Abstract
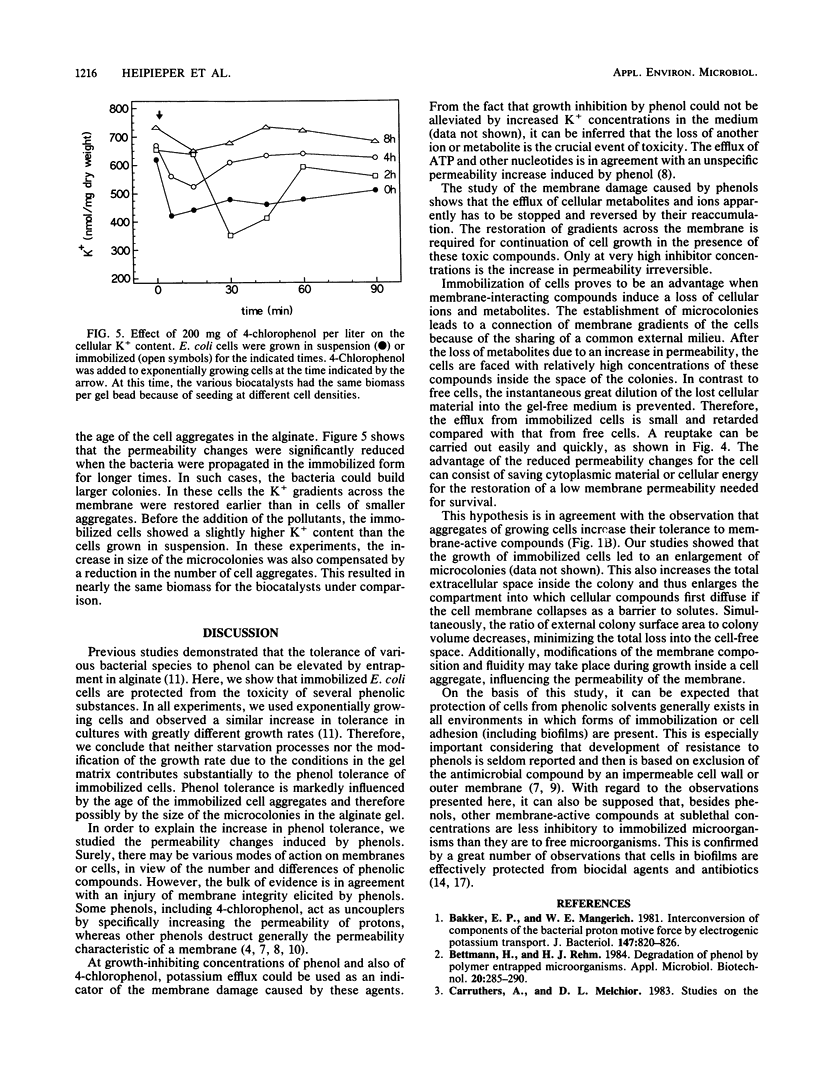
The inhibition of the exponential growth of Escherichia coli K-12 by different phenolic compounds was examined. Cells entrapped in calcium alginate showed a greater tolerance than cells grown in suspension. The extent of inhibition of growth of the immobilized cells depended on the period of growth in the gel matrix. After the addition of bacteriostatic concentrations of phenol or 4-chlorophenol, a dose-dependent efflux of metabolites such as ATP and of K+ ions was elicited. Provided that glucose was supplied as an energy substrate, a reaccumulation of K+ ions at low phenol concentrations was observed. The restoration of the membrane gradient for K+ always preceded the continuation of growth in the presence of the toxic compounds. Compared with free cells, those cells immobilized and grown in alginate suffered a smaller loss of cations after the addition of 4-chlorophenol. The reestablishment of gradients was observed at higher concentrations of the pollutants with entrapped cells than with free cells. Corresponding to the increase in tolerance, the membrane damage was reduced in cells grown in immobilized form for longer times. These data offer a mechanistic explanation of the protection of immobilized microorganisms from phenolic solvents. The data point to the membrane as an important cell component in the toxicity of these pollutants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. F., Krumme M. L., Boyd S. A., Tiedje J. M. Kinetics of phenol biodegradation by an immobilized methanogenic consortium. Appl Environ Microbiol. 1986 Aug;52(2):345–351. doi: 10.1128/aem.52.2.345-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Brown M. R. Influence of growth rate and nutrient limitation on the gross cellular composition of Pseudomonas aeruginosa and its resistance to 3- and 4-chlorophenol. J Bacteriol. 1978 Mar;133(3):1066–1072. doi: 10.1128/jb.133.3.1066-1072.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keweloh H., Weyrauch G., Rehm H. J. Phenol-induced membrane changes in free and immobilized Escherichia coli. Appl Microbiol Biotechnol. 1990 Apr;33(1):66–71. doi: 10.1007/BF00170572. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- LeChevallier M. W., Cawthon C. D., Lee R. G. Inactivation of biofilm bacteria. Appl Environ Microbiol. 1988 Oct;54(10):2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Thomson K., Kaiser K. L. Quantitative structure-toxicity relationship of halogenated phenols on bacteria. Bull Environ Contam Toxicol. 1982 Aug;29(2):130–136. doi: 10.1007/BF01606140. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]


