Abstract
Thioredoxin, an antioxidant protein, is a promising molecule for development of functional foods because it protects the gastric mucosa and reduces the allergenicity of allergens. To establish a method for obtaining an ample amount of yeast thioredoxin, we found here that thioredoxin is released from Saccharomyces cerevisiae by treatment with 20% ethanol. We also found that Japanese sake contains a considerable amount of thioredoxin.
Saccharomyces cerevisiae plays quite important roles in alcohol fermentation and bread-manufacturing processes. In addition, S. cerevisiae itself is used as a dietary supplement because it contains health-promoting molecules, such as vitamins, amino acids, and β-glucan (4). In addition to nonproteinaceous components, S. cerevisiae can be a source of proteinaceous factors that are promising molecules for the development of functional foods, and in our studies we have focused on thioredoxin (11, 17). Thioredoxin is a ubiquitous low-molecular-weight protein-disulfide oxidoreductase (6, 7). It functions as a reducing power for several peroxiredoxins to reduce hydrogen peroxide and lipid hydroperoxides (15). Besides its role as an antioxidant, thioredoxin has an anti-inflammatory function, and it has been reported to protect the gastric mucosa (2). On the other hand, thioredoxin catalyzes the cleavage of disulfide bonds of proteins, which leads to an increase in the digestibility of proteins by proteases. Therefore, this molecule has the potential to decrease the allergenicity of allergens (3). Consequently, oral administration of thioredoxin is of considerable interest for both clinical use and the development of functional foods. We examined conditions acceptable in the food industry under which thioredoxin can be extracted without disrupting yeast cells. Here we demonstrated that thioredoxin can be efficiently extracted from S. cerevisiae cells by treatment with ethanol.
Basically, S. cerevisiae YPH250 (MATa trp1-Δ1 his3-Δ200 leu2-Δ1 lys2-801 ade2-101 ura3-52) was used as a laboratory strain. CY4 (MATa ura3-52 leu2-3 leu2-112 trp1-1 ade2-1 his3-11 can1-100), isogenic derivatives of this strain carrying Trx1-3HA, Trx2-3HA, and Trx3-3Myc at the TRX loci (18), industrial baker's yeast (Oriental Yeast Co., Ltd., Japan), wine yeast strain OC2 (Institute of Enology and Viticulture, Yamanashi University), sake yeasts Kyokai no. 7, 10, and 11 (Japan Brewing Society), and sake yeast KZ06 (Kizakura Co., Ltd., Kyoto, Japan) were also used. Cells were cultured in YPD medium (2% glucose, 1% yeast extract, 2% peptone) to the log phase, and 30 A610 units of a culture was centrifuged to collect the cells (∼30 mg [wet weight]). After cells were washed with distilled water, they were suspended in 1 ml of distilled water containing 20% (vol/vol) ethanol, and the cell suspension was kept at 37°C for 2 h. Cells were removed by centrifugation, and 50 μl of a 100% (wt/vol) trichloroacetic acid solution was added to each supernatant. The resultant mixture was kept on ice or at 4°C to precipitate proteins. The trichloroacetic acid-treated mixture was centrifuged, and precipitates were washed with acetone. The dried material was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting to detect thioredoxin. Japanese sake (Japanese rice wine) was brewed on an industrial scale (45,000-liter tank) at the factory of Kizakura Co., Ltd., starting with 2.5 kg of yeast (strain KZ06), 9,150 kg of steamed rice, and 16,343 liters of water (total mash volume, 25,111 liters) and using the company's specifications (http://www.kizakura.co.jp/en/index.html). General components in sake were analyzed by the standard method established by the National Tax Administration Agency of Japan (13). To determine the amount of thioredoxin in sake, the sake mash (moromi) was centrifuged, and the supernatants (1 ml) were subjected to Western blotting for thioredoxin as described above.
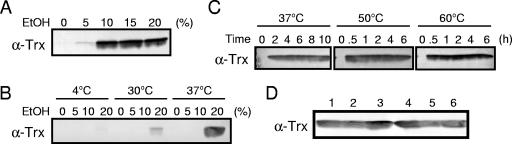
To circumvent the difficulty in isolating thioredoxin from the cellular components, we searched for conditions under which we could extract thioredoxin from yeast without disrupting the cells. We previously succeeded in extracting the enzyme glyoxalase I from the yeast Hansenula mrakii using several organic solvents, and ethanol was one of the most effective of these solvents (8, 9). To verify that thioredoxin is released from S. cerevisiae by ethanol, yeast cells were treated with various concentrations of ethanol at 37°C for 16 h, and the resultant supernatants were analyzed by Western blotting using antithioredoxin antibody. As shown in Fig. 1A, thioredoxin was detected in the supernatant of a cell suspension containing >10% ethanol. However, a 2-h incubation was not enough to extract thioredoxin with 10% ethanol at 37°C (Fig. 1B). Since thioredoxin is thermostable in general and heat treatment (65°C) has been used for purification of yeast thioredoxin (5), the extraction temperature was raised to 60°C. As shown in Fig. 1C, thioredoxin was extracted from yeast cells for 30 min at >50°C with 20% ethanol. At 37°C, almost the maximum amount of thioredoxin was extracted in 2 h.
FIG. 1.
Extraction of thioredoxin with ethanol. (A) Cells (30 A610 units) of S. cerevisiae YPH250 were suspended in 1-ml portions of various concentrations of ethanol (EtOH) at 37°C for 16 h. (B) Cells (30 A610 units) were treated with various concentrations of ethanol (1 ml) at 4, 30, or 37°C for 2 h. (C) Cells (30 A610 units) were treated with 20% ethanol (1 ml) at 37, 50, or 60°C for different times. (D) Various yeast strains (30 A610 units) were treated with 20% ethanol (1 ml) at 37°C for 2 h. Lane 1, sake yeast Kyokai no. 7; lane 2, sake yeast Kyokai no. 10; lane 3, sake yeast Kyokai no. 11; lane 4, YPH250; lane 5, industrial baker's yeast; lane 6, wine yeast OC2. The amounts of thioredoxin in the supernatants were determined by Western blotting. α-Trx, antithioredoxin antibody.
S. cerevisiae has been used in several industrial fields, including alcohol fermentation and baking. Japanese sake has a characteristically high alcohol concentration in its mash compared with other alcoholic drinks, such as beer and wine. Generally, sake yeasts show a higher tolerance to alcohol. Thus, we examined whether thioredoxin can be extracted from sake yeasts, as well as other industrial yeast strains, under the same conditions (20% ethanol at 37°C for 2 h). As shown in Fig. 1D, thioredoxin was extracted from sake yeasts (Kyokai no. 7, 10, and 11), baker's yeast, wine yeast OC2, and beer yeasts (data not shown).
To determine the amount of thioredoxin in an ethanol-extracted sample, we carried out extraction on a large scale and then removed the ethanol in the extracts with an evaporator in vacuo, followed by lyophilization. The dried material was dissolved in distilled water, and the protein concentration was determined by the method of Bradford (1). The proportion of thioredoxin in the protein extracted from yeast cells with 20% ethanol under our standard conditions (2 h at 37°C) was approximately 20 to 30%.
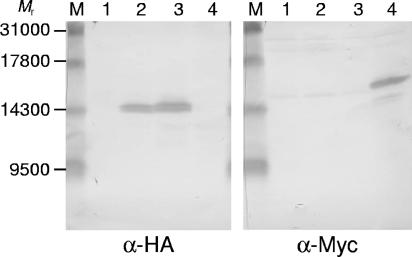
S. cerevisiae contains three isoforms of thioredoxin, Trx1 and Trx2 in the cytosol (and partially in the vacuole) (10, 18, 19) and Trx3 in the mitochondrial matrix (14). Although the antithioredoxin antibody used in the present study was raised against Trx2 in rabbits (10), it cannot distinguish between Trx1 and Trx2 because the level of identity between these two thioredoxins is 76%. On the other hand, this antibody does not react with mitochondrial Trx3 because no immunoreactive band was detected by Western blotting when cell extracts of a trx1Δ trx2Δ mutant were used (data not shown). To determine whether Trx3 is also extracted from yeast cells by ethanol treatment, we used a yeast strain expressing Myc-tagged Trx3 for the ethanol extraction test. As shown in Fig. 2, Myc-tagged Trx3 was detected by Western blotting using the anti-Myc antibody. The extraction of Trx1 and Trx2 was also confirmed using hemagglutinin (HA)-tagged thioredoxins by performing Western blotting with anti-HA monoclonal antibody. Therefore, all thioredoxin isoforms were extracted from yeast cells following ethanol treatment.
FIG. 2.
All isoforms of thioredoxin are extracted with ethanol. Log-phase cells (30 A610 units) of S. cerevisiae CY4 carrying either TRX1-3HA, TRX2-3HA, or TRX3-3Myc were suspended in 1 ml of 20% ethanol at 37°C for 2 h. The amounts of thioredoxin in the resultant supernatants were determined by Western blotting using anti-HA antibody 6E2 (α-HA) (Cell Signaling Technology) (left panel) or anti-Myc antibody A-14 (α-Myc) (Santa Cruz Biotechnology) (right panel). Lanes M, molecular weight markers; lanes 1, CY4 without tagged thioredoxin; lanes 2, CY4 with Trx1-3HA; lanes 3, CY4 with Trx2-3HA; lanes 4, CY4 with Trx3-3Myc. Since the thioredoxins were tagged with three copies of each epitope, they are slightly larger than the originals (11 to ∼14 kDa).
We demonstrated that ethanol extracted thioredoxin from all of the S. cerevisiae strains tested. With such an extraction, yeast cells in the logarithmic phase of growth under aerobic conditions (i.e., when alcohol fermentation is greatly repressed) are suddenly challenged with 20% ethanol. Thus, to examine whether thioredoxin can be extracted from yeast by the ethanol produced during fermentation, we determined thioredoxin levels in Japanese sake mash. In contrast to the alcohol concentrations during beer brewing and wine making, the alcohol concentration in Japanese sake mash reaches >17% due to the characteristic fermentation process; i.e., the hydrolysis of rice starch (saccharification) and alcohol fermentation occur simultaneously. Furthermore, the fermentation period is longer (∼30 days) than that for beer or wine.
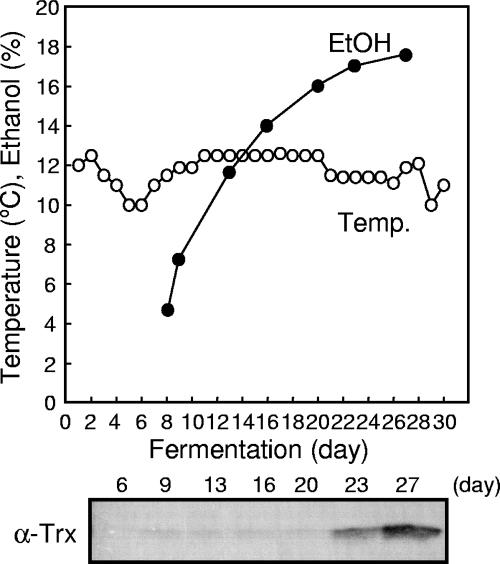
We determined the thioredoxin levels in sake mash withdrawn during industrial-scale brewing. As shown in Fig. 3, thioredoxin was not detected in the early stages of fermentation when the alcohol concentration was low (∼5%) and the yeast population was small. We began to detect thioredoxin when the alcohol concentration increased, and thioredoxin accumulated in the late stages of fermentation when a considerable number of yeast cells were challenged with a higher concentration of ethanol (>17%) for a longer period. In this brewing process, the fermentation temperature was controlled at around 10 to 13°C to block loss of the fruity flavor of the sake (12). As shown in Fig. 1B, temperature is one of the factors that determines the efficiency with which thioredoxin is extracted from yeast cells, and therefore, a longer period may be necessary for thioredoxin to accumulate in sake mash.
FIG. 3.
Extraction of thioredoxin during brewing of Japanese sake. Sake was brewed under standard industrial conditions (25,111 liters in a 45,000-liter tank). The amount of thioredoxin in the sake mash (moromi) was determined by Western blotting. The graph shows the ethanol (EtOH) concentrations and changes in temperature during the brewing process. α-Trx, antithioredoxin antibody.
We demonstrated in this study that thioredoxin can be extracted from yeast cells with 20% ethanol. We scaled up the procedures to a pilot scale (35 kg yeast/240 liters ethanol), with little change in performance in terms of extraction efficiency (20 to 30% thioredoxin in the protein). Since thioredoxin makes up just 0.015% of all cellular soluble protein (5), our method is efficacious for preparation of the starting material for purification of thioredoxin. Indeed, we have succeeded in concentrating thioredoxin in the ethanol-extracted sample by sequential use of hollow fibers having different extinction molecular sizes on a bulk scale (unpublished data). We believe that our method is a promising method for obtaining thioredoxin for developing functional foods. Furthermore, we found that Japanese sake contains thioredoxin (10 to 140 ng thioredoxin/ml sake, with the concentration depending on the brand). Since thioredoxin has the potential to protect the gastric mucosa, our results suggest that Japanese sake is a potential candidate for a thioredoxin-containing beverage.
Although we established the fundamental conditions for extracting thioredoxin from yeast without disrupting the cells, the mechanisms by which yeast thioredoxin is extracted by ethanol treatment remain to be elucidated. A wide variety of normal and/or neoplastic cells, such as monocytes, lymphocytes, fibroblasts, and airway epithelial cells, actively secrete thioredoxin (16). The mechanism of thioredoxin secretion in animal cells seems to be different from that of a typical endoplasmic reticulum-Golgi apparatus-dependent vesicle transport system, and the N terminus of thioredoxin does not contain hydrophobic amino acids that may serve as a secretory signal sequence (16). This is also the case with yeast thioredoxins. In addition, Trx3 in the mitochondrial matrix was extracted by ethanol (Fig. 2), even though there are three biological membranes (mitochondrial inner membrane, outer membrane, and cytoplasmic membrane). Furthermore, we found that yeast thioredoxin can be extracted with some environmental stimuli other than ethanol treatment (unpublished data). We are now attempting to determine the mechanism behind the release of thioredoxin from yeast cells.
Acknowledgments
We thank C. Grant, T. Miki, and the Oriental Yeast Co., Ltd., for yeast strains. We are grateful to Tadahiro Yasuoka for his technical assistance.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Dekigai, H., H. Nakamura, J. Bai, M. Tanito, H. Masutani, K. Hirota, H. Matsui, M. Murakami, and J. Yodoi. 2001. Geranylgeranylacetone promotes induction and secretion of thioredoxin in gastric mucosal cells and peripheral blood lymphocytes. Free Radic. Res. 35:23-30. [DOI] [PubMed] [Google Scholar]
- 3.del Val, G., B. C. Yee, R. M. Lozano, B. B. Buchanan, R. W. Ermel, Y. M. Lee, and O. L. Frick. 1999. Thioredoxin treatment increases digestibility and lowers allergenicity of milk. J. Allergy Clin. Immunol. 103:690-697. [DOI] [PubMed] [Google Scholar]
- 4.Eicher, S. D., C. A. McKee, J. A. Carroll, and E. A. Pajor. 2006. Supplemental vitamin C and yeast cell wall β-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J. Anim. Sci. 84:2352-2360. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez Porqué, P., A. Baldesten, and P. Reichard. 1970. Purification of a thioredoxin system from yeast. J. Biol. Chem. 245:2363-2370. [PubMed] [Google Scholar]
- 6.Holmgren, A. 1985. Thioredoxin. Annu. Rev. Biochem. 54:237-271. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren, A. 1989. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 264:13963-13966. [PubMed] [Google Scholar]
- 8.Inoue, Y., H. Tsuchiyama, N. Kosugi, and A. Kimura. 1992. Production of S-lactoylglutathione by organic-solvent-extracted glyoxalase I from Hansenula mrakii. Appl. Microbiol. Biotechnol. 36:469-472. [Google Scholar]
- 9.Inoue, Y., and A. Kimura. 1995. Methylglyoxal and regulation of its metabolism in microorganisms. Adv. Microb. Physiol. 37:177-227. [DOI] [PubMed] [Google Scholar]
- 10.Izawa, S., K. Maeda, K. Sugiyama, J. Mano, Y. Inoue, and A. Kimura. 1999. Thioredoxin deficiency causes the constitutive activation of Yap1, an AP-1-like transcription factor in Saccharomyces cerevisiae. J. Biol. Chem. 274:28459-28465. [DOI] [PubMed] [Google Scholar]
- 11.Maeta, K., W. Nomura, Y. Takatsume, S. Izawa, and Y. Inoue. 2007. Green tea polyphenols function as prooxidants to activate oxidative-stress-responsive transcription factors in yeasts. Appl. Environ. Microbiol. 73:572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minetoki, T. 1992. Alcohol acetyl transferase of sake yeast. J. Brew. Soc. Jpn. 87:334-340. [Google Scholar]
- 13.Nishiya, N. 1993. Standard method established by National Tax Administration Agency, 4th ed. Brewing Society of Japan, Tokyo, Japan.
- 14.Pedrajas, J. R., E. Kosmidou, A. Miranda-Vizuete, J. A. Gustafsson, A. P. Wright, and G. Spyrou. 1999. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 274:6366-6373. [DOI] [PubMed] [Google Scholar]
- 15.Rhee, S. G., H. Z. Chae, and K. Kim. 2005. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 38:1543-1552. [DOI] [PubMed] [Google Scholar]
- 16.Rubartelli, A., A. Bajetto, G. Allavena, E. Wollman, and R. Sitia. 1992. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267:24161-24164. [PubMed] [Google Scholar]
- 17.Takatsume, Y., K. Maeta, S. Izawa, and Y. Inoue. 2005. Enrichment of yeast thioredoxin by green tea extract through activation of Yap1 transcription factor in Saccharomyces cerevisiae. J. Agric. Food Chem. 53:332-337. [DOI] [PubMed] [Google Scholar]
- 18.Trotter, E. W., and C. M. Grant. 2005. Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae. Eukaryot. Cell 4:392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu, Z., and W. Wickner. 1996. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J. Cell Biol. 132:787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]