT-3′) in combination with the fluorescently labeled probe BuchGroEL-TP1 (5′-AAGCAGTAGCAGCTGGTATGAATCCAATGG-3′). The titers of B. aphidicola were not significantly different in the aphid clones and positively correlated with the total nitrogen concentration in the diet (see “clone” and “diet” data, respectively, in analysis of variance [ANOVA] results), although a clone-dependent difference was observed in aphids reared on the 150 mM diet. The general consistency of results obtained for the aphid clones indicates that variability in the degree of ploidy of the B. aphidicola genome is unlikely to be the underlying cause of the observed patterns, although this possibility cannot be excluded. The genome density of B. aphidicola in both clones IS and AISTIS also tended to increase with dietary nitrogen concentration, but the significant interaction term in the ANOVA again highlights the reduction in B. aphidicola density in clone IS on the 150 mM diet. Although little is known about the regulatory mechanisms that maintain B. aphidicola densities within aphids, these results demonstrate that (i) the proliferation of B. aphidicola is linked to the availability of nitrogen and (ii) the density of B. aphidicola is not maintained at a fixed level and can vary, possibly to meet the nutritional demands of the host. In particular, there is likely to be a high demand for the metabolic repertoire of the bacteria in the rapidly growing aphids reared on diets containing 150 mM nitrogen compared to the results seen with aphids reared on diets containing 25 mM nitrogen that grow very slowly. An additional factor is the increasing contribution of the reproductive tissues to aphid size with both developmental and chronological age. The proliferation of B. aphidicola is linked to reproductive condition—the density of B. aphidicola reaches a peak in actively reproducing young adults and declines in postreproductive adults (10). This relationship may explain the decrease in B. aphidicola density in clone IS reared on the 150 mM diet, although degrees of reproductive investment did not differ significantly between aphids reared on the different diets (T. L. Wilkinson, unpublished results). The symbiosis is known to disintegrate in postadult aphids through a reduction in the number of B. aphidicola cells and in the number of bacteriocytes (1, 8) and a decrease in the bacterial division rate in fourth instar larvae (20). Whatever the mechanisms involved, the regulation of B. aphidicola density on a day-to-day basis appears to be fine tuned to meet the metabolic demands of the host, as would be expected given the long evolutionary history of the host-symbiont association (15).
S. symbiotica is considered a facultative bacterial symbiont because its presence is not required for the survival and reproduction of the host insect (16). The population dynamics of S. symbiotica follow a simple logistic growth pattern during aphid development (10), suggesting a lack of strict control over the proliferation of the facultative symbiont. In the present study, S. symbiotica genome numbers were quantified in terms of groEL gene copies by use of primers PASSGroE-AF1 (5′-CCTCAAGGCTGTGGCCG-3′) and PASSGroE-AR1 (5′-GAGTTTGCAGAGATGGTGCCTA-3′) in combination with the fluorescently labeled probe PASSGroE-TP1 (5′-AAGCAGTTGTTGCGGCGGTTGAA-3′). The titer of S. symbiotica in the insects was an order of magnitude lower than that of B. aphidicola, reflecting both the numerical dominance and the genome polyploidy of B. aphidicola (11). While S. symbiotica titers were variable, particularly in clone AISTIS, there was no consistent pattern with respect to total nitrogen concentration in the diet. However, the number of S. symbiotica in clone AISTIS was significantly higher than in clone IS (see main effect “clone” in ANOVA). The population density of S. symbiotica in clone AISTIS was also significantly higher than in clone IS, and in both clones the density of S. symbiotica tended to decrease as dietary nitrogen concentration was elevated. The high population density of S. symbiotica in aphids reared on diets containing low concentrations of nitrogen was reflected in the contribution of S. symbiotica to total bacterial load. In clone IS, S. symbiotica represented approximately 4% of the total endosymbiont population when the aphids were reared on diets containing 25 mM total nitrogen, equivalent to a 3.2-fold increase compared to aphids reared on the 150 mM diet. Similarly, S. symbiotica contributed over 8% of the endosymbiont population in clone AISTIS reared on a diet containing 25 mM total nitrogen, a 5.5-fold increase over aphids reared on the 150 mM diet. The increase in S. symbiotica when aphid performance is limited by dietary nitrogen is surprising, since intuitively the proliferation of S. symbiotica must be dependent on nutritional resources from the aphid, the availability of which will be strongly affected by the quality of the diet. The most plausible explanation is that the proliferation rate of S. symbiotica is constant and independent of insect body size or nutritional condition, such that the density of S. symbiotica is higher in smaller insects of the same chronological age reared on the nutritionally restricted diets, as was observed. Similarly, one would expect newly emerged adult aphids (i.e., of the same developmental age) reared on the 25 mM diet to contain larger populations of S. symbiotica than aphids reared on the 150 mM diet, since development time is extended by approximately 6 days on the nitrogen-restricted diet. However, this was not observed in supplemental experiments (data not shown) in which there was no significant difference between the titers of S. symbiotica from adult aphids from either clone reared on the 25 mM and 150 mM nitrogen diets, suggesting that the proliferation of S. symbiotica is not independent of regulatory controls. Further experiments are required to determine (i) whether the response of other S. symbiotica strains to dietary nitrogen, from A. pisum and from other aphid species, is the same as reported here and (ii) whether other factors linked to nutritional stress, such as an impaired immune response, may be responsible for the proliferation of S. symbiotica.
Endosymbiont transmission.
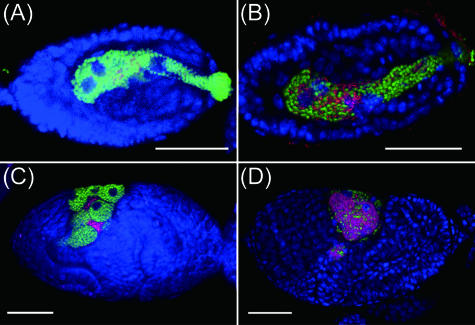
The density of S. symbiotica, but not that of B. aphidicola, was higher in the aphid clone AISTIS than in clone IS. The difference between the clones was consistent in aphids reared on the chemically defined diets (Table 1) and was also observed in 7-day-old aphids reared on the host plant Vicia faba (Table 2), indicating that the higher density of S. symbiotica arises from a difference between the aphid clones. To explore possible mechanisms that might give rise to this interclonal variation, the transmission of S. symbiotica to the parthenogenetic embryo was investigated. Early embryos were dissected from adult aphids reared on the plant and subjected to whole-mount fluorescent in situ hybridization as previously described (16). B. aphidicola and S. symbiotica were detected by specific probes targeting their 16S rRNA: Cy5-ApisP2a (Cy5-5′-CCTCTTTTGGGTAGATCC-3′) and Cy3-PASSisR (Cy3-5′-CCCGACTTTATCGCTGGC-3′), respectively. Host cell nuclei were stained with SYTOX Green (Molecular Probes). The specimens were mounted in SlowFade antifade solution (Molecular Probes) and observed under a laser confocal microscope (Pascal 5; Carl Zeiss). The localizations and densities of B. aphidicola were generally similar between the aphid clones (see Fig. 2), but in contrast, the number of S. symbiotica cells infecting embryos of the clone AISTIS was much greater than that infecting clone IS. At later stages of embryonic development, the S. symbiotica infection in clone IS remained localized to the interstitial areas between bacteriocytes and was at a much lower density than the infection in clone AISTIS, in which S. symbiotica occupied the same tissue location as B. aphidicola. These findings suggest that efficient transmission and rapid proliferation of S. symbiotica in the clone AISTIS may be the cause of the higher bacterial density observed in this study, a result which is probably linked to the history of the symbiotic association and/or the genetic background of the host insect.
TABLE 2.
Bacterial populations in 7-day-old pea aphids Acyrthosiphon pisum reared from birth on the host plant Vicia faba cv. The Suttona
| Aphid clone | Fresh wt (mg)b | Buchnera titer (no. of groEL gene copies per insect × 108)c | Serratia titer (no. of groEL gene copies per insect × 108)d | Buchnera density (no. of groEL gene copies per mg of body wt × 108)e | Serratia density (no. of groEL gene copies per mg of body wt × 108)f |
|---|---|---|---|---|---|
| IS | 2.1 ± 0.12 | 12.3 ± 1.21 | 0.3 ± 0.033 | 3.3 ± 0.36 | 0.2 ± 0.02 |
| AISTIS | 3.7 ± 0.40 | 13.3 ± 0.97 | 1.7 ± 0.58 | 3.5 ± 0.26 | 0.5 ± 0.16 |
All data represent means ± standard errors (n = 5).
Two-tailed t test result: t8 = 3.9 (0.01 > P > 0.001).
Two-tailed t test result: t8 = 0.7 (P not significant).
Two-tailed t test result: t8 = 2.3 (0.05 > P > 0.01).
Two-tailed t test result: t8 = 0.5 (P not significant).
Two-tailed t test result: t8 = 2.6 (0.05 > P > 0.01).
FIG. 2.
Symbiont infection in early embryos of the clones IS and AISTIS. (A) A stage 8 embryo of clone IS; (B) a stage 8 embryo of clone AISTIS; (C) a stage 12 embryo of clone IS; (D) a stage 12 embryo of clone AISTIS. Green, red, and blue signals indicate B. aphidicola, S. symbiotica, and nuclei of host cells, respectively. Bars, 50 μm. At stage 8, embryos are infected with the symbionts, and at stage 12, a large syncytial cytoplasm harboring the symbionts cellularizes into many bacteriocytes. Developmental staging of A. pisum is according to reference 14.
In this study, we demonstrated that, although cohabiting in the same insect, the obligate symbiont B. aphidicola and the facultative symbiont S. symbiotica respond to nutritional stress imposed on the host in different ways. This finding highlights the complex host-symbiont and symbiont-symbiont interactions in this endosymbiotic ecosystem and indicates the nutritional, physiological, and ecological relevance of endosymbiotic associates in natural insect populations. The natural diet of aphids is phloem sap, the availability and quality of which may vary temporally from diurnal changes through to seasonal changes and spatially between different parts of the plant and even different sieve elements (see reference 7 for a review). In polyphagous aphids, host plants of different taxonomic affiliation provide further variations in the quality and quantity of the food source. Under natural conditions, therefore, the endosymbiotic microbiota in aphids must be influenced by these nutritional fluctuations, possibly as observed in this study. Of particular relevance is the observation that the infection density of an unidentified facultative symbiont in the polyphagous aphid Aphis fabae was significantly higher on a host plant on which the aphid exhibited the poorest performance (22).
The biological consequences of such up- or down-regulation of endosymbiont populations are difficult to predict a priori, since a number of factors are affected simultaneously. For example, increasing the population density of a facultative symbiont could enhance both negative and positive aspects of the symbiont infection by acting directly on the host insect or at the same time indirectly by influencing the host via B. aphidicola or other microbial associates. These considerations are relevant to virtually all aphid species; B. aphidicola is ubiquitous in aphids that feed on phloem sap, and many aphids, but not all, possess facultative secondary bacteria (16). Indeed, there is also relevance for the approximately 10% of insect species that utilize the metabolic capabilities of symbiotic microorganisms in their nutrition. In summary, to improve our understanding of the physiology and ecology of aphids and other insects, we must take into account the complex interplay between the host insect, obligate and facultative symbionts, and the environment.
Acknowledgments
Financial support was provided by the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Baumann, L., and P. Baumann. 1994. Growth kinetics of the endosymbiont Buchnera aphidicola in the aphid Schizaphis graminum. Appl. Environ. Microbiol. 60:3440-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodbeck, B., and D. R. Strong. 1987. Amino acid nutrition of herbivorous insects and stress to host plants, p. 347-364. In P. Barbosa and J.C. Schultz (ed.), Insect outbreaks. Academic Press, New York, NY.
- 3.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Wiley, London, England.
- 4.Chen, D. Q., and A. H. Purcell. 1997. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 34:220-225. [DOI] [PubMed] [Google Scholar]
- 5.Darby, A. C., and A. E. Douglas. 2003. Elucidation of the transmission patterns of an insect-borne bacterium. Appl. Environ. Microbiol. 69:4403-4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas, A. E. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17-37. [DOI] [PubMed] [Google Scholar]
- 7.Douglas, A. E. 2006. Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57:747-754. [DOI] [PubMed] [Google Scholar]
- 8.Douglas, A. E., and A. F. G. Dixon. 1987. The mycetocyte symbiosis in aphids: variation with age and morph in virginoparae of Megoura viciae and Acyrthosiphon pisum. J. Insect Physiol. 33:109-113. [Google Scholar]
- 9.Fukatsu, T., N. Nikoh, R. Kawai, and R. Koga. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga, R., T. Tsuchida, and T. Fukatsu. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. Royal Soc. Lond. B. 270:2543-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komaki, K., and H. Ishikawa. 1999. Intracellular bacterial symbionts of aphids possess many genomic copies per bacterium. J. Mol. Evol. 48:717-722. [DOI] [PubMed] [Google Scholar]
- 12.Mattson, W. J. 1980. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. System. 11:119-161. [Google Scholar]
- 13.McLean, D. L., and E. J. Houk. 1973. Phase contrast and electron microscopy of the mycetocytes and symbiotes of the pea aphid Acyrthosiphon pisum. J. Insect Physiol. 19:625-633. [Google Scholar]
- 14.Miura, T., C. Braendle, A. Shingleton, G. Sisk, S. Kambhampati, and D. Stern. 2003. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. 295B:59-81. [DOI] [PubMed] [Google Scholar]
- 15.Moran, N. A., M. A. Munson, P. Baumann, and H. Ishikawa. 1993. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. Roy. Soc., Ser. B. 253:167-171. [Google Scholar]
- 16.Moran, N. A., J. A. Russell, R. Koga, and T. Fukatsu. 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71:3302-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munson, M. A., P. Baumann, and M. G. Kinsey. 1991. Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int. J. Syst. Bact. 41:566-568. [Google Scholar]
- 18.Ponder, K. L., J. Pritchard, R. Harrington, and J. S. Bale. 2001. Difficulties in location and acceptance of phloem sap combined with reduced concentration of phloem amino acids explain lowered performance of the aphid Rhopalosiphon padi on nitrogen deficient barley (Hordeum vulgare) seedlings. Entomol. Exp. Appl. 97:203-210. [Google Scholar]
- 19.Russell, J. A., A. Latorre, B. Sabater-Munoz, A. Moya, and N. A. Moran. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead, L. F., and A. E. Douglas. 1993. Populations of symbiotic bacteria in the parthenogenetic pea aphid (Acyrthosiphon pisum) symbiosis. Proc. Roy. Soc. Lond. Ser. B. 254:29-32. [Google Scholar]
- 21.Wilkinson, T. L., and A. E. Douglas. 2003. Phloem amino acids and the host plant range of the polyphagous aphid, Aphis fabae. Entomol. Exp. Appl. 106:103-113. [Google Scholar]
- 22.Wilkinson, T. L., D. Adams, L. B. Minto, and A. E. Douglas. 2001. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J. Exp. Biol. 204:3027-3038. [DOI] [PubMed] [Google Scholar]