Abstract
Our goal was to determine whether chlorpyrifos oxon, dichlorvos, diisopropylfluorophosphate (DFP), and sarin covalently bind to human albumin. Human albumin or plasma was treated with organophosphorus agent (OP) at alkaline pH, digested with pepsin at pH 2.3, and analyzed by MADLI-TOF mass spectrometry. Two singly charged peaks, 1718 and 1831 m/z, corresponding to the unlabeled peptide fragments containing the active site Tyr 411 residue, were detected in all samples. The sequences of the two peptides were VRYTKKVPQVSTPTL and LVRYTKKVPQVSTPTL. The peptide-OP adducts of these peptides were also found. They had masses of 1854 and 1967 for chlorpyrifos oxon, 1825 and 1938 for dichlorvos, 1881 and 1994 for DFP, and 1838 and 1938 for sarin; these masses fit a mechanism whereby OP bound covalently to Tyr 411. The binding of DFP to Tyr 411 of human albumin was confirmed by electrospray tandem mass spectrometry and analysis of product ions. None of the OP-albumin adducts lost an alkoxy group, leading to the conclusion that aging did not occur. Our results show that OP pesticides and nerve agents bind covalently to human albumin at Tyr 411. The presence of Tyr 411 on an exposed surface of albumin suggests that an antibody response could be generated against OP-albumin adducts.
Keywords: biomarker organophosphate exposure, pepsin, sarin, soman, dichlorvos, diisopropylfluorophosphate, chlorpyrifos oxon, nerve agents, pesticides
Introduction
The acute toxicity of organophosphorus toxicants (OP) is known to be due to inhibition of acetylcholinesterase. However, other proteins also bind OP though their role in toxicity is less defined (Casida and Quistad, 2004). Albumin is a potential new biomarker of OP exposure. Mice treated with a nontoxic dose of a biotinylated nerve agent analog, FP-biotin (10-fluoroethoxyphosphinyl-N-biotinamidopentyldecanamide), had 1000 times more FP-biotinylated albumin than FP-biotinylated butyrylcholinesterase in their blood (Peeples et al., 2005).
Albumin has been shown to covalently bind radiolabeled diisopropylfluorophosphate (DFP). Human albumin incorporated 1 mole of DFP per mole of albumin when 20–70 μM albumin was incubated with a 7-fold molar excess of DFP at pH 8.0 for 2 h at 23°C (Means and Wu, 1979; Hagag et al., 1983). Bovine albumin also incorporated 1 mole of DFP per mole of albumin (Murachi, 1963). The site of covalent binding of DFP to human albumin was identified by amino acid sequencing. The labeled peptide had the sequence ArgTyrThrLys with DFP bound to Tyr (Sanger, 1963). Later, when the complete amino acid sequence of human albumin was known, the active site tyrosine was identified as Tyr 411 (Tyr 435 when residue #1 is Met of the signal peptide). Mass spectrometry identified Tyr 410 of bovine albumin (equivalent to Tyr 411 of human albumin) as the covalent binding site for FP-biotin (Schopfer et al., 2005). The nerve agents soman and sarin were shown to bind covalently to human albumin on tyrosine (Black et al., 1999; Adams et al., 2004) and to be released by treatment with potassium fluoride (Adams et al., 2004).
Albumin has also been demonstrated to be an OP hydrolase, hydrolyzing chlorpyrifos oxon, O-hexyl O-2, 5-dichlorophenylphosphoramidate, and paraoxon at measurable rates (Erdos and Boggs, 1961; Ortigoza-Ferado et al., 1984; Sultatos et al., 1984; Sogorb et al., 1998a). The apparent Km of bovine albumin is 0.41 mM for chlorpyrifos oxon, 1.85 mM for paraoxon (Sultatos et al., 1984) and that of human albumin is 3.6 mM for DFP (Means and Wu, 1979). Despite this seemingly consistent body of results, some issues have been raised regarding the reaction of OP with albumin. It has been questioned whether the observed OP hydrolase activity was associated with the albumin molecule itself, or with minor phosphotriesterase contaminants in the albumin preparation (Erdos and Boggs, 1961). In addition, the possibility has been raised that DFP binds to one site in albumin, but that other OP bind to a different site (Mourik and de Jong, 1978; Sultatos et al., 1984).
Our goal was to determine whether Tyr 411 of human albumin was the site for covalent attachment of a variety of OP. For this purpose we developed a MALDI-TOF mass spectrometry assay applicable to purified human albumin and to human plasma.
Materials and Methods
Materials
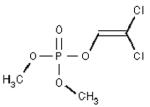
Purified human serum albumin, essentially fatty acid free (Fluka via Sigma, St. Louis, MO; cat no. 05418), pepsin (Sigma, St. Louis, MO; cat no. P6887 from porcine gastric mucosa), modified trypsin, sequencing grade (Promega, Madison, WI; cat no. V5113), diisopropylfluorophosphate (Sigma; cat no. D0879), dichlorvos and chlorpyrifos oxon (Chem Service Inc., West Chester, PA; cat no. PS-89, MET-674B), sarin treated human plasma from Dr. Patrick Masson, acetonitrile (HPLC grade 99% ACROS cat no. 61001-0040 from Fisher Scientific, Pittsburgh, PA), trifluoroacetic acid, sequencing grade (Beckman Instruments, Palo Alto, CA; cat no. 290203), 2,5-dihydroxybenzoic acid (DHBA) matrix (Applied Biosystems Foster City, CA), alpha-cyano 4-hydroxycinnamic acid (CHCA) (Sigma; cat no. 70990) was recrystallized before use. Calibration standards for MALDI-TOF were from New England Biolabs (Beverly, MA; cat no. P7720S). They included Angiotensin 1, 1297.51 amu, ACTH (7–38) 3660.19 amu, and ACTH (18–39) 2466.73 amu. Double distilled water was prepared in-house and autoclaved.
Sample preparation for diisopropylfluorophosphate, dichlorvos, and chlorpyrifos oxon treated samples
Fatty acid-free human albumin at a concentration of 10 mg/ml, which is 150 μM, was dissolved in 25 mM ammonium bicarbonate pH 8.6 and treated with an equimolar concentration of OP for 24 h at 37°C. The pH of 1000 μl reaction mixture was reduced to pH 2.3 by the addition of 500 μl of 1% trifluoroacetic acid. Pepsin was dissolved in 10 mM HCl to make 1 mg/ml and stored at −80°C. The albumin was digested with pepsin (1:250 ratio) for 2 hours at 37°C and diluted to 1 pmol/μl with 0.1% trifluoroacetic acid.
A 200 μl aliquot of human plasma was treated with 6.85 μl of 20 mM OP (660 μM final OP concentration) for 24 h at 37°C. The pH was adjusted to 2.3–2.5 by the addition of 200 μl of 1% TFA. Proteins were digested with 50 μl of 1 mg/ml pepsin for 2 hours at 37°C. Before spotting the digest on the target plate, a 10 μl aliquot of the digest was diluted with 390 μl of 0.1% TFA so that the final plasma dilution was 1000-fold.
MALDI-TOF
A 1 μl aliquot of diluted peptic digest was applied to a stainless steel target plate, air dried, and overlaid with 1 μl of 2,5-dihydroxybenzoic acid matrix. The CHCA matrix gave similar results. Mass spectra were acquired with the Voyager DE-PRO MALDI-TOF mass spectrometer (Applied Biosystems, MDS Sciex, Foster City, CA) in linear positive ion mode, 20,000 volts accelerating voltage, 94% grid voltage, 0.1% guide wire, 350 nsec extraction delay time, automated laser intensity adjustment from 1000 to 1600. The instrument was calibrated with a peptide calibration mixture from New England Biolabs. Mass accuracy for each standard was within 0.05% of the corresponding average molecular weight. Spectra were acquired in automatic mode, by examining signals from random spots on a target. The signals from the first 10 spots that met the acceptance criteria were summed into one final profile mass spectrum The acceptance criteria were signal intensities between 1000 and 55000 counts, with signal to noise ratios of 10 or greater, and minimum resolution of 50. The final spectrum was the average of 1000 shots.
The MS-Digest program from the UCSF Mass Spectrometry Facility was used to calculate the masses of the peptic peptides expected from digests of human serum albumin.
Quadrupole mass spectrometer
MS/MS spectra were acquired on a Q-Trap 2000 triple quadrupole linear ion trap mass spectrometer (Applied Biosystems, MDS Sciex, Foster City, CA) with a nano electrospray ionization source. DFP-labeled albumin digested with trypsin was infused into the mass spectrometer via a fused silica emitter (360 μm o.d., 20 μm i.d., 10 μm taper from New Objective, Woburn, MA), using a Harvard syringe pump to drive a 100 μl Hamilton syringe equipped with an inline 0.25 micron filter, at a flow rate of 1 μl/min. Samples were prepared in 50% acetonitrile, 0.1% formic acid. Positive ion spectra were obtained. Mass spectra were calibrated using fragment ions generated from collision-induced dissociation of Glu fibrinopeptide B (Sigma). Enhanced product ion scans were obtained with a collision energy of 40±5 volts and a nitrogen gas pressure of 4 x 10−5 Torr. The final enhanced product ion scan was the average of 212 scans.
Sample preparation of sarin treated plasma
100 μl of human plasma was treated with 600 μM sarin and stored at ambient temperature for 3 days. This concentration of sarin is equimolar with the concentration of albumin in plasma. Ten μl was digested with 0.5 μg of pepsin at 37°C for 2 h at pH 2.3, and the peptides separated by HPLC on a Waters 625 LC system. A C18 reverse phase column (Prodigy 5 μ ODS(2), 100 x 4.6 mm, 5 micron, 00D-3300-E0 from Phenomenex) was used to trap the peptides from the digest, which were then eluted with a 40 min gradient starting with 85% of buffer A (0.1 % trifluoroacetic acid in water) 15% buffer B (acetonitrile containing 0.07% trifluoroacetic acid) and ending with 65% buffer A, 35% buffer B. One ml fractions were reduced in volume to 200 μl in a vacuum centrifuge and 1 μl was analyzed by MALDI-TOF. A control plasma sample was treated identically, except that it was incubated with 3.5% isopropanol rather than with sarin.
Results
Reaction of pure human albumin with OP
The assay was developed with pure human albumin and later tested with human plasma. Fatty acid free-albumin was used because fatty acids and OP bind to the same albumin domain and therefore fatty acids could block the binding of OP (Means and Wu, 1979; Peters, 1996). The covalent attachment site for human albumin, Tyr 411, is located near the surface of the albumin molecule where it is accessible to proteases. Digestion with trypsin at pH 8.6 or with pepsin at pH 2–2.5 released peptides of the expected masses without the need to denature or to reduce and alkylate the disulfide bonds of albumin. Peptides containing Tyr 411 had the sequence YTK (411 m/z, singly charged mass) when the protease was trypsin, and the sequences VRYTKKVPQVSTPTL (1718 m/z) and LVRYTKKVPQVSTPTL (1831 m/z) when the protease was pepsin. Pepsin routinely missed one cleavage in our experiments.
The tryptic YTK peptide (411 m/z) and the dichlorvos, chlorpyrifos oxon, and DFP adducts had masses that overlapped with matrix peaks, which made them difficult to detect by MALDI mass spectrometry. Furthermore, the YTK peptide and YTK-OP adducts did not seem to ionize when irradiated by the nitrogen laser in the Voyager DE-PRO, though they did ionize in the electrospray source of the Q-Trap. By contrast, the larger peptides produced by digestion of albumin with pepsin separated well from matrix and ionized to give good signals in the Voyager DE-PRO. Therefore, samples intended for analysis by MALDI-TOF were digested with pepsin rather than with trypsin.
Table 1 lists the expected peptic peptide masses before and after covalent binding of dichlorvos, chlorpyrifos oxon, DFP, and sarin to Tyr 411 of human albumin.
Table 1.
Pepsin digested human albumin. The [M+H]+1 masses of peptic peptides containing Tyr 411 are listed before and after covalent binding of OP. Tyr 411 is underlined.
The accession # for human albumin is GI:28592 where Tyr 411 is Tyr 435 because numbering begins with the signal peptide.
The leaving group in Table 1 is that portion of the OP molecule that detaches from the OP upon covalent binding of the OP to protein. The mass of the leaving group is absent from the final adduct. The added OP mass comes from the phosphorus atom, the two phosphorus ligands, the phosphoryl oxygen atom, less one hydrogen.
Figure 1 shows the MALDI-TOF spectra obtained for pepsin-digested human albumin before and after treatment with OP. The top panel shows masses at 1718 and 1831 m/z, which are consistent with the peptides from unlabeled albumin that contain Tyr 411. Additional albumin peptides are also present but they do not contain Tyr 411 and are therefore not discussed. The dichlorvos panel shows peaks at 1718 and 1831 m/z, as well as two new peaks at 1826 and 1939 m/z. The two new peaks have the expected sizes for the dimethoxyphosphate adducts of the 1718 and 1831 m/z peptides. The amount of labeled albumin estimated from the relative peak areas is about 65%. The chlorpyrifos oxon panel shows two new peaks at 1854 and 1967 m/z for the diethoxyphosphate adducts. About 30% of the albumin is labeled. The DFP panel shows two new peaks at 1882 and 1995 m/z for the diisopropoxyphosphate adducts. About 70% of the albumin is labeled. Since the MALDI conditions disrupt non-covalent interactions, the OP-peptide adducts must be covalently formed. These results support the conclusion that human albumin is labeled by dichlorvos, chlorpyrifos oxon, and DFP; and are consistent with the site for covalent attachment being Tyr 411. The masses correspond to the dialkoxy adducts rather than the monoalkoxy adducts. Masses for monoalkoxy adducts were not found, supporting the conclusion that OP-albumin adducts do not age.
Figure 1.
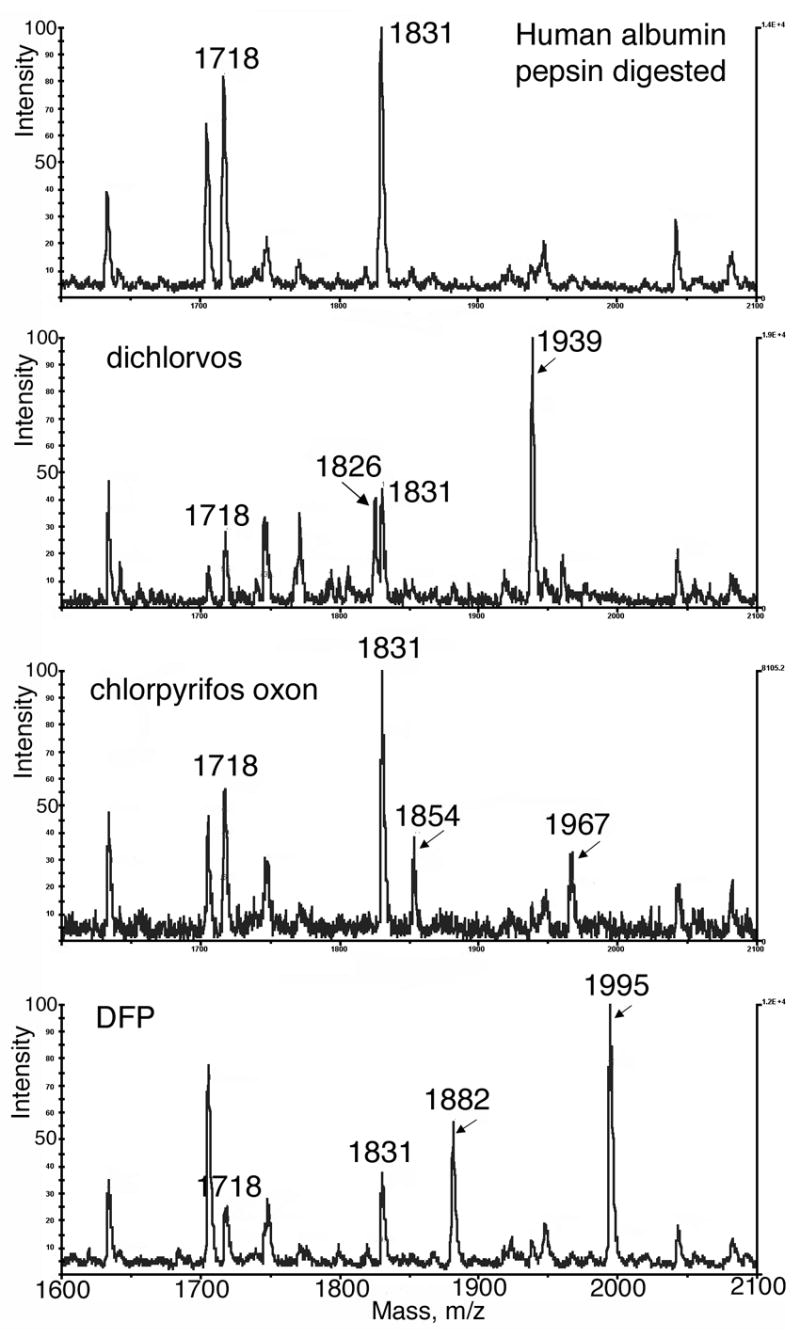
MALDI-TOF analysis of human albumin-OP adducts. (Top panel) Digestion of human albumin with pepsin at pH 2.3 yields 2 peptides containing Tyr 411 whose average mass to charge ratios are 1718 and1831 (singly protonated). (Dichlorvos panel) Incubation of human albumin with dichlorvos in ammonium bicarbonate pH 8.5, followed by digestion with pepsin at pH 2.3 yields dimethoxyphosphate adducts of 1826 and 1939 m/z. (Chlorpyrifos oxon panel) Incubation with chlorpyrifos oxon yields diethoxyphosphate adducts of 1854 and 1967 m/z. (DFP panel) Incubation with DFP yields diisopropoxyphosphate adducts of 1882 and 1995 m/z. Samples were diluted to 1 pmol/μl before plating 1 μl on the MALDI target with 2,5-dihydroxybenzoic acid matrix.
Saturating the albumin binding sites
Unlabeled 1718 and 1831 m/z peptides were always present when the concentration of OP was the same as the concentration of albumin. However, when the dichlorvos or DFP concentration was 40-fold higher than the albumin concentration, all the Tyr 411 sites were occupied and no unlabeled peptides of 1718 and 1831 m/z were detected. OP labeling reactions with albumin were performed at pH 8.5 because labeling occurs at high pH but is markedly decreased at neutral pH (Murachi, 1963; Means and Wu, 1979).
Limit of detection
It was essential to dilute the albumin and plasma digests before applying the sample on the target plate. Undiluted samples did not show the desired peptides due to ion suppression and charge competition effects. The limit of detection of OP-labeled peptide was determined from dilutions of peptides in which all of the Tyr 411 sites were occupied. The diluent was 0.1% trifluoroacetic acid in water. Peaks of interest were detected after 100, 1000, 3000, and 9,000 fold dilution of a sample whose starting concentration was 600 μM (40 mg/ml) albumin. The signal to noise ratio for the 1:9000 diluted sample was 3:1. At 18,000 fold dilution the peak height was only two fold above the noise. Thus, the minimum detectable signal from an OP-labeled peptide occurred at 0.07 pmol/μl.
Missed cleavage
We hoped to increase the sensitivity of the assay by changing digestion conditions so that pepsin would consistently cleave Leu from the N-terminus of the active site peptide. If there were no missed cleavage, the area of the 1721 peak would increase relative to the background noise, thus increasing the sensitivity of the assay. We increased the pepsin to albumin ratio up to 1:25, lowered the pH in increments down to 1.5, and increased the digestion time to 4 hours. A pepsin to albumin ratio of 1:25 at pH 1.8 incubated for 2.5 hours at 37°C did eliminate the 1831 peptide, but did not increase the signal for 1721.
Detection of dichlorvos bound to albumin in human plasma
The concentration of albumin in human plasma is about 40–50 mg/ml, which is 600–770 μM. No other protein in plasma is present at such a high concentration. This overwhelming concentration of albumin in plasma suggested that it might be possible to detect OP-albumin adducts without separating albumin from other proteins in plasma. This was tested by incubating an aliquot of human plasma with a concentration of dichlorvos equimolar to the albumin concentration in plasma. The pH was not adjusted and no buffer was added for the reaction with dichlorvos. Next the plasma was digested with pepsin at pH 2.3, and diluted 600 fold with 0.1% trifluoroacetic acid to yield an albumin peptide concentration of about 1 pmole/μl. A 1 μl aliquot of the diluted digest was applied to the MALDI target. The control sample was human plasma treated in parallel with everything but dichlorvos. Figure 2 shows that albumin peptides at 1718 and 1831 m/z stand out, despite the presence of normal plasma components, and that the OP-labeled form of these peptides at 1826 and 1939 m/z can be detected by MALDI-TOF. We conclude that human plasma can be assayed for OP bound to albumin. The 1826 and 1939 masses are 108 amu larger than their unlabeled counterparts, which identifies the OP as a dimethoxy OP, thus classifying it as a pesticide rather than a nerve agent. For forensic purposes it is valuable to know whether the OP is a pesticide or a nerve agent.
Figure 2.
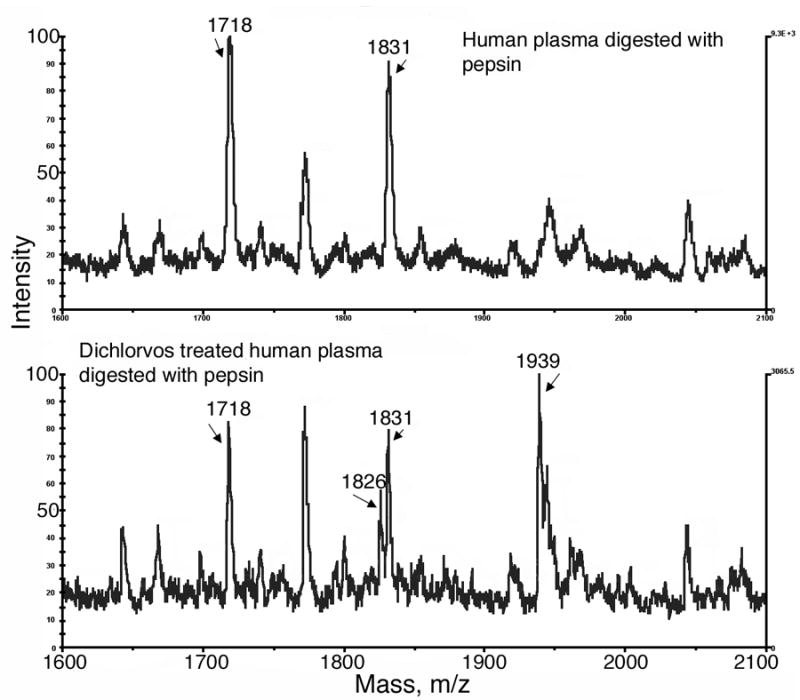
MALDI-TOF analysis of OP-albumin adducts in human plasma. The top panel is the control sample. Human plasma digested with pepsin shows the Tyr 411 containing, albumin peptides of 1718 and 1831 m/z. The bottom panel shows two new peaks at 1826 and 1939 m/z in dichlorvos treated human plasma, representing peptides containing dichlorvos covalently bound to Tyr 411 of albumin.
Detection of sarin bound to albumin in human plasma
MALDI-TOF analysis of peptic digests of human plasma labeled by reaction with 600 μM sarin did not show the sarin-labeled peptides after simple dilution. We suspected that the absence of signal was due to ion suppression. Therefore the digests were fractionated by reverse phase chromatography, prior to mass spectral analysis, as shown in Figure 3. Fractions were collected at one minute intervals. Each HPLC fraction was analyzed by MALDI-TOF. Peptides of interest eluted between 8–16 min. The unlabeled active site peptides of albumin, of mass 1718 and 1831, eluted at 8–10 min. The sarin-labeled peptides of mass 1838 and 1951 eluted at 14–16 min (fractions 14 and 16, Figure 4). The peptides of interest separated from a large peak of UV absorbing material in the HPLC. Elimination of this material would be expected to reduce ion suppression, thereby accounting for the appearance of the sarin-labeled peptide signals in MALDI-TOF analysis.
Figure 3.
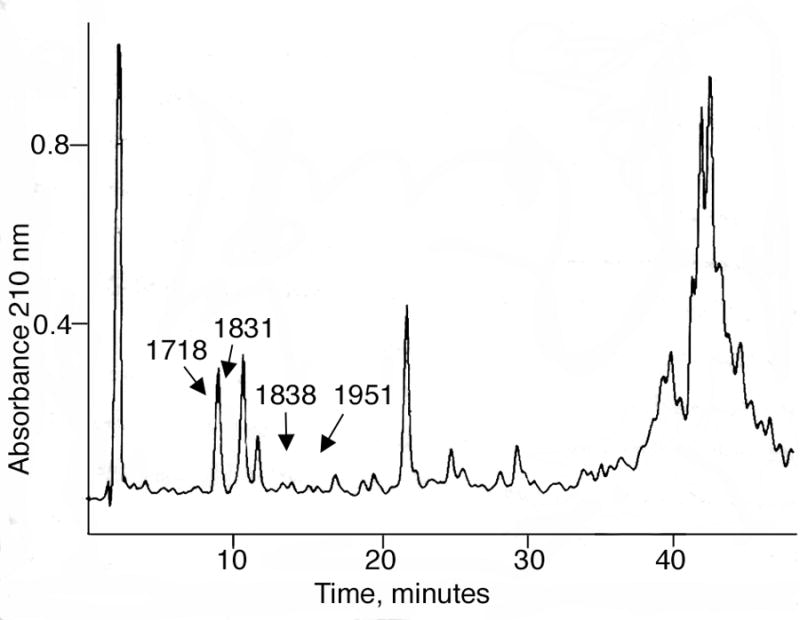
HPLC trace. A peptic digest of human plasma (10 μl) that had been treated with sarin was subjected to reverse phase chromatography. Unlabeled active site albumin peptides of mass 1718 and 1831separated from sarin-labeled albumin peptides of mass 1838 and 1951 and from a large peak of UV absorbing material. The marked peaks contain a mixture of peptides and therefore the relative UV intensities do not represent the relative amounts of labeled and unlabeled albumin.
Figure 4.
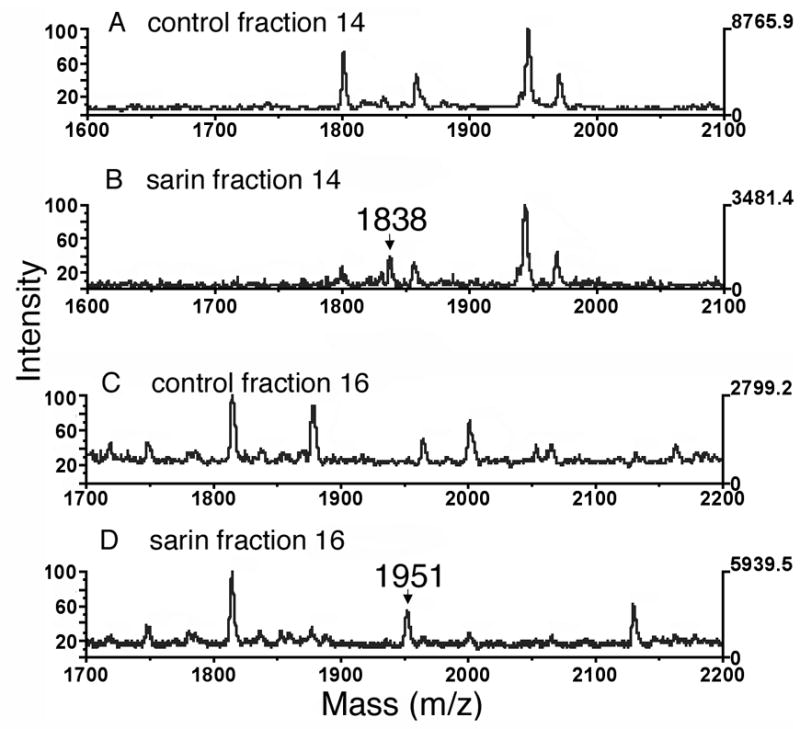
MALDI-TOF analysis of sarin covalently attached to human albumin. Panels A and C, control human plasma digested with pepsin and fractionated by HPLC, but not treated with sarin. Panels B and D, human plasma treated with sarin before digestion with pepsin and fractionation by HPLC. Fractions were collected at one minute intervals. Sarin-labeled albumin peptides of 1838 and 1951 amu include 120 amu from sarin.
Figure 4 shows the MALDI-TOF spectra for the two sarin-labeled peptides of human albumin with masses of 1838 and 1951 m/z. These masses are consistent with the 1718 and 1831 albumin peptides to which 120 amu from sarin have been added. That the sarin-peptide complex survived the MALDI conditions indicates that sarin has made a covalent complex. These results show that sarin covalently binds to human albumin on Tyr 411. They also show that binding can be detected in plasma, and that the sarin adduct of albumin does not lose an alkoxy group during storage for 3 days at room temperature.
MS/MS analysis confirms DFP binding to Tyr 411 of the YTK peptide
DFP-labeled human albumin was digested with trypsin and the digest was infused into the Q-Trap mass spectrometer. The enhanced mass spectrum showed a peak at 575.4 m/z, which is consistent with the singly charged YTK peptide covalently bound to DFP ([M+H]+1 = 411 amu for the YTK peptide plus 164 amu added mass from DFP, see table 1). This peptide was subjected to collision-induced dissociation with nitrogen as the collision gas. The resulting enhanced product ion spectrum yielded amino acid sequence information consistent with the sequence YTK where DFP is covalently bound to tyrosine. See Figure 5. A mass was found at 147.2 amu, indicative of the C-terminal lysine from a y-series. This was followed by masses at 248.3, for the ThrLys dipeptide, and at 575.4, for the Tyr*ThrLys tripeptide, including the N-terminus, from a y-series (where Tyr* represents the diisopropylphospho-adduct of tyrosine). No relevant signals were found at higher mass. No evidence for the diisopropylphospho-adduct of threonine was found.
Figure 5.
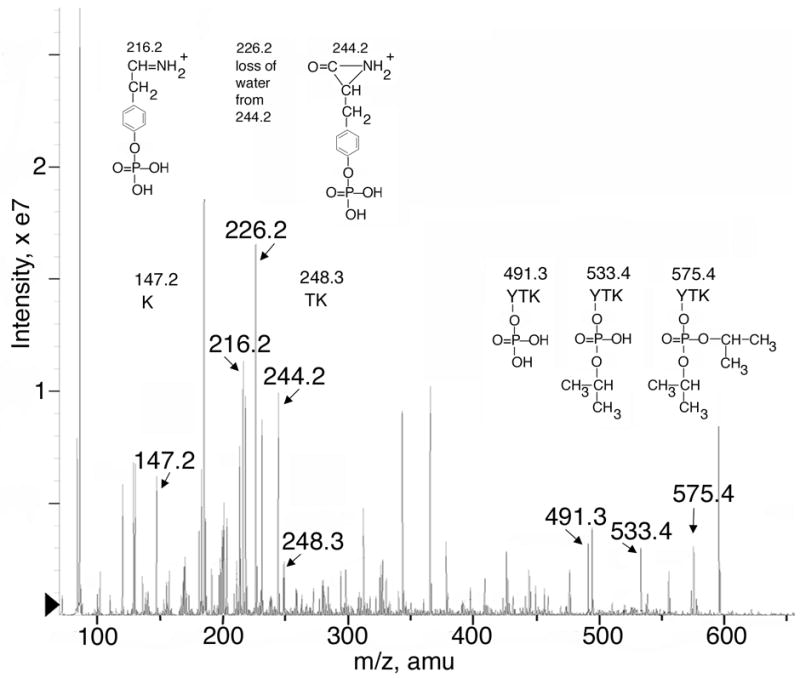
Product ion spectrum of DFP-labeled human albumin peptide 575.4. DFP-labeled albumin was digested with trypsin and infused into the Q-Trap mass spectrometer. The singly charged parent ion of 575.4 m/z has the sequence YTK and has the diisopropylphosphate group covalently bound to tyrosine. Fragment masses and their corresponding structures are shown. In addition to information on the DFP-YTK peptide, fragments from a second albumin peptide (LVNEVTEFAK, doubly protonated) can be extracted from the fragmentation information.
Further, convincing evidence for covalent binding of DFP to Tyr 411 comes from the presence of 6 masses that are all consistent with various fragments of DFP attached to tyrosine, alone or in conjunction with the YTK peptide. The structures of these 6 ions are shown in Figure 5. As mentioned before, the ion at 575.4 amu is consistent with the singly protonated YTK peptide plus the added mass from covalent attachment of DFP. Neutral loss of 42 amu yields the 533.4 amu ion. Loss of 42 amu is predicted for β-elimination of propylene from the diisopropylphosphate adduct. This β-elimination type reaction, also referred to as a McLafferty rearrangement (McLafferty, 1959; Fredriksson et al., 1995) is a facile reaction commonly seen during collision induced dissociation of phosphopeptides (McLachlin and Chait, 2001). A second neutral loss of 42 amu yields the 491.3 amu ion; this mass is consistent with a phosphorylated YTK peptide. In theory, all three of these masses are consistent with DFP adducts of either tyrosine or threonine. However, the mass at 244.2 amu is characteristic of an N-terminal phosphotyrosine, b-series aziridone ion, and the 226.2 amu ion is consistent with its dehydration product (Mann et al., 2001). In addition, the mass at 216.2 amu is characteristic of a phosphotyrosine immonium ion (Steen et al., 2001). Furthermore, no indication of phosphorylated or organophosphorylated threonine was found. These results prove that DFP covalently binds to Tyr 411 of human albumin.
No evidence for any form of the dephosphorylated YTK peptide was found. This probably reflects the relative difficulty of releasing OP from tyrosine, compared to the other fragmentation pathways available to the diisopropylphospho-YTK peptide. Facile dephosphorylation of phospho-tyrosine via a β-elimination type mechanism is not available because tyrosine does not afford a suitable environment. Beta-elimination would require the shift of a proton from the beta carbon of the leaving group to the phosphate oxygen, with concomitant formation of a double bond between the alpha and beta carbons of the leaving group. For phospho-tyrosine, the beta-proton of the leaving tyrosine is aromatic and therefore not readily released; furthermore, formation of the double bond requires introduction of a triple bond into the aromatic ring of tyrosine, another difficult operation. Though phosphate can be released from tyrosine during collision induced dissociation, it seems to require the presence of hydroxyl moieties on the phosphate. Even then, the yield of dephosphorylation is poor (Fredriksson et al., 1995; Tholey et al., 1999). Thus, it would appear that elimination of propylene from the diisopropoxy-moieties and fragmentation of the peptide backbone provide energetically more acceptable routes for utilization of the collision energy than dephosphorylation of the tyrosine.
The spectrum in Figure 5 contains a number of major peaks that were not used in the analysis of the DFP-YTK adduct. Most of these are attributable to fragments from a second albumin peptide. The doubly charged form of LVNEVTEFAK appears at 575 m/z. The peaks at 218, 365, 494, and 595 amu (plus additional peaks at higher mass: 694, 823, 937 and 1036 amu) correspond to the complete y-series for this contaminating peptide. Peaks at 213, 327, 456 and 555 amu can be assigned to a b-series from this same peptide, while peaks at 312, 343, 426, and 444 amu reflect internal fragments. The large peak at 86 amu is the immonium ion of Leu/Ile.
The presence of two isopropyl groups in parent ion 575.4 supports the conclusion that the DFP-albumin adduct does not age.
The unique set of 6 phosphorylated fragment ions in Figure 5 for DFP-albumin could be useful for identifying exposure to DFP in a mass spectrometry method that selectively searches for characteristic fragment ions.
Only one covalent binding site for OP
Human albumin labeled with dichlorvos or DFP and digested with pepsin was searched for additional peptides that might bind OP. MALDI-TOF and Q-Trap analysis revealed no other OP adducts. The only identified OP binding site was Tyr 411.
Discussion
Mechanism of OP labeling of albumin
Each of the OP tested in this work labeled Tyr 411 of human albumin. The stoichiometry of labeling has been shown to be one mole 3H-DFP or 14C-DFP incorporated per mole of albumin (Murachi, 1963; Means and Wu, 1979; Hagag et al., 1983; Sultatos et al., 1984). The specific labeling of one tyrosine in a molecule that contains 18 tyrosines suggests that Tyr 411 is in a special environment. This tyrosine has an unusually low pKa of 7.9–8.3 (Means and Wu, 1979; Ahmed et al., 2005), in contrast to the pKa of 10 for the average tyrosine.
Tyr 411 is the active site residue not only for reaction with OP, but also for reaction with esters such as p-nitrophenyl acetate, carbamates such as carbaryl, and amides such as o-nitroacetanilide (Means and Wu, 1979; Sogorb et al., 1998b; Sogorb et al., 2004; Manoharan and Boopathy, 2006). The esterase and amidase activity of albumin can be inhibited by pretreatment with DFP. Conversely, labeling with DFP can be prevented by pretreatment with p-nitrophenyl acetate, which forms a stable acylated albumin adduct (Means and Wu, 1979). The sensitivity of albumin esterase activity to ionic strength led Means and Wu to conclude that the reactive tyrosine residue is located on the surface of albumin in an apolar environment adjacent to several positively charged groups. This description of the OP binding site of albumin was proven to be correct when the crystal structure was solved (He and Carter, 1992; Sugio et al., 1999). Subdomain IIIa of albumin contains a pocket lined by hydrophobic side chains. The hydroxyl of Tyr 411 is close to the side chains of Arg 410 and Lys 414.
Site-directed mutagenesis experiments have shown that albumin esterase activity is abolished when Tyr 411 is mutated to Ala, and severely diminished when Arg 410 is mutated to Ala (Watanabe et al., 2000). These results support Tyr 411 as the active site for albumin esterase activity and support a role for Arg 410 in stabilizing the reactive anionic form of Tyr 411. The negatively charged Tyr 411 is available for nucleophilic attack on ester and amide substrates. Though crystal structures of several ligand albumin complexes have been solved (Ghuman et al., 2005), the crystal structure of an OP-albumin adduct is not yet available.
No aging of OP-albumin adducts
Aging of OP-labeled acetylcholinesterase and butyrylcholinesterase is defined as the loss of an alkoxy group from the OP-labeled active site serine (Benschop and Keijer, 1966; Michel et al., 1967). The nerve agents sarin, soman, and VX yield the same aged OP derivative, so that these agents may be difficult to distinguish when bound to acetylcholinesterase or butyrylcholinesterase (Millard et al., 1999a; Millard et al., 1999b).
Three of the OP studied in this work, sarin, DFP, and chlorpyrifos oxon, are known to age when bound to acetylcholinesterase and butyrylcholinesterase. However these OP did not age when bound to albumin. Aging is a catalytic process that requires the participation of nearby histidine and glutamic acid residues (Kovach et al., 1997). Residues that promote aging are not present in the active site pocket of albumin. The absence of aging allows the bound OP to be spontaneously released from Tyr 411. This makes albumin an OP hydrolase, though a very slow one. Albumin hydrolyzes chlorpyrifos oxon, O-hexyl O-2,5-dichlorophenylphosphoramidate, and paraoxon (Erdos and Boggs, 1961; Ortigoza-Ferado et al., 1984; Sultatos et al., 1984; Sogorb et al., 1998a). We have recently measured the hydrolysis of soman by human albumin and found a deacylation rate of 0.0052 per hour (unpublished).
The observation that OP-albumin adducts do not age is supported by the findings of others (Black et al., 1999; Adams et al., 2004). Using LC/MS, Black et al found O-(pinacolyl methylphosphonyl)tyrosine in human plasma as well as in albumin samples that had been treated with soman. They found O-(isopropyl methylphosphonyl)tyrosine in samples treated with sarin. If aging had occurred the products would have been (methylphosphonyl)tyrosine for both soman and sarin. Adams et al used GC-MS to measure sarin and soman recovered from human plasma and albumin samples. The plasma and albumin were reacted with sarin or soman, excess agent was removed by solid phase extraction, and the samples were treated with potassium fluoride to release the bound OP. Intact sarin and soman were recovered, demonstrating that aging had not occurred. Absence of aging in OP-albumin adducts suggests that albumin could be a useful biomarker to distinguish between soman and sarin exposure. In the same manner, OP-albumin adducts could distinguish between pesticide and nerve agent exposure.
Advantages and disadvantages of MALDI-TOF mass spectrometry
The MALDI-TOF mass spectrometer is an easy instrument to use. Samples have to be free of salt and the concentration of peptide has to be approximately 1 pmol/μl. As little as 0.5 μl of a 1 pmol/μl solution gives a good signal. Results are acquired in seconds.
The disadvantage of MALDI is that not all peptides ionize when the sample contains a mixture of peptides. For example the FP-biotin labeled bovine albumin YTR peptide gave an intense signal at 1012 amu (data not shown). In contrast FP-biotin labeled human albumin YTK peptide gave no signal. In both experiments the sample was a mixture of tryptic peptides. Ion suppression is a common problem in mass spectrometry and one way to solve the problem is to separate the peptide of interest from other peptides by HPLC before examining it by MALDI-TOF mass spectrometry. This strategy allowed us to detect sarin-labeled albumin in human plasma. Alternatively, the peptides can be separated by step elution from a C18 ziptip, a procedure that was successful for nerve agent adducts of acetylcholinesterase (Elhanany et al., 2001).
OP-albumin as a biomarker of OP exposure
Many proteins in human plasma are labeled by OP. Mass spectrometry assays have been developed for OP-butyrylcholinesterase adducts (Fidder et al., 2002; Van Der Schans et al., 2004; Tsuge and Seto, 2006) . The present report provides an assay for OP-albumin adducts. Albumin is far less reactive with OP than butyrylcholinesterase, but the 10,000 fold higher concentration of albumin in plasma compared to butyrylcholinesterase (40,000–50,000 mg/ml versus 4–5 mg/ml), means that both albumin and butyrylcholinesterase will be labeled when a person is exposed to OP. New assays that use precursor and fragment ion m/z values in selected reaction monitoring experiments are expected to be capable of diagnosing low dose exposure (Tsuge and Seto, 2006).
Antibody to OP-albumin
The information presented in this work could have application to the monitoring of individuals exposed to OP. It may be possible to detect exposure through use of an antibody detection assay directed toward the OP-albumin adduct at Tyr 411. There is precedent for the generation of antibodies to very small haptens bound to protein. For example, antibodies that distinguish between phosphotyrosine, phosphoserine, and phosphothreonine have been successfully produced (Glenney et al., 1988; Levine et al., 1989). Antibodies to soman, sarin, and VX bound to carrier proteins through a chemical linker have been produced (Grognet et al., 1993; Zhou et al., 1995; Johnson et al., 2005) . The proposed OP-albumin epitope could be more useful for detection of OP exposure than existing antibodies because the OP-albumin adduct has no chemical linker and no foreign protein environment.
An OP-albumin adduct at Tyr 411 may generate an antibody response in exposed individuals and the antibody could be detected to determine a history of exposure to OP. This would facilitate monitoring exposure to OP long after the exposure incident and long after the antigen has disappeared.
Acknowledgments
Mass spectra were obtained with the support of the Protein Structure core Facility at the University of Nebraska Medical Center. This work was supported by U.S. Army Medical Research and Materiel Command Contract W81XWH-06-1-0102, Edgewood Biological Chemical Center Contract W911SR-04-C-0019, Eppley Cancer Center grant P30CA36727, NIH grant 1 U01 NS058056-01 and grants DGA/DSP/STTC-PEA 010807 and EMA/LR 06 from France.
abbreviations
- FP-biotin
10-fluoroethoxyphosphinyl-N-biotinamidopentyldecanamide
- DFP
diisopropylfluorophosphate
- DHBA
2,5-dihydroxybenzoic acid
- CHCA
α-cyano 4-hydoxycinnamic acid
- OP
organophosphorus toxicant
- MS/MS
tandem mass spectra
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams TK, Capacio BR, Smith JR, Whalley CE, Korte WD. The application of the fluoride reactivation process to the detection of sarin and soman nerve agent exposures in biological samples. Drug Chem Toxicol. 2004;27:77–91. doi: 10.1081/dct-120027901. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem. 2005;280:5724–5732. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- Benschop HP, Keijer JH. On the mechanism of ageing of phosphonylated cholinesterases. Biochim Biophys Acta. 1966;128:586–588. doi: 10.1016/0005-2744(69)90441-0. [DOI] [PubMed] [Google Scholar]
- Black RM, Harrison JM, Read RW. The interaction of sarin and soman with plasma proteins: the identification of a novel phosphonylation site. Arch Toxicol. 1999;73:123–126. doi: 10.1007/s002040050596. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Elhanany E, Ordentlich A, Dgany O, Kaplan D, Segall Y, Barak R, Velan B, Shafferman A. Resolving pathways of interaction of covalent inhibitors with the active site of acetylcholinesterases: MALDI-TOF/MS analysis of various nerve agent phosphyl adducts. Chem Res Toxicol. 2001;14:912–918. doi: 10.1021/tx0100542. [DOI] [PubMed] [Google Scholar]
- Erdos EG, Boggs LE. Hydrolysis of paraoxon in mammalian blood. Nature. 1961;190:716–717. doi: 10.1038/190716a0. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Fredriksson SA, Hammarstrom LG, Henriksson L, Lakso HA. Trace determination of alkyl methylphosphonic acids in environmental and biological samples using gas chromatography/negative-ion chemical ionization mass spectrometry and tandem mass spectrometry. J Mass Spectrom. 1995;30:1133–1143. [Google Scholar]
- Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353:38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Zokas L, Kamps MP. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988;109:277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Grognet JM, Ardouin T, Istin M, Vandais A, Noel JP, Rima G, Satge J, Pradel C, Sentenac-Roumanou H, Lion C. Production and characterization of antibodies directed against organophosphorus nerve agent VX. Arch Toxicol. 1993;67:66–71. doi: 10.1007/BF02072038. [DOI] [PubMed] [Google Scholar]
- Hagag N, Birnbaum ER, Darnall DW. Resonance energy transfer between cysteine-34, tryptophan-214, and tyrosine-411 of human serum albumin. Biochemistry. 1983;22:2420–2427. doi: 10.1021/bi00279a018. [DOI] [PubMed] [Google Scholar]
- He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Cerasoli DM, Lenz DE. Role of immunogen design in induction of soman-specific monoclonal antibodies. Immunol Lett. 2005;96:121–127. doi: 10.1016/j.imlet.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kovach IM, Akhmetshin R, Enyedy IJ, Viragh C. A self-consistent mechanism for dealkylation in soman-inhibited acetylcholinesterase. Biochem J. 1997;324 ( Pt 3):995–996. doi: 10.1042/bj3240995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L, Gjika HB, Van Vunakis H. Antibodies and radioimmunoassays for phosphoserine, phosphothreonine and phosphotyrosine. Serologic specificities and levels of the phosphoamino acids in cytoplasmic fractions of rat tissues. J Immunol Methods. 1989;124:239–249. doi: 10.1016/0022-1759(89)90360-8. [DOI] [PubMed] [Google Scholar]
- Mann M, Hendrickson RC, Pandey A. Analysis of proteins and proteomes by mass spectrometry. Annu Rev Biochem. 2001;70:437–473. doi: 10.1146/annurev.biochem.70.1.437. [DOI] [PubMed] [Google Scholar]
- Manoharan I, Boopathy R. Diisopropylfluorophosphate-sensitive aryl acylamidase activity of fatty acid free human serum albumin. Arch Biochem Biophys. 2006;452:186–188. doi: 10.1016/j.abb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- McLachlin DT, Chait BT. Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr Opin Chem Biol. 2001;5:591–602. doi: 10.1016/s1367-5931(00)00250-7. [DOI] [PubMed] [Google Scholar]
- McLafferty FW. Mass spectrometric analysis, molecular rearrangements. Anal Chem. 1959;31:82–87. [Google Scholar]
- Means GE, Wu HL. The reactive tyrosine residue of human serum albumin: characterization of its reaction with diisopropylfluorophosphate. Arch Biochem Biophys. 1979;194:526–530. doi: 10.1016/0003-9861(79)90647-7. [DOI] [PubMed] [Google Scholar]
- Michel HO, Hackley BE, Jr, Berkowitz L, List G, Hackley EB, Gillilan W, Pankau M. Ageing and dealkylation of Soman (pinacolylmethylphosphonofluoridate)-inactivated eel cholinesterase. Arch Biochem Biophys. 1967;121:29–34. doi: 10.1016/0003-9861(67)90006-9. [DOI] [PubMed] [Google Scholar]
- Millard CB, Koellner G, Ordentlich A, Shafferman A, Silman I, Sussman J. Reaction products of acetylcholinesterase and VX reveal a mobile histidine in the catalytic triad. J Am Chem Soc. 1999a;121:9883–9884. [Google Scholar]
- Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999b;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- Mourik J, de Jong LP. Binding of the organophosphates parathion and paraoxon to bovine and human serum albumin. Arch Toxicol. 1978;41:43–48. doi: 10.1007/BF00351768. [DOI] [PubMed] [Google Scholar]
- Murachi T. A general reaction of diisopropylphosphorofluoridate with proteins without direct effect on enzymic activities. Biochim Biophys Acta. 1963;71:239–241. doi: 10.1016/0006-3002(63)91021-7. [DOI] [PubMed] [Google Scholar]
- Ortigoza-Ferado J, Richter RJ, Hornung SK, Motulsky AG, Furlong CE. Paraoxon hydrolysis in human serum mediated by a genetically variable arylesterase and albumin. Am J Hum Genet. 1984;36:295–305. [PMC free article] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr . All about albumin. Biochemistry, genetics, and medical applications. Academic Press Ltd; London: 1996. [Google Scholar]
- Sanger F. Amino-acid sequences in the active centers of certain enzymes. Proc Chem Soc. 1963;5:76–83. [Google Scholar]
- Schopfer LM, Champion MM, Tamblyn N, Thompson CM, Lockridge O. Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410) Anal Biochem. 2005;345:122–132. doi: 10.1016/j.ab.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Carrera V, Vilanova E. Hydrolysis of carbaryl by human serum albumin. Arch Toxicol. 2004;78:629–634. doi: 10.1007/s00204-004-0584-x. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Diaz-Alejo N, Escudero MA, Vilanova E. Phosphotriesterase activity identified in purified serum albumins. Arch Toxicol. 1998a;72:219–226. doi: 10.1007/s002040050492. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Monroy A, Vilanova E. Chicken serum albumin hydrolyzes dichlorophenyl phosphoramidates by a mechanism based on transient phosphorylation. Chem Res Toxicol. 1998b;11:1441–1446. doi: 10.1021/tx980015z. [DOI] [PubMed] [Google Scholar]
- Steen H, Kuster B, Fernandez M, Pandey A, Mann M. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal Chem. 2001;73:1440–1448. doi: 10.1021/ac001318c. [DOI] [PubMed] [Google Scholar]
- Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999;12:439–446. doi: 10.1093/protein/12.6.439. [DOI] [PubMed] [Google Scholar]
- Sultatos LG, Basker KM, Shao M, Murphy SD. The interaction of the phosphorothioate insecticides chlorpyrifos and parathion and their oxygen analogues with bovine serum albumin. Mol Pharmacol. 1984;26:99–104. [PubMed] [Google Scholar]
- Tholey A, Reed J, Lehmann WD. Electrospray tandem mass spectrometric studies of phosphopeptides and phosphopeptide analogues. J Mass Spectrom. 1999;34:117–123. doi: 10.1002/(SICI)1096-9888(199902)34:2<117::AID-JMS769>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Tsuge K, Seto Y. Detection of human butyrylcholinesterase-nerve gas adducts by liquid chromatography-mass spectrometric analysis after in gel chymotryptic digestion. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:21–30. doi: 10.1016/j.jchromb.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Van Der Schans MJ, Polhuijs M, Van Dijk C, Degenhardt CE, Pleijsier K, Langenberg JP, Benschop HP. Retrospective detection of exposure to nerve agents: analysis of phosphofluoridates originating from fluoride-induced reactivation of phosphylated BuChE. Arch Toxicol. 2004;78:508–524. doi: 10.1007/s00204-004-0568-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Tanase S, Nakajou K, Maruyama T, Kragh-Hansen U, Otagiri M. Role of arg-410 and tyr-411 in human serum albumin for ligand binding and esterase-like activity. Biochem J 349 Pt. 2000;3:813–819. doi: 10.1042/bj3490813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YX, Yan QJ, Ci YX, Guo ZQ, Rong KT, Chang WB, Zhao YF. Detection of the organophosphorus nerve agent sarin by a competitive inhibition enzyme immunoassay. Arch Toxicol. 1995;69:644–648. doi: 10.1007/s002040050226. [DOI] [PubMed] [Google Scholar]