Abstract
Measuring the chemotactic response of Borrelia burgdorferi, the bacterial species that causes Lyme disease, is relatively more difficult than measuring that of other bacteria. Because these spirochetes have long generation times, enumerating cells that swim up a capillary tube containing an attractant by using colony counts is impractical. Furthermore, direct counts with a Petroff-Hausser chamber is problematic, as this method has a low throughput and necessitates a high cell density; the latter can lead to misinterpretation of results when assaying for specific attractants. Only rabbit serum and tick saliva have been reported to be chemoattractants for B. burgdorferi. These complex biological mixtures are limited in their utility for studying chemotaxis on a molecular level. Here we present a modified capillary tube chemotaxis assay for B. burgdorferi that enumerates cells by flow cytometry. Initial studies identified N-acetylglucosamine as a chemoattractant. The assay was then optimized with respect to cell concentration, incubation time, motility buffer composition, and growth phase. Besides N-acetylglucosamine, glucosamine, glucosamine dimers (chitosan), glutamate, and glucose also elicited significant chemoattractant responses, although the response obtained with glucose was weak and variable. Serine and glycine were nonchemotactic. To further validate and to exploit the use of this assay, a previously described nonchemotactic cheA2 mutant was shown to be nonchemotactic by this assay; it also regained the wild-type phenotype when complemented in trans. This is the first report that identifies specific chemical attractants for B. burgdorferi and the use of flow cytometry for spirochete enumeration. The method should also be useful for assaying chemotaxis for other slow-growing prokaryotic species and in specific environments in nature.
Lyme disease is caused by the motile spirochete Borrelia burgdorferi. This disease is the most prevalent arthropod-borne infection in the United States, with 19,804 cases reported in 2004 (11). The clinical course of B. burgdorferi infections includes symmetrical spread of the spirochetes through the dermis resulting in a rash referred to as erythema migrans and invasion of the blood and deep organs (6). Disease manifestations also include arthritis, cardiac abnormalities, and neuropathies. The life cycle of B. burgdorferi involves transmission from a tick vector to mammals or birds and back to the tick over the course of several seasons (54).
A robust motility-and-chemotaxis system is likely to be vital for B. burgdorferi in its overall life cycle. Many of the motility and chemotaxis genes are expressed in both the tick and the mammalian host, and several are upregulated in the laboratory that mimic in vivo conditions (8, 16, 21, 45, 48, 57). Furthermore, approximately 6% of its chromosomal genome encodes putative chemotaxis and motility genes (18) and between 10 and 14% of the total cellular protein is composed of flagellar filament proteins (42). Thus, this system is both evidently important but energetically expensive to the spirochete. Motility and chemotaxis have been postulated as being important for the spirochetes to migrate from the tick gut to salivary glands for deposition into the new host upon infection (12, 16). In the mammalian host, motility may be important for the spirochetes to penetrate the bloodstream after being deposited in the skin by the tick bite and also for specific tissue and organ localization (32, 55). For the cycle to be completed, salivary proteins and other compounds could conceivably serve as attractants during tick feeding (12, 32, 53). This chemotactic signaling would result in the spirochetes concentrating at the site of the tick bite for cycle continuation. Chemotaxis and motility have been extensively studied in several species of bacteria and are best understood in the enteric species Escherichia coli and Salmonella enterica serovar Typhimurium (3, 33, 47, 59). Bacterial chemotaxis involves a sensory transduction system that enables cells to swim toward a favorable environment or away from one that is toxic.
We have begun to understand the basic movements of B. burgdorferi motility (12). These spirochetes have two bundles of periplasmic flagella (PFs) located between the cell cylinder and outer membrane sheath. Each bundle consists of 7 to 11 PFs that overlap in the center of the cell. As with other spirochete species, the PFs are attached to basal bodies similar to those of other bacteria (44); these basal bodies are subterminally positioned at each pole of the cell cylinder. A model of B. burgdorferi motility states that during a run there is a coordinated rotation of the two PF bundles that results in backward-moving flat waves (12, 22, 23, 31). These waves propel the spirochete in a given direction. To balance the rotation of the PFs, the cell body rolls around its axis in the opposite direction. During the run, the two bundles rotate in opposite directions, i.e., asymmetrically, one going clockwise (the frame of reference is viewing the PF from its distal end to its insertion into the cell) and the other counterclockwise. Cells reverse directions; they also have a stop mode referred to as a flex when the bundles are believed to rotate in the same direction (clockwise or counterclockwise).
The subpolar localization of the bundles of PFs with their consequential asymmetrical rotation during runs adds a level of complexity in deciphering B. burgdoferi's chemotactic response (12). Nonchemotactic cheA mutants of B. burgdorferi constantly run (30), as do cheA mutants of the spirochete Treponema denticola (34) and also E. coli and S. enterica serovar Typhimurium (46). In addition, inactivation of B. burgdorferi cheX, which encodes a specific cheY phosphatase, was recently found to result in constant flexing (41). However, a chemosensory model that integrates the phenotypes of these cheA2 and cheX chemotaxis mutants has yet to be formulated.
Identifying compounds that are chemoattractants for B. burgdorferi will not only permit a further understanding of its motility and chemotaxis on a molecular level but should also facilitate better understanding of how these organisms shuttle between their mammalian or avian and arthropod hosts. The capillary tube assay is a well-documented procedure for quantitatively assessing bacterial chemotaxis and identifying attractants (1). In this assay, bacteria that have entered capillary tubes containing attractants are usually counted by colony formation. However, measuring B. burgdorferi chemotaxis with the capillary tube assay has been difficult. B. burgdorferi bacteria are fastidious and slow growing, with approximately 2 to 3 weeks required for colony formation. While direct counting of B. burgdorferi bacteria in capillary tubes with Petroff-Hausser chambers has been used to demonstrate that rabbit serum and tick saliva are attractants (52, 53), this approach is highly labor-intensive and has a low throughput. In addition, it requires a high density of cells (at least 1 × 108/ml in the motility buffer medium) (30, 52). This high cell density can lead to misinterpretation of results. Specifically, cell metabolism could conceivably lead to an alteration in the gradient such that attractant depletion (1) or the generation of a secondary metabolite could interfere with or even promote chemotaxis. To overcome these limitations, a flow cytometry approach was developed to accurately and rapidly enumerate B. burgdorferi bacteria at a relatively low cell concentration. This method was optimized and used to identify specific chemicals that elicit a chemotactic response in B. burgdorferi. In addition, it was also exploited to further characterize the chemotaxis cheA2 mutant by complementation (30). A brief description of the use of this method to analyze a B. burgdorferi cheX mutant has recently been published (41).
MATERIALS AND METHODS
Bacterial strains and culture.
A single clone of high-passage B. burgdorferi B31 (B31A) and its cheA2::kan deletion mutant (LC-A2, referred to as the cheA2 mutant) were grown at 33 to 34°C in liquid BSK-II medium (7, 30). Kanamycin (350 μg/ml) and streptomycin (80 μg/ml) were used as needed. Swarm plate chemotaxis assays were carried out as previously described (30, 40).
DNA manipulation, primers, and cheA2 complementation.
Restriction mapping, enzyme modification, and transformation were carried out by standard procedures. Based on the published B. burgdorferi genome sequence (18), DNA amplifications with the PCR primers listed in Table 1 were carried out with Taq polymerase. cheA2 mutant cells were transformed with the intact cheA2 gene for construction of the complemented strain, referred to as cheA2+, as follows. A 409-bp B. burgdorferi flgB promoter fragment with PstI and NdeI sites at the 5′ and 3′ ends, respectively, was PCR amplified and cloned into pGEM-T Easy (Promega Co., Madison, WI). The entire cheA2 gene was PCR amplified with primers engineered with NdeI and SphI sites at the 5′ and 3′ ends, respectively, and ligated into pGEM-T Easy. This cheA2 fragment was digested from the pGEM-T easy vector and fused to the 3′ end of the flgB promoter at the NdeI site. The resultant flgB-cheA2 fusion fragment was excised with PstI/SphI and subcloned into B. burgdorferi shuttle vector pKFSS1 (17) that was modified by the insertion of a T7 terminator at SphI/HindIII, generating the complementation plasmid pFlgBA2com. DNA sequencing confirmed that the insert was present in the proper orientation. The preparation of B. burgdorferi competent cells and electrotransformation were performed as previously described (40, 49). Approximately 10 μg of the pFlgBA2com plasmid was transformed into B. burgdorferi cheA2 mutant cells by electroporation. After 2 to 3 weeks of incubation in BSK-II agar plates containing 80 μg/ml streptomycin, antibiotic-resistant colonies were picked, transferred to BSK-II medium, and further analyzed.
TABLE 1.
Primers used to amplify genetic elements
| Fragment | Primer name | Primer sequencea |
|---|---|---|
| flgB promoter | pflgB1-forward | 5′-CTGCAG TAATACCCGAGCTTCAAG-3′ |
| pflgB2-reverse | 5′-CATATGACCTCCCTCATTTAAAATTGC-3′ | |
| cheA2 | FPA2-forward | 5′-CATATGATTAAAGAGGAGAAATTAACTATG-3′ |
| RPA2-reverse | 5′-GCATGCTCAGCTAATTAAGCTTGGCTG-3′ | |
| T7 terminator | pFT7-forward | 5′-GCATGCGCTAACAAAGCCCGAAAGGAAGCT-3′ |
| pRT7-reverse | 5′-AAGCTTTGCAGATCCGGATATAGTTCCTCC-3′ | |
| cheA2 probe | DFA2-forward | 5′-AAAAGACAATCCTATGGCTAC-3′ |
| DFA2-reverse | 5′-TAACTCTCTCTTCAACTGTTTC-3′ |
Engineered restriction sites are underlined.
Western and Southern blotting.
Western blotting was carried out as previously described, by the ECL detection method (Amersham Pharmacia, Piscataway, NJ) (19). P. Matsumura (University of Illinois, Chicago) and J. Benach (State University of New York at Stony Brook) generously provided rabbit antiserum to E. coli CheA (used at a 1:1,500 dilution) and monoclonal antibodies to B. burgdorferi DnaK (used at a 1:3,000 dilution), respectively. Southern blot analysis was used to determine whether the intact cheA2 gene in complemented cells resided on the chromosome or a plasmid (50). Chromosomal DNA was isolated by phenol-chloroform extraction; plasmids were isolated with a Wizard plus SV Minipreps Kit (Promega Co., Madison, WI) and a HiSpeed Plasmid Midi Kit (QIAGEN Co., Hilden, Germany). A 906-bp fragment within cheA2 was PCR amplified (Table 1), labeled with digoxigenin (DIG DNA Labeling and Detection Kit; Boehringer, Mannheim, Germany), and used as a probe.
Flow cytometry enumeration of B. burgdorferi.
All culture media and solutions, except methylcellulose, were filtered (0.1 μm) before flow cytometry. B. burgdorferi cells were diluted in counting solution (0.01 M HEPES, 0.15 M NaCl, pH 7.4, 10 nM fresh Syto61 [Molecular Probes Inc., Eugene, OR]), with or without 6-μm-diameter polystyrene beads (final concentration, 3 × 103 to 5 × 103/ml; Duke Scientific Co., Palo Alto, CA). Syto61 is a membrane-permeating, nucleic acid-binding, fluorescent dye excitable at 635 nm with emission at 647 nm. The beads were used in the initial experiments as an internal standard to ensure consistent volumes. Samples were analyzed in a Becton-Dickinson FACScalibur with 15-mW air-cooled argon and red diode lasers operating at 488 and 635 nm, respectively, at a rate of 12 or 16 μl/min for 60 or 120 s to ensure at least 100 events in the gate where cells were observed. Compensation was unnecessary, as there was no spectral overlap between detectors. All data acquisition and analysis was performed with CellQuestPro (Becton-Dickinson, San Jose, CA) and Excel (Microsoft Co., Redman, WA).
Modified capillary tube chemotaxis assay.
The capillary tube assay developed by Pfeffer and described by Adler was optimized to measure B. burgdorferi chemotaxis (1). B. burgdorferi cells were grown to late logarithmic phase (∼7.5 × 107 cells/ml) from an initial concentration of 2 × 105 cells/ml and centrifuged at 23°C for 8 min at 1,800 × g and gently resuspended in a motility buffer consisting of 136.9 mM NaCl, 8.10 mM Na2HPO4, 2.7 mM KCl, 1.47 mM KH2PO4, 2% recrystallized bovine serum albumin (BSA; Sigma-Aldrich Co., St. Louis, MO), and 0.1 mM EDTA and adjusted to pH 7.4. Cells were first enumerated by flow cytometry and then diluted to 1 × 107/ml in motility buffer containing 1.0% methylcellulose (400 mesh; Sigma-Aldrich Co., St. Louis, MO), yielding a final viscosity of 224 cP (33°C, Cannon-Fenske viscometer). Attractants were dissolved in motility buffer-1.0% methylcellulose and readjusted to pH 7.4. Chemotaxis chambers consisted of either 2.0-ml Microfuge tubes with a Parafilm sheet closed under a perforated cap or two 96-well plates sandwiched face to face with tape and with holes on one side for inserting capillary tubes in well bottoms (Fig. 1). Cells were transferred to Microfuge tubes (0.3 ml) or 96-well plates (0.15 to 0.2 ml), and attractant-filled 70-μl capillary tubes (catalog no. 22-362-574; Fisher Scientific, Pittsburgh, PA) plugged with silicone grease were inserted into the chambers. After the chambers were incubated at 33°C in 3% CO2 for 120 min, the capillary tubes were removed, the outsides were carefully wiped with a paper towel, and the contents were expelled by centrifugation at 1,000 × g for 3 to 4 s. Approximately 0.01-ml volumes of expelled solutions were mixed with 0.49 ml of counting solution, and bacteria were enumerated by flow cytometry. The mean of four to five replicas was determined, and the results are expressed as the mean relative increase over a buffer control containing no attractant (referred to as the relative chemotactic response) or as the number of cells that migrated into the capillary tube. At least three independent experiments were done for each test compound, and the results are expressed as the mean ± the standard deviation (SD) for individual experiments or the standard error of the mean for multiple experiments. An increase in the number of spirochetes equal to or greater than twice that of the buffer control was considered significant (26, 27, 35, 43). All chemicals were obtained from Sigma-Aldrich Chemical Co., St. Louis, MO, with the exception of chitosan dimers [β-(1-4)-linked d-glucosamine; U.S. Biological, Swampscott, MA] and N-N-diacetyl-chitobiose [β-(1-4)-linked N-acetyl d-glucosamine; Associates of Cape Cod, Inc., East Falmouth, MA].
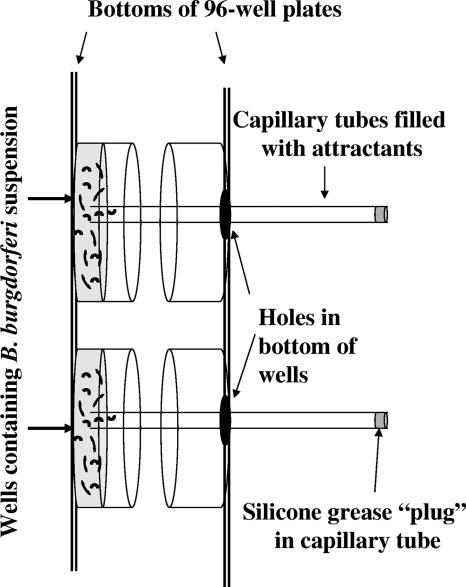
FIG. 1.
Schematic of chemotaxis assay using two 96-well plates. B. burgdorferi cells in motility buffer plus methylcellulose were placed in wells of a 96-well plate. Holes were formed in the corresponding bottoms of wells of another 96-well plate with a heated metal cylinder. These 96-well plates were taped together face to face, with the plate containing the cells lying face up. Capillary tubes filled with attractant in motility buffer plus methylcellulose were plugged at one end with silicone grease and placed through the holes in the top 96-well plate. The ends of the capillary tubes dipped into the cell suspension in the corresponding wells of the other 96-well plate. The sandwiched plates were incubated such that the attractant-filled capillary tubes were horizontal but still remained inserted in the cell suspension.
Motion analysis.
To track cells, the spirochetes were prepared as described for the chemotaxis assay and then visualized at ×200 magnification with a Zeiss Axioskop 2 under dark-field illumination (Carl Zeiss Inc., Oberkochen, Germany) equipped with a 35°C heated stage (Physitemp Inc., Clifton, NJ). The Hobson BacTracker was used in the initial tracking experiments (30), and the software package Volocity (Improvision Inc., Coventry, United Kingdom) was used in later analyses. With Volocity, video sequences of swimming cells were captured with iMovie on a PowerMac G4 (Apple Computer Inc., Cupertino, CA) with a Scion LG-5 (Scion Inc., Fredrick, MD) frame grabber card and a Dage MTI (Dage-MTI Inc., Michigan City, IN) black-and-white video camera. Videos were exported as QuickTime (Apple Computer Inc., Cupertino, CA) movies and imported into OpenLab (Improvision Inc., Coventry, United Kingdom) where the frames were cropped, calibrated with a stage micrometer, and saved as LIFF files. Volocity was then used to track the LIFF files.
RESULTS
Flow cytometry enumeration of B. burgdorferi bacteria.
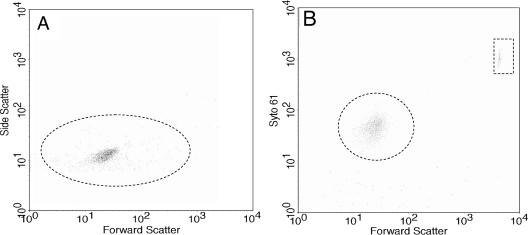
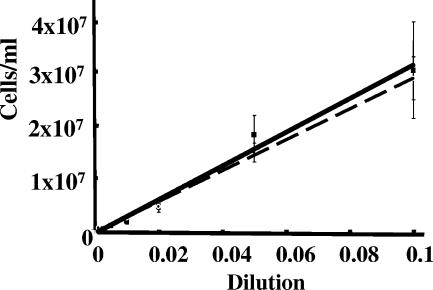
Although flow cytometry has been previously used to assay for gene expression of B. burgdorferi (2, 9, 10, 13-15), it has not been used to enumerate cells. We developed a flow cytometry method to quantitate B. burgdorferi. In the absence of fluorescent dyes in our chemotaxis assays, flow cytometric analysis by side versus forward scatter indicated a distinct cell population but with considerable background (Fig. 2A). This background had several causes, including electronic noise, residual particulate material in the BSK-II medium, and B. burgdorferi's flat-wave morphology and variable length. To separate B. burgdorferi from this background, the fluorescent, nucleic acid-staining dye Syto61 was tested. We first determined that neither the debris nor B. burgdorferi cells autofluoresced with the FLH-4 fluorescence detector. In addition, the debris did not take up the dye. Staining B. burgdorferi cells yielded a highly uniform population (Fig. 2B) well resolved from the latex bead control (rectangle) and the background. These beads were initially incorporated into the counting solution as an internal control for sample flow rates through the cytometer, but because of high reproducibility, the beads were eliminated in later studies. To test the validity of this assay for enumerating B. burgdorferi bacteria, several different concentrations of cells were counted both with the Petroff-Hausser counting chamber and by flow cytometry. As shown in Fig. 3, the correlation between the two methods was excellent, with a correlation coefficient greater than 0.98.
FIG. 2.
Flow cytometric analysis of B. burgdorferi. Wild-type cells were analyzed by flow cytometry in the absence (A) or presence (B) of Syto61. The y axis represents side scatter in panel A and intensity of Syto61 staining in panel B. The dotted circle in panel A indicates that B. burgdorferi cells are detected by side scatter, and that in panel B indicates detection of cells stained with Syto61. Polystyrene beads formed a distinct fluorescent population (dotted box) in the presence of Syto61 (B). Results identical to those in panel B were also obtained with cheA2 mutant cells (not shown).
FIG. 3.
Comparison of cell enumeration by flow cytometry and the Petroff-Hausser counting chamber. B. burgdorferi bacteria were collected by centrifugation, suspended in motility buffer, and serially diluted. Cell concentrations were determined manually with a Petroff-Hausser counting chamber (squares, solid line) or by flow cytometric analysis (circles, dashed line). The coefficient of correlation between the two methods was >0.98.
Optimization of the capillary tube chemotaxis assay for B. burgdorferi.
The capillary tube chemotaxis assay using flow cytometry was optimized for B. burgdorferi. We found that 100 mM N-acetylglucosamine served as a relatively strong and consistent attractant. Accordingly, the parameters for the chemotaxis assay were established with this compound. A concentration of 1 × 107 cells/ml in the buffer chamber was found to be optimal. If we used a lower concentration (1 × 105 to 1 × 106 cells/ml), the results were variable due to the lower number of cells that entered the capillary tubes. Assays using 1 × 107 cells/ml in the motility buffer provided sufficient cell numbers for reproducible counts, and this low number limited the possibility that metabolism of the test compound could influence the chemotaxis assay. This cell concentration is in the range (6 × 107 cells/ml) of the standard chemotaxis assay used for E. coli (1). The number of B. burgdorferi bacteria entering capillary tubes containing motility buffer alone (control) or motility buffer with N-acetylglucosamine (100 mM) steadily increased as a function of time up to 2 h. The relative chemotactic response to N-acetylglucosamine was greatest at 2 h, which was adopted as the standard incubation time; Shi et al. (52) also used 2 h in their B. burgdorferi chemotaxis assay.
Several other parameters were examined to optimize the chemotaxis assay. Gentle resuspension of the cells in the motility buffer after centrifugation was essential for the cells to maintain motility. At least 90% of the resuspended cells were motile at the beginning of the assay, and at least 50% remained motile when the assay was stopped. A second wash and recentrifugation markedly inhibited motility in the buffer solution. Furthermore, preincubating or starving the centrifuged cells in motility buffer longer than the 45 min required to set up the assay resulted in a diminished chemotactic response. Initial experiments indicated that the composition of the buffer was critical for the cells to maintain motility throughout the 2-h incubation period. Specifically, suspending the cells in buffer containing recrystallized rather than Cohn fraction V BSA, or fatty acid-free BSA, was essential. Because B. burgdorferi and other spirochetes increase their velocity in a gel-like medium (23, 29), we tested whether methylcellulose added to the motility buffer and attractant solutions influenced the chemotaxis assay. We found that the addition of 1% methylcellulose resulted in more reproducible results from one experiment to another. Thus, methylcellulose was incorporated in all assays. We also determined whether the age of the cells influenced the chemotactic response. Adler found early logarithmic-phase cells of E. coli to be optimal (1). In contrast, we found that cells taken from the late logarithmic phase of growth responded best to the attractant (Fig. 4). In addition, these cells were of more uniform size than stationary-phase cells and were thus easily counted with the gating parameters chosen. While conventional bacterial capillary tube chemotaxis assays use small (usually 1-μl) capillary tubes with heat sealing of one end (1), we used 70-μl microhematocrit capillary tubes (30, 41). These tubes provided the volume and numbers of cells necessary for flow cytometric analysis. In addition, these tubes can be more readily filled and plugged at one end with silicone than the 1-μl capillary tubes. Because the filling solution contains BSA, heat sealing of the tubes was not a reasonable option. In our initial chemotaxis assays, we used the standard U-shaped tube sandwiched between a cover glass, a capillary tube, and a slide (1). However, Eppendorf tubes, as well as sandwiched 96-well plates with submerged capillary tubes, containing attractant yielded results comparable to those of the standard assay. The latter assays were adopted, as the materials were easier to manipulate and consequently were less likely to lead to a laboratory-acquired infection.
FIG. 4.
Growth curve dependence of chemotaxis. B. burgdorferi cells were assayed for chemotaxis during different phases of growth. The chemotactic response is represented by the bars, and cell numbers per milliliter are represented by the dashed line. The cell division time during logarithmic growth was 9.33 h. Error lines represent SDs of quadruplicate samples.
Defined attractants for B. burgdorferi.
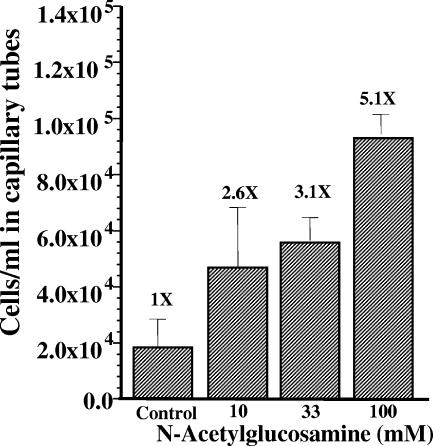
In many bacterial species, compounds that are transported and are also metabolized by cells are often chemoattractants. Genomic analysis indicated that a putative N-acetylglucosamine transporter is encoded in the B. burgdorferi genome (18). In addition, either N-acetylglucosamine or the N-acetylglucosamine dimer chitobiose is an essential nutrient for B. burgdorferi (56). Accordingly, N-acetylglucosamine was tested as a chemoattractant. N-Acetylglucosamine induced a concentration-dependent increase in the number of cells that migrated into the capillary tube compared to the buffer control (Fig. 5). The relative chemotactic response to 200 mM N-acetylglucosamine was not significantly greater than that to 100 mM N-acetylglucosamine (data not shown), suggesting that the chemotactic response may have been saturated at 100 mM N-acetylglucosamine. Although these results suggested that N-acetylglucosamine was a chemoattractant, other explanations are possible. One possibility is that N-acetylglucosamine increased the velocity of the cells, and this increase resulted in more cells migrating into the capillary tube. We found that the velocity of the cells with different concentrations of N-acetylglucosamine were not different than the buffer control (3 to 5 μm/s). We also tested if a gradient of attractant was necessary for the cells to migrate into the capillary tube (37). When cells were suspended in the buffer containing the same concentration of N-acetylglucosamine as found in the capillary tube, there was no increase in cells in the capillary tube compared to the control (Fig. 6, no-gradient control). Finally, we tested nonchemotactic cheA2 mutant cells in the capillary tube assay. This mutant has been previously shown to be nonchemotactic to rabbit serum, and it had a constantly running phenotype (30). We found that this mutant failed to show an increase in cell numbers in the capillary tube compared to the buffer control (Fig. 6). Taken together, the results indicate that N-acetylglucosamine is a chemoattractant for B. burgdorferi.
FIG. 5.
Dependence of chemotaxis on N-acetylglucosamine concentration. Capillary tubes filled with motility buffer alone (control) or the indicated concentrations of N-acetylglucosamine were incubated with B. burgdorferi cells and assayed for chemotaxis. The results of a representative experiment are depicted, showing the mean and SD of quadruplicate samples. The relative chemotactic response is indicated as a times value at the top of each bar.
FIG. 6.
CheA2 and attractant gradient dependence of B. burgdorferi on chemotaxis to N-acetylglucosamine. Capillary tubes filled with motility buffer containing 100 mM N-acetylglucosamine were assayed for chemotaxis with wild-type B. burgdorferi, the cheA2 mutant, or complemented strain cheA2+. The relative chemotactic response compared to the buffer control was determined. Results are expressed as the mean of four experiments ± the standard error. “No gradient” indicates a control in which both the capillary tube and the suspending motility buffer contained equal concentrations of N-acetylglucosamine.
Based on known B. burgdorferi nutritional requirements and/or the presence of putative transporters in the genome (5, 18), other compounds were assessed for a chemotactic response. In each experiment, a nongradient control and cheA2 mutant cells were used to verify the chemotactic response. The putative N-acetylglucosamine transporter is postulated to also be involved in the transport of glucosamine (18). However, glucosamine is not an essential nutrient as is N-acetylglucosamine. We found that glucosamine elicited a concentration-dependent response, similar to N-acetylglucosamine. Besides N-acetylglucosamine and glucosamine, chitosan dimers, glucose, and L-glutamate were found to be chemoattractants for B. burgdorferi (Table 2). These compounds induced a relative chemotactic response equal to or greater than twice the buffer control in a concentration-dependent manner. Both glutamate and glucose were weak attractants, as their response approximated twice the buffer control. Furthermore, the response to glucose was variable whereas that for glutamate was consistent. Several of the compounds tested showed no chemotactic response, and these included glycine and β-alanine.
TABLE 2.
Chemotactic responses of B. burgdorferi to specific compoundsa
| Compound | Relative chemotactic response ± SEM |
|---|---|
| Chitosan dimer [β-(1-4)-linked d-glucosamine] | 4.5 ± 1.4 |
| Glucosamine | 3.5 ± 0.3 |
| N-Acetylglucosamine | 2.7 ± 0.3 |
| Glutamate | 2.2 ± 0.1 |
| Glucose | 2.0 ± 0.5 |
| β-Alanine | 1.4 ± 0.1 |
| Glycine | 1.2 ± 0.2 |
The relative chemotactic response is the mean response to the test compound relative the buffer control for at least three experiments. All test compounds were at 100 mM, except the chitosan dimer, which was at 10 mM. Lower concentrations of the above compounds did not elicit a stronger response (not shown except in Fig. 5). All of the compounds shown except β-alanine and glycine elicited relative chemotactic responses of ≥2 and were considered chemoattractants.
Complementation of cheA2.
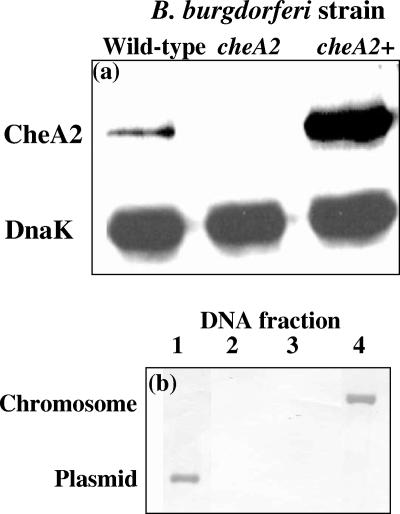
We used the flow cytometry chemotaxis assay to test for complementation of cheA2 mutant cells. We previously reported that inactivation of cheA2 by targeted mutagenesis with either a kan or an erm cassette inhibited CheA2 synthesis and chemotaxis toward rabbit serum (30). These mutants failed to reverse and constantly ran. The kan cassette inserted into cheA2 did not affect expression of the downstream gene cheY3, but insertion of the erm cassette resulted in a decrease of CheY3 accumulation by one-third (30). Although these results pointed toward cheA2 being involved in chemotaxis, it was not rigorously determined whether the resulting phenotype in the cheA2 mutant cells was due to inactivation of cheA2 or to a secondary mutation. Accordingly, we complemented this strain with shuttle plasmid pFlgBA2com, a derivative of pKFSS1 (17), and analyzed the resulting genotype and phenotype. The strong flgB promoter (20) was used to drive transcription of cheA2 in the complemented strain (referred to as cheA2+). Western blot analysis indicated that CheA2 was produced in the cheA2+ strain but not in cheA2 mutant cells (Fig. 7a). Using reactivity to DnaK to normalize the amount of protein loaded in the gels, we found that the level of CheA2 produced in cheA2+ cells was greater than that produced in wild-type cells. These results are consistent with reports that the flgB promoter is quite strong (20).
FIG. 7.
cheA2 inactivation and complementation. (a) Ten micrograms of protein from lysates of wild-type, cheA2, and cheA2+ cells were electrophoresed on sodium dodecyl sulfate-polyacrylamide gels, blotted onto nitrocellulose, and reacted with E. coli CheA and B. burgdorferi DnaK antibodies. (b) Southern blot analysis of chromosomal or plasmid DNA from cheA2+, wild-type, and cheA2 mutant cells (see the text). Southern blot analysis was carried out with a digoxigenin-labeled DNA probe complementary to a fragment of cheA2 deleted in the mutant (Table 1). The chromosomal and plasmid DNAs migrated at rates represented by the vertical axis.
We also determined if pFlgBA2com is extrachromosomal in cheA2+ or if it is integrated into the chromosome. Southern blot analysis was used to localize intact cheA2. Chromosomal and plasmid DNAs from wild-type, cheA2 mutant, and cheA2+ cells were separated from one another. With a probe complementary to the deleted fragment in the cheA2 mutant (Table 1), positive reactions were detected with chromosomal DNA isolated from wild-type cells (Fig. 7b, lane 4) and plasmid DNA from cheA2+ cells (lane 1). No reaction was detected with plasmid DNA isolated from cells of the wild type (lane 2) or with chromosomal DNA isolated from cheA2+ (not shown) or cheA2 mutant (lane 3) cells. These results indicate that pFlgBA2com replicated as a plasmid in cheA2+ cells.
We determined if pFlgBA2com restored the wild-type phenotype in the cheA2 mutant. With the flow cytometry chemotaxis assay, we found that cheA2+ cells had a strong chemotactic response to N-acetylglucosamine (Fig. 6). Restoration of the chemotactic response in cheA2+ cells was corroborated by the swarm plate chemotaxis assay (30); wild-type and cheA2+ cells both formed 17- to 18-mm-diameter swarm rings in diluted growth medium at 3 days of incubation. These swarm ring diameters were considerably larger than that of the cheA2 mutant, which was 3 to 5 mm (not shown).
We also tracked the swimming behavior of cheA2+ cells (30) and compared it to that of the wild type. Whereas cheA2 mutant cells failed to reverse and continuously translated in one direction, cheA2+ cells readily reversed. The reversal frequency of cheA2+ cells (22 ± 1.0 reversals/min) was the same that of wild-type cells (21 ± 1.5 reversals/min). These results indicate that pFlgBA2com restored the wild-type phenotype in the cheA2 mutant cells and that a secondary mutation was not responsible for its altered phenotype. In addition, these results also illustrate that the flow cytometry chemotaxis assay is applicable in analyzing the chemotactic response of B. burgdorferi.
DISCUSSION
The routinely used capillary tube chemotaxis assay has obvious limitations when adopted for use with slow-growing bacteria. To facilitate identification of defined chemoattractants and to begin to define the complex molecular events involved in B. burgdorferi chemotaxis, we developed a rapid capillary tube chemotaxis assay in combination with flow cytometry. The enumeration of Sty061-stained cells by flow cytometry produced results identical to those obtained with a Petroff-Hausser chamber. The main advantages of flow cytometry over manual counting are that it enables the use of a lower concentration of cells and it has a much higher throughput.
Results of capillary tube assays are often reported as the “relative chemotactic response” (26, 27, 35, 43). This response is defined as the ratio of cells that migrate into the attractant versus buffer-filled capillary tubes, and it is used to normalize day-to-day variability. A relative chemotactic response of ≥2 is frequently used to indicate a significant chemotactic response (26, 27, 35, 43). We identified a number of compounds as being chemoattractants by this criterion (Table 2). Furthermore, other results also indicate that these compounds are chemoattractants. Specifically, no response occurred with these compounds in the absence of a concentration gradient. In addition, cheA2 mutant cells failed to show a positive response to these compounds. We show here that trans complementation of this mutant resulted in cells regaining the chemotaxis response. Finally, two of these compounds, N-acetylglucosamine and glucosamine, were tested for the ability to increase cell speed and no increase was detected. Thus, the positive results found with these two test compounds were not a consequence of a general physiological effect that resulted in an increase in cell velocity.
The compounds identified to date as B. burgdorferi chemoattractants elicited relative chemotactic responses ranging from ∼2 to 5 (Table 2), which is at the lower end of the range relative to other bacteria. For example, the relative chemotactic responses of several bacterial species are approximately the following (calculated): E. coli, 10 to 72 (1, 38); Spirochaeta aurantia, 4 to 155 (24); Pseudomonas aeruginosa, 2.5 to 25 (26, 43); Brachyspira hyodysenteriae, 2 to 16 (27). The apparent lower relative chemotactic responses found with B. burgdorferi may be attributed, in part, to shallower attractant gradients with the larger capillary tubes in the modified chemotaxis assay; a decrease in the number of bacteria entering larger capillary tubes in chemotaxis assays occurs for E. coli (1). The assay described herein uses 70-μl capillary tubes, whereas 1-μl capillary tubes are used in the standard assay (1). Seventy-microliter capillary tubes have an aperture ∼7.6 times the area of 1-μl capillary tubes. Calculations of diffusion gradients indicate a gradient that is shallower with 70-μl capillary tubes than with 1-μl capillary tubes but is still present at 2 h of incubation (4). With a different chemotaxis assay, in which B. burgdorferi bacteria were inoculated into one side of a U-shaped tube partially filled with semisolid medium and attractants were placed at the other end, somewhat higher relative chemotactic responses (∼10 to 25) were reported for salivary gland extracts prepared from ticks that fed on hosts over a 12- to 48-h assay period (53). This higher response may be attributed to the different type of assay or to more potent chemoattractants in the salivary gland extracts.
The use of capillary tubes with large apertures, producing shallow gradients, may also have required higher concentrations of most attractants (100 mM) to elicit significant chemotactic responses in B. burgdorferi than would be required for smaller capillary tubes. However, similar concentrations of attractants (∼100 mM) elicited the maximal chemotactic response for E. coli and S. enterica serovar Typhimurium to alanine, asparagine, glycine, and several other amino acids (25, 36, 37). Furthermore, maximal chemotactic responses were observed with 100 mM chemoattractants for S. aurantia (24) and for B. hyodysenteriae (27) to 12 of 21 and to 3 of 9 defined chemoattractants, respectively.
Both rabbit serum and tick saliva have been identified as B. burgdorferi chemoattractants, but these attractants are complex and the specific attractants in these mixtures have not been identified (52, 53). Here we identified the first specific chemoattractants of B. burgdorferi: N-acetylglucosamine, glucosamine, chitosan dimers [β-(1-4)-linked d-glucosamine], and glutamate; the response to glucose was variable and weak. Both genetic and physiological analyses indicate that N-acetylglucosamine, glucose, and glutamate are metabolized by B. burgdorferi (5, 18, 58). N-Acetylglucosamine dimer (chitobiose) was reported to support B. burgdorferi growth (56), but we found no evidence that it is an attractant at 10 mM (data not shown), a concentration approximately 50-fold greater than that reported to support B. burgdorferi growth (56). Glucose, N-acetylglucosamine, and N-acetylglucosamine dimers were reported to be nutrients utilized by B. burgdorferi, while glucosamine was not (58); however, glucosamine was an attractant. We found that glutamate was a weak attractant, but Shi et al. found that it was not (52). Our positive response was at 100 mM, whereas Shi et al. tested glutamate at 10 mM. When we tested lower concentrations of glutamate (25 mM), we also obtained a negative response 1.02 ± 0.19 (not shown), suggesting that the threshold concentration of glutamate was higher than that of most of the sugars that were identified as chemoattractants (Table 2). It is not clear if any of the B. burgdorferi attractants identified to date play a role in the life cycle of the spirochete as it shuttles between tick and mammalian hosts. However, some of the pathologies associated with Lyme disease are attributed to B. burgdorferi colonization of the nervous system and joints. It is tempting to speculate that glutamate, a major central nervous system neurotransmitter, and N-acetylglucosamine and glucosamine, major components of connective tissue in joints, may play a role in the migration of B. burgdorferi to these tissues.
The development of this assay is timely. Recent microarray results indicate that several motility and chemotaxis genes are markedly up-regulated in mammalian infection or under in vitro conditions that mimic infection (8, 16, 48, 57). Our results set the stage for the detailed phenotypic analysis of targeted mutations of these genes and other chemotaxis genes and their roles in chemotaxis and virulence (41). Defined chemoattractants will also be important in sorting out how B. burgdorferi coordinates rotation of the two PF bundles. For example, the response of individual cells to an immediate release of an attractant (28) can be determined by using glutamate as a caged compound, and pulsed release of an attractant at one cell pole will facilitate determination of the response speed to the other cell pole (51). The modified capillary tube flow cytometry chemotaxis assay is likely to have important uses of a general nature: First, it should be quite useful in enumerating spirochetes and in measuring the chemotaxis of other slow-growing or difficult-to-manipulate bacteria such as anaerobes. Second, the assay should also be helpful in measuring chemotaxis of bacteria in natural settings where motile bacteria are present (39). Specifically, capillary tubes containing various putative attractants could be inserted and incubated in specific environments in nature. Flow cytometry could then be used to rapidly enumerate, and even identify, the organisms responding to the attractants.
Acknowledgments
We thank S. F. Goldstein for assistance with calculating diffusion gradients with 70-μl capillary tubes and G. Benach and P. Matsumura for antibodies. We also thank M. Motaleb, M. Sal, K. Tilly, and D. Yelton for helpful discussions, suggestions, and comments and G. Hobbs for statistical assistance.
This research was supported by U.S. Public Health Service grants AI29743 (to N.W.C.), AR050656-01 (to C.L.), and RR16440 (to the West Virginia University Flow Cytometric Core Facility); West Virginia University Health Science Center internal grants, Office of Research and Graduate Education, to M.R.M.; and a grant from the WVU School of Medicine MD/Ph.D. program to R.B.
Footnotes
Published ahead of print on 15 December 2006.
REFERENCES
- 1.Adler, J. 1973. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J. Gen. Microbiol. 74:77-91. [DOI] [PubMed] [Google Scholar]
- 2.Babb, K., J. D. McAlister, J. C. Miller, and B. Stevenson. 2004. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J. Bacteriol. 186:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, M. D., P. M. Wolanin, and J. B. Stock. 2006. Signal transduction in bacterial chemotaxis. Bioessays 28:9-22. [DOI] [PubMed] [Google Scholar]
- 4.Bakker, R. G. 2006. Measuring chemotaxis in Borrelia burgdorferi, the Lyme disease spirochete. Ph.D. dissertation. West Virginia University, Morgantown.
- 5.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 6.Berger, B. W., R. C. Johnson, C. Kodner, and L. Coleman. 1992. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J. Clin. Microbiol. 30:359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 182:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caimano, M. J., C. H. Eggers, C. A. Gonzalez, and J. D. Radolf. 2005. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 187:7845-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease and Control Prevention. 2006. Summary of notifiable diseases—2004. Morbid. Mort. Wkly. Rep. 53:19. [Google Scholar]
- 12.Charon, N. W., and S. F. Goldstein. 2002. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu. Rev. Genet. 36:47-73. [DOI] [PubMed] [Google Scholar]
- 13.Crowley, H., and B. T. Huber. 2003. Host-adapted Borrelia burgdorferi in mice expresses OspA during inflammation. Infect. Immun. 71:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 15.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2006. Sigma factor selectivity in Borrelia burgdorferi: RpoS recognition of the ospE/ospF/elp promoters is dependent on the sequence of the −10 region. Mol. Microbiol. 59:1859-1875. [DOI] [PubMed] [Google Scholar]
- 16.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, and J. Gocayne. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 19.Ge, Y., C. Li, L. Corum, C. A. Slaughter, and N. W. Charon. 1998. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J. Bacteriol. 180:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge, Y., I. G. Old, I. Saint Girons, and N. W. Charon. 1997. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus σ70 promoter. J. Bacteriol. 179:2289-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, S. F., K. F. Buttle, and N. W. Charon. 1996. Structural analysis of Leptospiraceae and Borrelia burgdorferi by high-voltage electron microscopy. J. Bacteriol. 178:6539-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein, S. F., N. W. Charon, and J. A. Kreiling. 1994. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 91:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg, E. P., and E. Canale-Parola. 1977. Chemotaxis in Spirochaeta aurantia. J. Bacteriol. 130:485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedblom, M. L., and J. Adler. 1983. Chemotactic response of Escherichia coli to chemically synthesized amino acids. J. Bacteriol. 155:1463-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly-Wintenberg, K., and T. C. Montie. 1994. Chemotaxis to oligopeptides by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy, M. J. and R. J. Yancey, Jr. 1996. Motility and chemotaxis in Serpulina hyodysenteriae. Vet. Microbiol. 49:21-30. [DOI] [PubMed] [Google Scholar]
- 28.Khan, S., F. Castellano, F. Spudich, J. L. McCray, R. S. Goody, and G. P. Reid. 1993. Excitatory signaling in bacteria probed by caged chemoeffectors. Biophys. J. 65:2368-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimsey, R. B., and A. Spielman. 1990. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 162:1205-1208. [DOI] [PubMed] [Google Scholar]
- 30.Li, C., R. G. Bakker, M. A. Motaleb, M. L. Sartakova, F. C. Cabello, and N. W. Charon. 2002. Asymmetrical flagellar rotation in Borrelia burgdorferi nonchemotactic mutants. Proc. Natl. Acad. Sci. USA 99:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, C., M. A. Motaleb, M. Sal, S. F. Goldstein, and N. W. Charon. 2000. Spirochete periplasmic flagella and motility. J. Mol. Microbiol. Biotechnol. 2:345-354. [PubMed] [Google Scholar]
- 32.Lux, R., A. Moter, and W. Shi. 2000. Chemotaxis in pathogenic spirochetes: directed movement toward targeting tissues? J. Mol. Microbiol. Biotechnol. 2:355-364. [PubMed] [Google Scholar]
- 33.Lux, R., and W. Shi. 2004. Chemotaxis-guided movements in bacteria. Crit. Rev. Oral Biol. Med. 15:207-220. [DOI] [PubMed] [Google Scholar]
- 34.Lux, R., J. Sim, J. P. Tsai, and W. Shi. 2002. Construction and characterization of a cheA mutant of Treponema denticola. J. Bacteriol. 184:3130-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazumder, R., T. J. Phelps, N. R. Krieg, and R. E. Benoit. 1999. Determining chemotactic responses by two subsurface microaerophiles using a simplified capillary assay method. J. Microbiol. Methods 37:255-263. [DOI] [PubMed] [Google Scholar]
- 36.Melton, T., P. E. Hartman, J. P. Stratis, T. L. Lee, and A. T. Davis. 1978. Chemotaxis of Salmonella typhimurium to amino acids and some sugars. J. Bacteriol. 133:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesibov, R., and J. Adler. 1972. Chemotaxis toward amino acids in Escherichia coli. J. Bacteriol. 112:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesibov, R., G. W. Ordal, and J. Adler. 1973. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range. Weber law and related phenomena. J. Gen. Physiol. 62:203-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell, J. G., and K. Kogure. 2006. Bacterial motility: links to the environment and a driving force for microbial physics. FEMS Microbiol. Ecol. 55:3-16. [DOI] [PubMed] [Google Scholar]
- 40.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 97:10899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motaleb, M. A., M. R. Miller, C. Li, R. G. Bakker, S. F. Goldstein, R. E. Silversmith, R. B. Bourret, and N. W. Charon. 2005. CheX is a phosphorylated CheY phosphatase essential for Borrelia burgdorferi chemotaxis. J. Bacteriol. 187:7963-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motaleb, M. A., M. S. Sal, and N. W. Charon. 2004. The decrease in FlaA observed in a flaB mutant of Borrelia burgdorferi occurs posttranscriptionally. J. Bacteriol. 186:3703-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moulton, R. C., and T. C. Montie. 1979. Chemotaxis by Pseudomonas aeruginosa. J. Bacteriol. 137:274-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy, G. E., J. R. Leadbetter, and G. J. Jensen. 2006. In situ structure of the complete Treponema primitia flagellar motor. Nature 442:1062-1064. [DOI] [PubMed] [Google Scholar]
- 45.Narasimhan, S., M. J. Caimano, F. T. Liang, F. Santiago, M. Laskowski, M. T. Philipp, A. R. Pachner, J. D. Radolf, and E. Fikrig. 2003. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc. Natl. Acad. Sci. USA 100:15953-15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkinson, J. S. 1977. Behavioral genetics in bacteria. Annu. Rev. Genet. 11:397-414. [DOI] [PubMed] [Google Scholar]
- 47.Parkinson, J. S., P. Ames, and C. A. Studdert. 2005. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 8:116-121. [DOI] [PubMed] [Google Scholar]
- 48.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 176:6045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segall, J. E., A. Ishihara, and H. C. Berg. 1985. Chemotactic signaling in filamentous cells of Escherichia coli. J. Bacteriol. 161:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, W., Z. M. Yang, Y. Geng, L. E. Wolinsky, and M. A. Lovett. 1998. Chemotaxis in Borrelia burgdorferi. J. Bacteriol. 180:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shih, C. M., L. L. Chao, and C. P. Yu. 2002. Chemotactic migration of the Lyme disease spirochete (Borrelia burgdorferi) to salivary gland extracts of vector ticks. Am. J. Trop. Med. Hyg. 66:616-621. [DOI] [PubMed] [Google Scholar]
- 54.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szczepanski, A., M. B. Furie, J. L. Benach, B. P. Lane, and H. B. Fleit. 1990. The interaction between Borrelia burgdorferi and endothelium in vitro. J. Clin. Investig. 85:1637-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilly, K., A. F. Elias, J. Errett, E. Fischer, R. Iyer, I. Schwartz, J. L. Bono, and P. Rosa. 2001. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J. Bacteriol. 183:5544-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Lackum, K., and B. Stevenson. 2005. Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol. Lett. 243:173-179. [DOI] [PubMed] [Google Scholar]
- 59.Wadhams, G. H., and J. P. Armitage. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5:1024-1037. [DOI] [PubMed] [Google Scholar]