Abstract
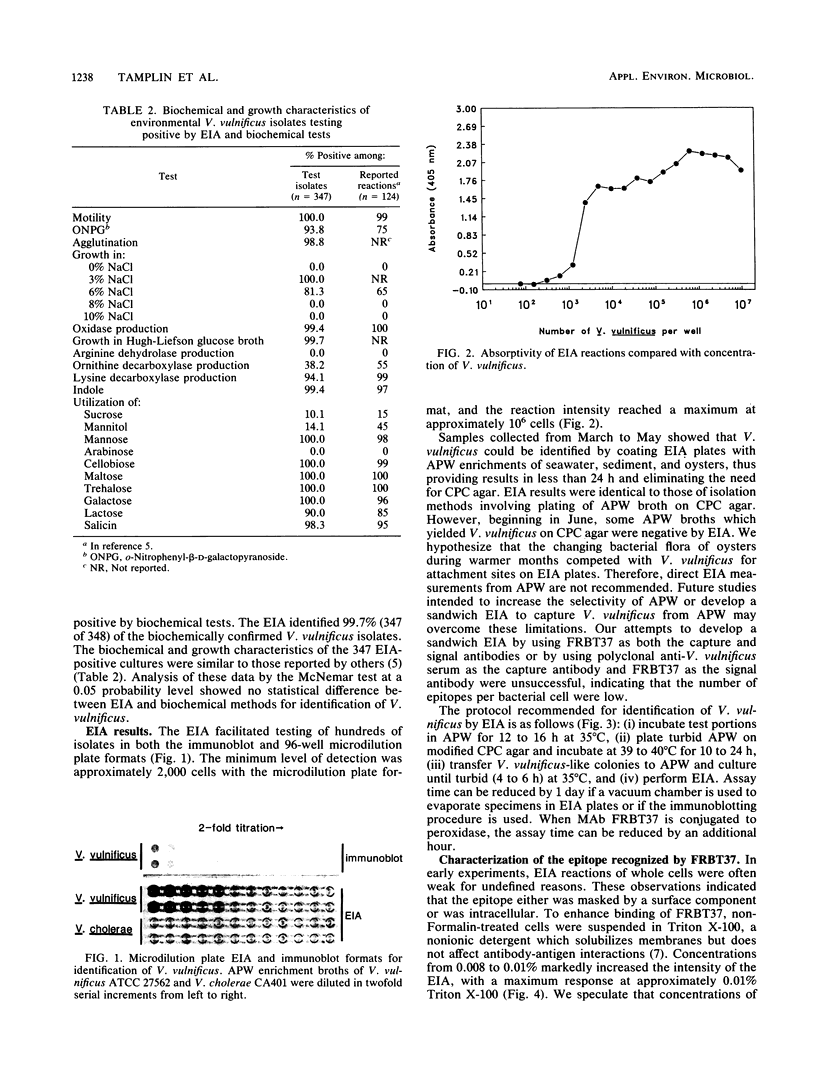
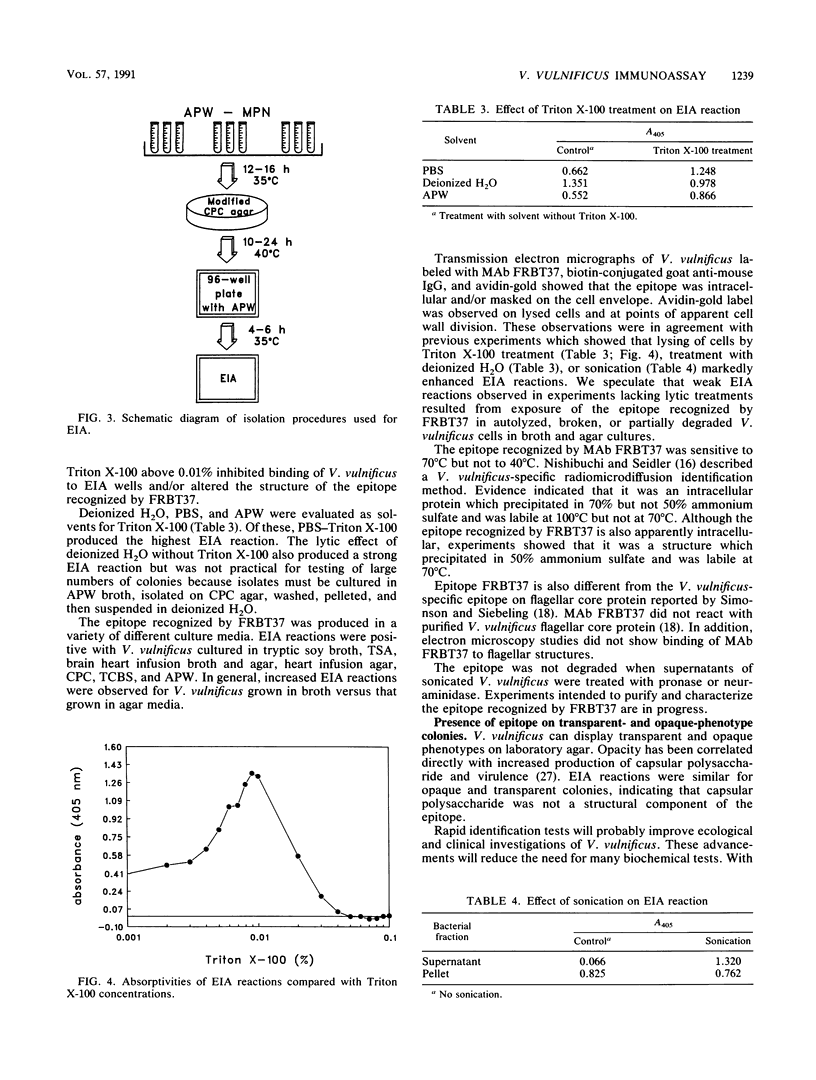
Historically, methods used to identify Vibrio vulnificus in environmental samples have been inadequate because isolation and identification procedures are time-consuming and fail to separate V. vulnificus from other bacterial species. We describe an enzyme immunoassay (EIA) and culture techniques which identified V. vulnificus in seawater, sediment, and oysters. The EIA used monoclonal antibody FRBT37 to a species-specific epitope of V. vulnificus. No cross-reactions were observed among 72 non-V. vulnificus strains comprising 34 species and 15 genera. In field trials, the EIA identified correctly 99.7% of 348 biochemically confirmed V. vulnificus isolates. The epitope corresponding to FRBT37 was found in cells lysed by Triton X-100, deionized H2O, and ultrasonication but was not found in culture supernatants, indicating that its location was intracellular. In addition, electron micrographs of V. vulnificus labeled with FRBT37-biotin-avidin-gold showed that epitope FRBT37 reacted with fragments of lysed cells but not whole cells. FRBT37 was expressed when V. vulnificus was cultured in different growth media. The minimum level of detection of the EIA was approximately 2,000 V. vulnificus cells per EIA well. Epitope FRBT37 was labile at 70 degrees C for 30 min. Immunoblot and EIA plate formats reduced assay time and facilitated handling large numbers of test samples.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brayton P. R., Tamplin M. L., Huq A., Colwell R. R. Enumeration of Vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl Environ Microbiol. 1987 Dec;53(12):2862–2865. doi: 10.1128/aem.53.12.2862-2865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. G., Jarvis J., Janda J. M. Use of sodium dodecyl sulfate-polymyxin B-sucrose medium for isolation of Vibrio vulnificus from shellfish. Appl Environ Microbiol. 1987 Jul;53(7):1556–1559. doi: 10.1128/aem.53.7.1556-1559.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. W., Pabst G. S., Jr Recommended modification of dilution procedure used for bacteriological examination of shellfish. J Assoc Off Anal Chem. 1984 Jan-Feb;67(1):197–198. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar C. W., Hartman P. A. Production and specificity of monoclonal antibodies and polyclonal antibodies to Escherichia coli. J Appl Bacteriol. 1987 Oct;63(4):335–341. doi: 10.1111/j.1365-2672.1987.tb02711.x. [DOI] [PubMed] [Google Scholar]
- Kaysner C. A., Abeyta C., Jr, Wekell M. M., DePaola A., Jr, Stott R. F., Leitch J. M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987 Jun;53(6):1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight I. T., Shults S., Kaspar C. W., Colwell R. R. Direct detection of Salmonella spp. in estuaries by using a DNA probe. Appl Environ Microbiol. 1990 Apr;56(4):1059–1066. doi: 10.1128/aem.56.4.1059-1066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. J., Tamplin M. L., Rodrick G. E. Thiosulfate-citrate-bile salts-sucrose agar and its selectivity for clinical and marine vibrio organisms. Ann Clin Lab Sci. 1983 Jan-Feb;13(1):45–48. [PubMed] [Google Scholar]
- Massad G., Oliver J. D. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol. 1987 Sep;53(9):2262–2264. doi: 10.1128/aem.53.9.2262-2264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Seidler R. J. Demonstration of a common antigen in sonicated cells for identification of Vibrio vulnificus serotypes. J Clin Microbiol. 1985 Jan;21(1):97–101. doi: 10.1128/jcm.21.1.97-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl Environ Microbiol. 1982 Dec;44(6):1404–1414. doi: 10.1128/aem.44.6.1404-1414.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson J., Siebeling R. J. Rapid serological identification of Vibrio vulnificus by anti-H coagglutination. Appl Environ Microbiol. 1986 Dec;52(6):1299–1304. doi: 10.1128/aem.52.6.1299-1304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Flesher B., Mansfield H. R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988 May;54(5):1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Rodrick G. E., Blake N. J., Bundy D. A., Alexander L. Public health aspects of halophilic Vibrios in Jamaica. West Indian Med J. 1983 Sep;32(3):147–151. [PubMed] [Google Scholar]
- Tamplin M., Rodrick G. E., Blake N. J., Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982 Dec;44(6):1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Ogawa M., Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985 Feb;47(2):446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




