Abstract
Campylobacter jejuni and C. coli isolates from poultry, cattle, and humans were studied using pulsed-field gel electrophoresis (PFGE) and PCR of candidate livestock-associated marker genes. Human isolates showed 5.7 and 61% overlap with cattle and poultry isolates, respectively, by use of PFGE. No unambiguous association was found between marker genes (the Cj1321 and Cj1324 genes) and livestock-associated isolates.
Campylobacter jejuni and C. coli constitute the leading cause of bacterial gastroenteritis in Finland (www.ktl.fi/ttr) (13), and tasting or eating raw or undercooked meat, drinking water from a dug well, and swimming in natural sources of water have been reported as the most important risk factors for domestically acquired sporadic infections (15), which show a clear seasonal peak, especially in July and August (8, 13). The relative contributions of different sources of infection are not well known. Molecular typing methods, including pulsed-field gel electrophoresis (PFGE), targeted at the identification of possible reservoirs and sources of infection have been widely utilized to study the molecular epidemiology of Campylobacter infections. More recently, a multilocus sequence typing (MLST) method has been described for the study of the population biology of C. jejuni and C. coli (3, 4). PFGE using SmaI restriction digestion has been shown to be more discriminatory than MLST for outbreak investigations (14). In addition, PFGE utilizing KpnI restriction digestion has been shown to be more discriminatory than SmaI typing for comparison of C. jejuni isolates (7, 11). Recently, a cluster of six genes (the Cj1321 gene to the Cj1326 gene) within the O-linked flagellin glycosylation locus was proposed to be a suitable genetic marker predictive of sources of C. jejuni infections based on comparative microarray analysis of strains isolated from humans, chickens, bovines, ovines, and the environment (1).
The aims of our study were (i) to evaluate the discriminatory power of PFGE with KpnI restriction digestion for typing Finnish C. jejuni and C. coli isolates previously characterized by MLST (8); (ii) to study the overlap of PFGE profiles of isolates from human domestically acquired sporadic infections from the Helsinki-Uusimaa area with those of isolates from cattle fecal and chicken retail meat samples during the same seasonal peak, from July to September 2003, in Finland; and (iii) to study the association of two genetic markers, the Cj1321 and Cj1324 genes, which have been suggested to be livestock-associated C. jejuni markers predictive of infection source (1), with the source of isolation.
Altogether, 70 human, 32 chicken and 4 turkey retail meat, and 20 cattle fecal isolates were collected during the seasonal peak from July to September in 2003 and were typed by MLST as previously reported (8). All human strains were non-outbreak related and were isolated from patients who had acquired their infections from domestic sources and were diagnosed at the Helsinki University Central Hospital Laboratory. During the same seasonal peak, poultry isolates were collected from the Helsinki area and cattle fecal samples were collected from different Finnish slaughterhouses all over the country.
PFGE was performed as described previously (6, 10) using KpnI (New England Biolabs Inc.; 20 U per sample) restriction digestion. PFGE data were analyzed with BioNumerics V. 4.01 software (Applied Maths, Kortrijk, Belgium) using the Dice similarity coefficient, with 0.5% optimization and 1% tolerance. Clustering was done with the unweighted pair group method using arithmetic averages. New PFGE KpnI profiles were assigned for each pattern differing by at least one band.
PCR primers and reactions for the amplification of the Cj1321 and Cj1324 loci were as described by Champion et al. (1). The cycling conditions were as follows: denaturation at 95°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 1 min for 35 cycles in total. Strain NCTC11168 was used as a positive control for the PCRs.
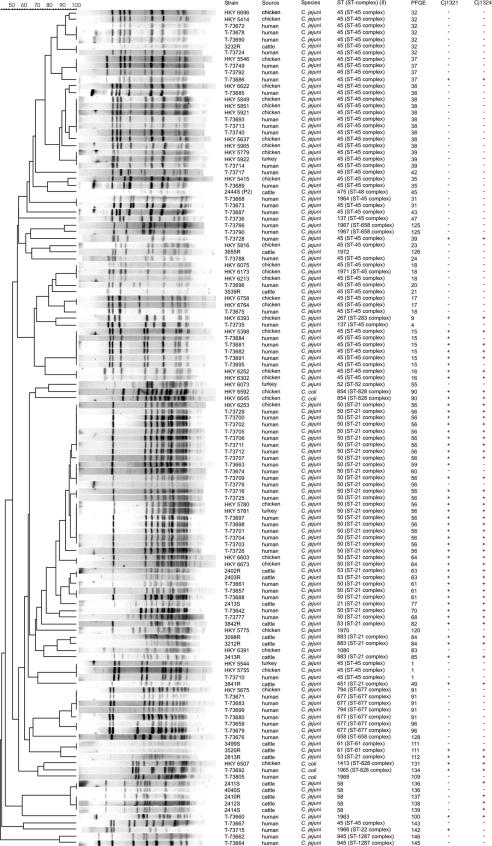
The distribution of MLST sequence types (STs) and PFGE and PCR profiles of human, cattle, and poultry isolates are shown in Fig. 1. In most cases, PFGE analysis further discriminated between isolates among MLST STs. This was especially pronounced among ST-45 isolates. PFGE analysis using KpnI resulted in 5.7 and 61% overlap between human and cattle isolates and human and poultry isolates, respectively. This is in line with the data reported in the EU showing that 3.3 and 30% of the outbreaks in 2004 were attributable to bovine and poultry meat, respectively (5), and with numerous case-control studies in which poultry meat has been identified as a major risk factor for sporadic cases of campylobacteriosis (13). However, more-accurate estimations for source attribution of the sporadic domestically acquired cases could have been obtained by comparing larger numbers of isolates from humans with those from both types of meat at the retail level, as all strains may not survive to be found on the final products (12), and by considering the temporal association of the isolates (7).
FIG. 1.
Results for PFGE using KpnI restriction enzyme and PCR for two genetic markers the Cj1321 and Cj1324 genes (1), from typing Finnish human, cattle, and poultry isolates of C. jejuni and C. coli collected during the seasonal peak from July to September in 2003.
Overall, a large percentage of the poultry and cattle isolates were shown to be PCR negative for the Cj1321 and Cj1324 genes (Fig. 1). The percentages of isolates positive for the genes by PCR among human, cattle, chicken, and turkey isolates were 47, 50, 34 and 25%, respectively, for the Cj1321 gene and 41, 70, 34 and 25%, respectively, for the Cj1324 gene. The ST-45 complex, previously found to be overrepresented among poultry isolates (2, 8, 9), was significantly associated with the absence of both genes (P < 0.05; χ2 test). In most cases, the PCR results for both genetic loci were concordant (i.e., either positive or negative). However, cattle isolates were significantly associated with the Cj1324 gene. Our findings diverged from those reported by Champion et al. (1), who suggested the Cj1321-Cj1326 gene cluster to be livestock associated based on microarray analysis of 70 C. jejuni strains isolated from humans, 17 from chickens, 13 from bovines, 5 from ovines, and 6 from beaches. This may reflect differences between the C. jejuni populations in livestock in Finland and those in the United Kingdom; however, our results stress the need to confirm the results obtained by microarray comparative genomic hybridization using a variety of C. jejuni strains from geographically diverse origins.
In conclusion, MLST and especially the more discriminatory PFGE analysis using the KpnI restriction enzyme suggested a role for poultry more important than that for cattle as a reservoir of Campylobacter subtypes identified in domestically acquired sporadic infections during the seasonal peak in 2003 in Finland. In addition, our study concerning a variety of Campylobacter isolates from Finland did not show a clear relationship between livestock-associated isolates and the previously (1) proposed Cj1321 and Cj1324 genetic markers.
Acknowledgments
This study was funded by the Academy of Finland, and R. Kärenlampi is funded by the Finnish Graduate School on Applied Bioscience.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Champion, O. L., M. W. Gaunt, O. Gundogdu, A. Elmi, A. A. Witney, J. Hinds, N. Dorrell, and B. W. Wren. 2005. Comparative phylogenomics of the food-borne pathogen Campylobacter jejuni reveals genetic markers predictive of infection source. Proc. Natl. Acad. Sci. USA 102:16043-16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colles, F. M., K. Jones, R. M. Harding, and M. C. J. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 69:7409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dingle, K. E., F. M. Colles, D. Falush, and M. C. J. Maiden. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dingle, K. E., F. M. Colles, D. R. A. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. L. Willems, R. Urwin, and M. C. J. Maiden. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority. 2005. The community summary report on trends and sources of zoonoses, zoonotic agents and antimicrobial resistance in the European Union in 2004. EFSA J. 310:1-278. [Google Scholar]
- 6.Hänninen, M.-L., P. Perko-Mäkelä, A. Pitkälä, and H. Rautelin. 2000. A three-year study of Campylobacter jejuni genotypes in humans with domestically acquired infections and in chicken samples from the Helsinki area. J. Clin. Microbiol. 38:1998-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kärenlampi, R., H. Rautelin, M. Hakkinen, and M.-L. Hänninen. 2003. Temporal and geographical distribution and overlap of Penner heat-stable serotypes and pulsed-field gel electrophoresis genotypes of Campylobacter jejuni isolates collected from humans and chickens in Finland during a seasonal peak. J. Clin. Microbiol. 41:4870-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kärenlampi, R., H. Rautelin, D. Schönberg-Norio, L. Paulin, and M.-L. Hänninen. 2007. Longitudinal study of Finnish Campylobacter jejuni and C. coli isolates from humans, using multilocus sequence typing, including comparison with epidemiological data and isolates from poultry and cattle. Appl. Environ. Microbiol. 73:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning, G., C. G. Dowson, M. C. Bagnall, I. H. Ahmed, M. West, and D. G. Newell. 2003. Multilocus sequence typing for comparison of veterinary and human isolates of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maslow, J. N., A. M. Slutsky, and R. D. Arbeit. 1993. Application of pulsed-field gel electrophoresis to molecular epidemiology, p. 563-572. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. ASM Press, Washington, DC.
- 11.Michaud, S., S. Menard, C. Gaudreau, and R. D. Arbeit. 2001. Comparison of SmaI-defined genotypes of Campylobacter jejuni examined by KpnI: a population-based study. J. Med. Microbiol. 50:1075-1081. [DOI] [PubMed] [Google Scholar]
- 12.Newell, D. G., J. E. Shreeve, M. Toszeghy, G. Domingue, S. Bull, T. Humphrey, and G. Mead. 2001. Changes in the carriage of Campylobacter strains by poultry carcasses during processing in abattoirs. Appl. Environ. Microbiol. 67:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rautelin, H., and M.-L. Hänninen. 2000. Campylobacters: the most common bacterial enteropathogens in the Nordic countries. Ann. Med. 32:440-445. [DOI] [PubMed] [Google Scholar]
- 14.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 41:4733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schönberg-Norio, D., J. Takkinen, M.-L. Hänninen, M.-L. Katila, S.-S. Kaukoranta, L. Mattila, and H. Rautelin. 2004. Swimming and Campylobacter infections. Emerg. Infect. Dis. 10:1474-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]