Abstract
Recent developmental studies demonstrate that early fossil hominins possessed shorter growth periods than living humans, implying disparate life histories. Analyses of incremental features in teeth provide an accurate means of assessing the age at death of developing dentitions, facilitating direct comparisons with fossil and modern humans. It is currently unknown when and where the prolonged modern human developmental condition originated. Here, an application of x-ray synchrotron microtomography reveals that an early Homo sapiens juvenile from Morocco dated at 160,000 years before present displays an equivalent degree of tooth development to modern European children at the same age. Crown formation times in the juvenile's macrodont dentition are higher than modern human mean values, whereas root development is accelerated relative to modern humans but is less than living apes and some fossil hominins. The juvenile from Jebel Irhoud is currently the oldest-known member of Homo with a developmental pattern (degree of eruption, developmental stage, and crown formation time) that is more similar to modern H. sapiens than to earlier members of Homo. This study also underscores the continuing importance of North Africa for understanding the origins of human anatomical and behavioral modernity. Corresponding biological and cultural changes may have appeared relatively late in the course of human evolution.
Keywords: dental development, human evolution, human origins, synchrotron microtomography, tooth growth
Because of increasing evidence of sophisticated material culture, new fossil material, and refined dating techniques, paleoanthropologists have renewed investigations of modern human origins in Africa, in particular the question of when early Homo sapiens†† became fully modern (1–6). Whereas cranial and dental features demonstrate that African Middle Pleistocene Homo underwent an anatomical transition from a more primitive form (i.e., Homo heidelbergensis/Homo rhodesiensis) to a relatively modern form (H. sapiens) (1), little is known about changes in the timing of somatic development, reproductive scheduling, or lifespan (7). Analyses of dental development, molar eruption, and age at death based on incremental features of the dentition are the most accurate means of identifying developmental change in the human fossil record (7–10).
Studies of dental development in early Homo document a life history pattern more similar to African apes than to modern humans (8, 10), and research on brain growth in Homo erectus also suggests a shorter period of early development than is seen in modern human populations (11). Aspects of H. heidelbergensis, Homo antecessor, and Homo neanderthalensis anterior tooth development also differ when compared with Upper Paleolithic/Mesolithic populations (12), although Neanderthal molar development may more closely resemble that of modern humans (13). Recent work on a single permanent Neanderthal molar tooth reported a shorter crown formation time than modern humans, but similar rates of root extension; this was interpreted as evidence that life history was similar between Neanderthals and modern humans (13). However, overlap in molar crown formation times and root extension rates have been found between chimpanzees and humans (10, 14), despite differences in life history scheduling. Furthermore, it is still unknown when Neanderthals erupt their first molars, an event which is highly correlated with other life history variables (7). In short, given well documented technological innovations during the Pleistocene, dietary changes during the Holocene, and dental reduction in modern populations, it is unclear whether dental development may have undergone corresponding changes, and when the unique, prolonged modern human pattern of growth and development originated (7, 10).
North African Middle and early Upper Pleistocene hominins are typically characterized as having macrodont dentitions and a combination of primitive traits shared with early Homo and derived traits shared with later Homo, including H. sapiens. Early descriptions of the hominins from Jebel Irhoud (Morocco) emphasized similarities with Neanderthals; however, recent analyses demonstrate a number of synapomorphies shared with modern humans, establishing the presence of H. sapiens sensu stricto in North Africa 130,000–190,000 years before present (ybp) (1). The juvenile individual from Jebel Irhoud (Irhoud 3) is represented by a well preserved mandible (15) that dates from just less than the geological ages of the earliest evidence for early H. sapiens in East Africa (5). Recent direct uranium series/electron spin resonance dates on the specimen confirm earlier dates, suggesting an age of 160,000 ± 16,000 ybp [supporting information (SI) Methods and SI Figs. 4–6]. This study aimed to characterize dental development and age at death in Irhoud 3, and to compare it with fossil hominins and living human populations to determine whether the modern human condition of prolonged dental development was present. The preservation of an erupted first molar, erupting lateral incisor and unerupted canine, and the application of high-resolution propagation phase contrast x-ray synchrotron microtomography (phase contrast SR-mCT) (16–18), facilitates accurate nondestructive estimation of the age at death, such that this specimen represents the earliest member of H. sapiens for which the timing of tooth eruption, duration of crown formation, and scheduling of life history may be inferred.
Tooth development is characterized by features representing subdaily, daily, supradaily, and annual rhythms permanently recorded in hard tissues of the crown and root (reviewed in ref. 19). Estimates of crown formation time and age at death based on these incremental features may yield highly accurate results from histologically sectioned material (9). The duration of enamel formation is typically assessed by counts of long-period lines (Retzius lines internally or perikymata externally) multiplied by their periodicity (number of daily cross-striations between successive Retzius lines), which is added to an estimate of cuspal enamel formation based on linear thickness and secretion rates. This study represents the most comprehensive nondestructive assessment of crown formation in a single fossil hominin specimen, combining data on external incremental features (perikymata) with internal variables (cuspal enamel thickness and long-period line periodicity) derived from high-resolution laboratory microtomography and phase contrast SR-mCT. This unique nondestructive approach yields crown formation times based on more directly determined growth parameters than most previous studies of fossil hominins (e.g., refs. 8, 12, and 20–23).
Results
Perikymata (Fig. 1) were evident on the surface of the Irhoud 3 lower right lateral incisor, lower left canine, and mesiobuccal cusp of the lower left molar. The periodicity of long-period incremental features was 10 days in this individual (Fig. 2 and SI Fig. 7), determined from phase contrast SR-mCT. Data on perikymata number, cuspal enamel thickness, and crown formation time for each of the three permanent teeth are given in Table 1. Crown formation times were estimated to be 5.13, 6.68, and 3.96 years for the lower lateral incisor, canine, and mesiobuccal cusp of the first molar, respectively. Twenty periradicular bands, which are believed to be equivalent to other long-period lines (23–25), were counted on the canine root from the end of crown formation until death; thus, the duration of root formation was 200 days. The age at death, determined by adding the age of modern human canine initiation (26) to the duration of canine crown and root formation observed in this specimen, was 7.78 years (2,839 days). Initiation ages of early-forming teeth do not seem to vary greatly between modern humans and chimpanzees; because initiation ages in both taxa are quite similar for early-forming teeth [less than a few days to ≈2 months apart depending on tooth type (26, 27)], the use of the modern human value for this variable is unlikely to greatly affect the accuracy of estimates.
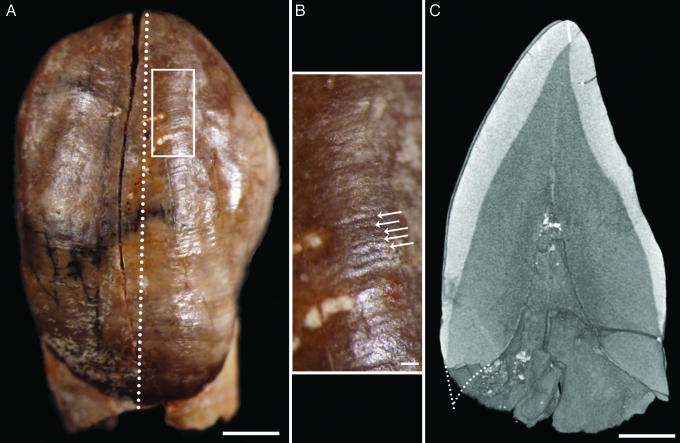
Fig. 1.
Unerupted lower left canine germ of the Irhoud 3 juvenile. (A) Stereo microscope overview with position of area enlarged in B (white box) and virtual plane of section in C (dotted line). (B) Perikymata (white arrows), surface manifestations of long-period Retzius lines, were counted from the cusp tip to the cervix on the original tooth. (C) Cuspal enamel thickness (white line near top) and estimated root length (dotted line near bottom) were taken from this virtual section. Labial root length estimate was made by projecting the curvatures of the developing root cone and the enamel-dentine junction. (Scale bars: 2 mm in A and C and 0.2 mm in B.)
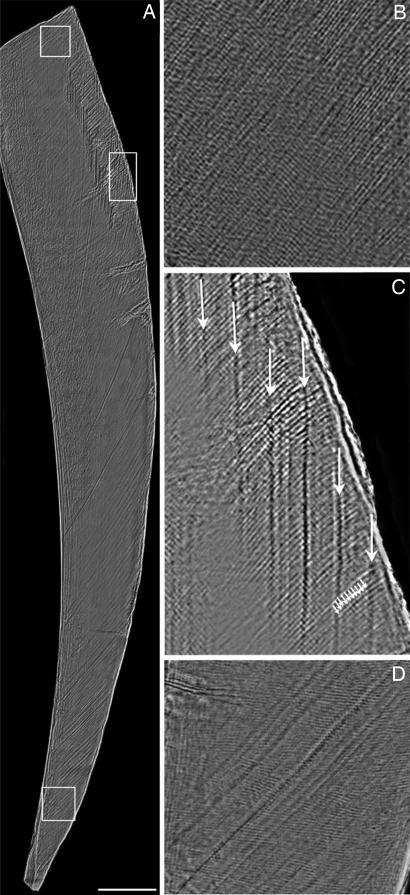
Fig. 2.
Enamel microstructure in an incisor fragment from Irhoud 3. (A) Virtual section (70 μm thick) of labial enamel generated by using phase contrast SR-mCT. (Scale bar: 0.5 mm.) (B–D) The enlarged areas from top to bottom show daily cross-striations (light and dark bands crossing diagonal enamel prisms) in the lateral enamel (B), the periodicity of Retzius lines: 10 daily cross-striations (small arrows) between successive Retzius lines (large arrows) (C) (see also SI Fig. 7), and an area of cervical enamel showing accentuated lines and slight prism decussation (D).
Table 1.
Crown formation times and age at crown completion in the mandibular permanent teeth of Irhoud 3
| Tooth | PK | Per | LT | Thick | Min CT | Max CT | CFT | Init | Age |
|---|---|---|---|---|---|---|---|---|---|
| I2 | 167 | 10 | 1,670 | 645 | 170 | 234 | 1,872 | 146 | 2,018 |
| C | 217 | 10 | 2,170 | 885 | 233 | 305 | 2,439 | 200 | 2,639 |
| M1 | 114 | 10 | 1,140 | 1,080 | 263 | 350 | 1,446 | −18 | 1,428 |
I2, right lateral incisor; C, left canine; M1, mesiobuccal cusp of the left first molar; PK, perikymata number; Per, periodicity (number of daily cross-striations between long-period lines); LT, lateral formation time in days; Thick, linear cuspal enamel thickness in micrometers; Min CT, minimum cuspal formation time in days; Max CT, maximum cuspal formation time in days; CFT, crown formation time in days; Init, initiation age in days; Age, age at crown formation in days.
Virtual extractions of the permanent dentition reveal that both premolars and the second permanent molar had completed crown formation and had just begun root formation (Fig. 3). Comparisons with modern European populations at a similar developmental stage (lower second premolar and second molar root initiation) suggests an average age of 7.2–7.3 years for a female or 7.3–7.6 years for a male juvenile (28). This result is consistent with the stage of lateral incisor (I2) eruption, which is a few mm short of being in a fully erupted position; modern European I2 eruption typically occurs between seven and eight years of age (29). Thus, the Irhoud 3 juvenile seems to have a developmental stage quite similar to average modern European children.
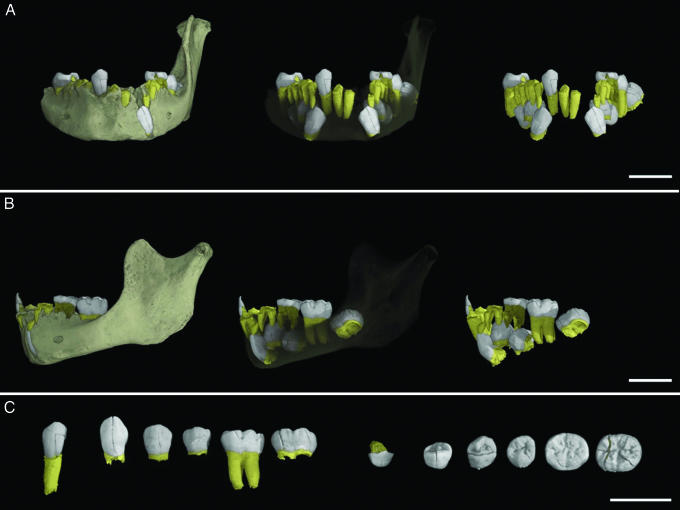
Fig. 3.
The mandibular dentition of the Irhoud 3 juvenile; crowns are in white, roots in yellow. (A and B) Virtual mandible showing the dentition from anterior (A) and lateral (B) perspectives, with the dentition virtually extracted in situ on the right. (C) Isolated and virtually extracted teeth in labial/buccal orientation on the left and occlusal orientation on the right (teeth left to right: right lateral incisor, left canine, left first premolar, left second premolar, left first permanent molar, left second permanent molar). Bright white speckles are from a dense mineral that invaded the mandible during fossilization. (Scale bars: 2 cm.)
Discussion
The nondestructive application of phase contrast SR-mCT to determine long-period line periodicity represents a valuable tool for studies of dental development. Uncertainty about this variable has historically led to broad confidence intervals for estimates of hominin crown formation times (e.g., refs. 8, 21, and 22), complicating comparisons with modern human populations. The long-period line periodicity of 10 in Irhoud 3 is the highest value yet reported in a fossil hominin; this high value may be related to its large body and/or tooth size. Long-period line periodicity in modern humans is highly variable; a recent study of 365 modern humans found a mean value of 8 days, with a range of 6–12 days (14).
Perikymata numbers were higher than mean values for equivalent teeth of early East African fossil Homo, southern African Middle Stone Age H. sapiens, H. neanderthalensis, and two modern human populations (Table 2), but were not outside the European population range for modern humans. Comparisons between the cuspal enamel thickness of Irhoud 3 and modern humans demonstrate similarities (14, 30, 31), although the Irhoud 3 permanent teeth have slightly thinner cuspal enamel. Crown formation times in Irhoud 3 are higher than modern human means, exceeding known ranges of the two modern human comparative samples. The incisor and canine formation times are more similar to modern Europeans than to southern African populations of modern humans (31), unlike the more rapid pattern reported for Neanderthals (21). Global variation in histologically determined crown formation times is poorly understood, for example modern Japanese dentitions may form over an even longer period than the Irhoud 3 individual (32). Long crown formation times in Irhoud 3 may be due in part to the absolutely large size of the teeth, which are larger than many macrodont fossil hominins (15). Canine height in Irhoud 3 (13.6 mm vertical height, 15.0 mm curvilinear length) is greater than mean values for H. heidelbergensis, H. neanderthalensis, H. antecessor, Upper Paleolithic/Mesolithic H. sapiens, and living H. sapiens, in many cases outside of 95% confidence intervals (12, 31). The finding of long crown formation times in a macrodont hominin dentition is not unexpected; given that secretion rates seem to be fairly constrained among apes and humans (e.g., refs. 8 and 14), increasing the duration of crown formation may be necessary to form larger teeth, suggesting that crown formation time alone may not be a reliable predictor of life history. Evidence of tooth eruption ages and the attainment of developmental stages represent more robust markers of life history (7), providing strong evidence for a prolonged life history in H. sapiens 160,000 years ago.
Table 2.
Long-period (incremental line) numbers and crown formation times in living and fossil Homo
| Taxon | LP |
CFT, days |
|||||
|---|---|---|---|---|---|---|---|
| N | LI2 | LC | LM1 | LI2 | LC | LM1 | |
| Early Homo | 2–3 | 92–113 | 110 | — | 893–1,292 | 1,142–1,362 | — |
| H. neanderthalensis | 5–10 | 151 (22) | 159 (15) | 92 (16) | — | — | 1,041 |
| Irhoud 3 | 1 | 167 | 217 | 114 | 1,872 | 2,439 | 1,446 |
| MSA Homo | 2 | — | — | 94 | — | — | 1,128 |
| Modern EUR | 6–13 | 130 (19) | 199 (22) | 92 (14) | 1,376 (46) | 2,066 (73) | 1,160 (34) |
| Modern SA | 6–23 | 111 (12) | 155 (24) | 93 (10) | 1,224 (25) | 1,712 (47) | 1,161 (31) |
In the Taxon column, the following applies: Early East African Homo (8); Neanderthal data are from refs. 13 and 21 and from D. Guatelli-Steinberg and D.J.R., unpublished data; MSA Homo are Middle Stone Age humans (22); Modern EUR are modern clinically extracted teeth from Newcastle, U.K. (14, 30, 31). Modern SA are extracted modern human teeth from a skeletal collection of mixed South African tribal populations (14, 30, 31). N, sample sizes, which varied within taxa for tooth types and developmental variables; LP, long-period line numbers, either perikymata from external surfaces or Retzius lines from histological sections of modern teeth. LI2, LC, and LM1, lower lateral incisor, lower canine, and lower molar mesiobuccal cusps, respectively; CFT, crown formation time, determined for some fossil taxa based on estimated periodicity and cuspal formation times (except for data from ref. 13), or for modern samples from values derived from histological sections. Numbers in parentheses represent standard deviations when given.
The fossil record of early representatives of H. sapiens is difficult to interpret because of the lack of secure dates and the mosaic of primitive and derived features found on many of the well preserved fossils (6). Given the strong genetic component to dental development (reviewed in ref. 33), as well as the high correlation with other aspects of life history (7), evidence from dental eruption and tooth growth represents a robust character for the identification of modern humans in the Middle or early Upper Pleistocene. Relatively few contemporaneous African specimens have the potential to provide information about dental development. An exception is the East African Herto child (BOU-VP-16/5) (4), which preserves a maxillary dentition at a similar developmental stage as the Irhoud 3 mandible. Although numerous juvenile Neanderthal dentitions have been recovered, age at death assignments have often been based on comparison with modern European developmental standards (34), which may be inappropriate if they have a more accelerated period of dental development than Upper Paleolithic/Mesolithic humans (12) or modern Europeans (35). The application of phase contrast SR-mCT allows for greater resolution of this issue, as it is now possible to nondestructively determine the long-period line periodicity values of fossil hominin dentitions.
Because of the permanent developmental records preserved in teeth and their potential for identifying developmental change in the human fossil record, the innovative studies of Dean and colleagues (e.g., 8, 10, 13, 20, 23, 35) have substantially altered reconstructions of fossil hominin ontogeny, pointing to differences among members of the genus Homo. This is further supported by suggestions of short anterior tooth formation times in H. antecessor, H. heidelbergensis, and H. neanderthalensis (12), and short molar formation time in a Neanderthal first molar (13). The juvenile mandible from Jebel Irhoud is, at present, the oldest-known member of Homo to show a pattern of eruption and period of dental development more similar to modern H. sapiens. This extended period of development, and by implication childhood, implies the advent of corresponding social, biological, and cultural changes necessary to support highly dependent children with prolonged opportunities for social learning in early childhood (7, 11, 36).
Materials and Methods
Perikymata counts were made on the erupting lower right lateral incisor, unerupted lower left canine, and erupted lower left first molar (mesiobuccal cusp) by using stereomicroscopy at a magnification of ×50; the canine was isolated as the mandible was fractured through the canine crypt, permitting physical extraction (15). The long-period line periodicity was determined from a fragment of the permanent incisor (Fig. 2 and SI Fig. 7) imaged at a 0.7-μm voxel size by using phase contrast SR-mCT performed with beamline ID 19 at the European Synchrotron Radiation Facility (Grenoble, France). Lateral (imbricational) enamel formation time was calculated by multiplying the number of perikymata by the periodicity. Cuspal enamel thickness was determined from microtomographic slices (detailed below); measurements were made from the tip of the dentine horn to the approximate position of the first perikymata at the cusp surface (Fig. 1).
Cuspal formation time was calculated by two different methods and an average value was determined. A minimum estimate was calculated as cuspal thickness divided by the modern human mean cuspal daily secretion rate: 3.80 μm/day for anterior teeth (37) and 4.11 μm/day for molar teeth (14). It was not possible to directly quantify cuspal daily secretion rates in Irhoud 3 as only the labial enamel fragment of the incisor was imaged with phase contrast SR-mCT. However, given the similarity of mean cuspal rates between humans, chimpanzees, and Neanderthals (13, 14), estimates derived from modern human populations are unlikely to affect accuracy. A maximum cuspal formation time estimate was calculated with regression equations for modern human cuspal enamel formation times for anterior (38) and posterior (8) teeth. Crown formation time was calculated by adding the lateral enamel formation time to the average of the two methods for cuspal formation time estimation. Age at crown completion was determined by adding modern human initiation ages (26) to the duration of crown formation time.
Canine root formation was determined by following an accentuated periradicular band, formed just after crown completion on the labial surface, to the longest portion of the root preserving the entire developing surface (on the original specimen); counts were made of long-period periradicular bands (23–25) from the accentuated band to the end of root formation, and this number was multiplied by the periodicity of long-period lines determined from enamel (10 days) to yield root formation in days. Labial canine root length was estimated from the original specimen and mCT slices, and a slight correction was made for the fractured developing surface (Fig. 1). The estimated canine root length of 1.56 mm was divided by the duration of formation to yield an average extension rate of 7.8 μm/day. This rate is approximately twice as high as that found in a small sample of recent humans (SI Table 3) and suggests that, relative to modern humans, the dentition of Irhoud 3 showed a long crown formation and a rapid root extension, leading to similar ages at tooth eruption. Age at death was determined as the sum of age at canine crown completion and the duration of root formation. Based on this age, root extension rates were estimated for 11 mm of lower lateral incisor root as 13.4 μm/day, and 9.6 μm/day for the first 13.5 mm of the first molar root. These values are intermediate between those for living and fossil hominins (8, 10, 13) and explain how teeth with relatively long crown formation times may erupt at the same age as modern humans.
The mandible was scanned by using a BIR Actis 300/225 microtomograph [Bio-Imaging Research, Inc. (BIR), Lincolnshire, IL] at an isotropic voxel size of 109 μm, and the developing canine was scanned at a voxel size of 13.7 μm with a Skyscan 1172 microtomograph (Kontich, Belgium), performed at the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany. Virtual extraction of the mandibular dentition was performed by using VG Studio Max 1.2.1 software (Heidelberg, Germany). For each tooth, enamel and dentine were segmented by using the 3D magic wand region growing tool or by manual segmentation. A three-pixel Gaussian filter was subsequently applied to each subvolume to obtain a smooth 3D rendering. Dense metallic oxide particles were partially removed by modifying the rendering ramp; the whitest parts of the slices were set to zero. In some cases, it was not possible to remove the oxide particles without altering the surface of the teeth (Fig. 3), which was avoided.
Supplementary Material
Acknowledgments
We give special thanks to Debbie Guatelli-Steinberg, Anthony Olejniczak, Fernando Ramirez Rozzi, Gina Steding, Heiko Temming, Andreas Winzer, Monsieur le Doyen de la Faculté des Sciences, the University of Rabat, the German Embassy in Morocco, the Government of Morocco, and the ID 19 beamline staff. We thank Chris Dean for helpful comments on this project. Allison Cleveland, Anthony Olejniczak, Gary Schwartz, and two anonymous reviewers provided helpful comments on the manuscript. Funding was supplied by the Max Planck Society, European Virtual Anthropology Marie Curie Research Training Network MRTN-CT-019564, the Centre National de la Recherche Scientifique, and the European Synchrotron Radiation Facility.
Abbreviations
- Phase contrast SR-mCT
Phase contrast X-ray synchrotron microtomography
- ybp
years before present.
Footnotes
††In this article, early H. sapiens includes African fossils postdating 200,000 ybp that are variably referred to as “ancestors of modern humans,” “early anatomically modern humans,” or “early modern humans” (1–6).
The authors declare no conflict of interest.
See Commentary on page 6093.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700747104/DC1.
References
- 1.Hublin J-J. In: Human Roots. Barham L, Robson-Brown K, editors. Bristol, UK: Western Academic and Specialist; 2001. pp. 99–121. [Google Scholar]
- 2.Klein R. The Human Career. Chicago: University of Chicago; 1999. [Google Scholar]
- 3.McBrearty S, Brooks A. J Hum Evol. 2000;39:453–563. doi: 10.1006/jhev.2000.0435. [DOI] [PubMed] [Google Scholar]
- 4.White TD, Asfaw B, DeGusta D, Gilbert H, Richards GD, Suwa G, Howell FC. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- 5.McDougall I, Brown FH, Fleagle JG. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 6.Trinkaus E. Annu Rev Anthropol. 2005;34:207–230. [Google Scholar]
- 7.Smith BH, Tompkins RL. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 8.Dean C, Leakey MG, Reid DJ, Schrenk F, Schwartz GT, Stringer C, Walker A. Nature. 2001;414:628–631. doi: 10.1038/414628a. [DOI] [PubMed] [Google Scholar]
- 9.Smith TM, Reid DJ, Sirianni JE. J Anat. 2006;208:125–138. doi: 10.1111/j.1469-7580.2006.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean MC. Proc R Soc London Ser B. 2006;273:2799–2808. [Google Scholar]
- 11.Coqueugniot H, Hublin J-J, Veillon F, Houët F, Jacob T. Nature. 2004;431:299–302. doi: 10.1038/nature02852. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez Rozzi FV, Bermudez de Castro JM. Nature. 2004;428:936–939. doi: 10.1038/nature02428. [DOI] [PubMed] [Google Scholar]
- 13.Macchiarelli R, Bondioli L, Debénath A, Mazurier A, Tournepiche J-F, Birch W, Dean C. Nature. 2006;444:748–751. doi: 10.1038/nature05314. [DOI] [PubMed] [Google Scholar]
- 14.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Ferrell RJ, Martin LB. In: Dental Perspectives on Human Evolution: State of the Art Research in Dental Paleoanthropology. Bailey SE, Hublin J-J, editors. The Netherlands: Springer, Dordrecht; in press. [Google Scholar]
- 15.Hublin J-J, Tillier AM. In: Aspects of Human Evolution. Stringer CB, editor. London: Taylor and Francis; 1981. pp. 167–185. [Google Scholar]
- 16.Tafforeau P. Montpellier, France: Université de Montpellier II; 2004. PhD thesis. [Google Scholar]
- 17.Tafforeau P, Boistel R, Boller E, Bravin A, Brunet M, Chaimanee Y, Cloetens P, Feist M, Hoszowska J, Jaeger J-J, et al. Appl Phys A. 2006;83:195–202. [Google Scholar]
- 18.Tafforeau P, Bentaleb I, Jaeger J-J, Martin C. Palaeogeogr Palaeoclimatol Palaeoecol. in press. [Google Scholar]
- 19.Smith TM. J Anat. 2006;208:99–113. doi: 10.1111/j.1469-7580.2006.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bromage TG, Dean MC. Nature. 1985;317:525–527. doi: 10.1038/317525a0. [DOI] [PubMed] [Google Scholar]
- 21.Guatelli-Steinberg D, Reid DJ, Bishop TA, Larsen CS. Proc Natl Acad Sci USA. 2005;102:14197–14202. doi: 10.1073/pnas.0503108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith TM, Olejniczak AJ, Tafforeau P, Reid DJ, Grine FE, Hublin J-J. S Afri J Sci. in press. [Google Scholar]
- 23.Dean MC. In: Aspects of Dental Biology: Palaeontology, Anthropology, and Evolution. Moggi-Cecchi J, editor. Florence, Italy: Intl Inst Study Man; 1995. pp. 239–265. [Google Scholar]
- 24.Newman HN, Poole DFG. Arch Oral Biol. 1974;19:1135–1143. doi: 10.1016/0003-9969(74)90242-8. [DOI] [PubMed] [Google Scholar]
- 25.Berkovitz BKB, Grigson C, Dean MC. Am J Med Gen. 1998;76:343–348. [PubMed] [Google Scholar]
- 26.Reid DJ, Beynon AD, Ramirez Rozzi FV. J Hum Evol. 1998;35:463–477. doi: 10.1006/jhev.1998.0233. [DOI] [PubMed] [Google Scholar]
- 27.Reid DJ, Schwartz GT, Dean C, Chandrasekera MS. J Hum Evol. 1998;35:427–448. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- 28.Smith BH. In: Advances in Dental Anthropology. Kelley MA, Larsen CS, editors. New York: Wiley; 1991. pp. 143–168. [Google Scholar]
- 29.Liversidge H. In: Patterns of Growth and Development in the Genus Homo. Thompson JL, Krovitz GE, Nelson AJ, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 73–113. [Google Scholar]
- 30.Reid DJ, Dean MC. Am J Phys Anthropol. 2000;113:135–139. doi: 10.1002/1096-8644(200009)113:1<135::AID-AJPA13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Reid DJ, Dean MC. J Hum Evol. 2006;50:329–346. doi: 10.1016/j.jhevol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Kajiyama S. Nihon Univ Dent J. 1965;39:77–83. [Google Scholar]
- 33.Scott GR, Turner CG. The Anthropology of Modern Human Teeth. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 34.Tillier AM. Anthropologie XXXVIII. 2000;1:109–120. [Google Scholar]
- 35.Stringer CB, Dean MC, Martin RD. In: Primate Life History and Evolution. De Rousseau CJ, editor. New York: Wiley–Liss; 1990. pp. 115–152. [Google Scholar]
- 36.Walker R, Burger O, Wagner J, Von Rueden CR. J Hum Evol. 2006;51:480–489. doi: 10.1016/j.jhevol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz GT, Reid DJ, Dean C. Int J Primatol. 2001;22:837–860. [Google Scholar]
- 38.Schwartz GT, Dean C. Am J Phys Anthropol. 2001;115:269–283. doi: 10.1002/ajpa.1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.