Abstract
Campylobacter is the leading cause of bacterium-associated diarrhea in the United States and most developed countries. While this disease is considered a food-borne disease, many clinical cases cannot be linked to a food source. In rural and agrarian areas environmental transmission may be an important factor contributing to case loads. Here we investigated the waterborne prevalence of campylobacters in a mixed-use rural watershed in the coastal plain of southern Georgia (United States). Six sites representing various degrees of agricultural and human influence were surveyed biweekly to monthly for 1 year for the presence of culturable thermophilic campylobacters and other measures of water quality. Campylobacters were frequently present in agriculture- and sewage-impacted stretches of streams. The mean campylobacter counts and overall prevalence were highest downstream from a wastewater treatment plant that handled both human and poultry slaughterhouse waste (≤595 CFU ml−1; 100% positive); the concentrations were significantly higher than those for the four upstream sites (P < 0.05). The counts were significantly correlated with the number of fecal coliform bacteria, conductivity, pH, and concentrations of nutrients (NO3−, PO43−, and NH3). Campylobacters were isolated more frequently and larger numbers were present during the summer months, similar to the occurrence of clinical cases of campylobacteriosis in this region. A multivariate model showed that the levels were significantly influenced by increasing precipitation, which also peaked in the summer. The results indicate that loading from both human and domestic animal waste may be high in the watershed studied during the summer months. Mixed-use watersheds supporting agriculture production, human populations, and wildlife may be at risk for contamination by campylobacters and may be an important route for human exposure.
The family Campylobacteraceae includes the genera Campylobacter and Arcobacter (50). The campylobacter group (campylobacters and campylobacter-like organisms) more commonly includes a number of taxonomically related genera, including Campylobacter, Arcobacter, Helicobacter, and Sutterella (10). Campylobacters have been characterized as gram-negative, non-spore-forming, motile, microaerobic, and ‘S’ or spiral-shaped organisms (50). Campylobacters, particularly Campylobacter jejuni and Campylobacter coli, are the etiological agents of campylobacteriosis, which is recognized as one of the most frequent causes of acute diarrheal disease throughout the world (47, 51). As much as 1% of the population is thought to be infected with Campylobacter spp. every year in Europe and the United States (53).
Most campylobacteriosis cases in the United States and throughout the world occur in the summer, and there are declines in the fall and winter (5, 43, 48). Handling and consumption of poultry or poultry-related products are considered to be a primary source of Campylobacter infection (14). It has been suggested that changes in food handling and consumption in the summer months (i.e., outdoor barbecuing) may lead to this predictable seasonality (43); however, this assessment does not always hold (26, 33) and does not explain the fact that the same pattern occurs in different nations and cultures (16, 33, 36, 47). Furthermore, the pathways involved in campylobacter contamination of poultry flocks also remain unclear. Given that the majority of human infections are sporadic, sources other than food (poultry) may be important in disease transmission, especially in areas where the food-borne disease burden has declined. Other reported sources of infection include contaminated water and raw milk (22, 32); however, in most cases the exact source of disease is not determined (43). Environmental loading and transmission may be factors associated with nonoutbreak cases, particularly those related to contamination of ambient waters (4, 28, 41, 42).
Although they require a host for growth, campylobacters are common in natural waters, such as streams, rivers, and lakes, due to discharges from wastewater treatment plants, runoff from agricultural lands, and direct contamination by feces of wild birds and animals (8, 13, 22, 32). Once Campylobacter spp. are introduced into the environment, the survival or persistence of these organisms depends on many factors, such as oxygen content, the presence of nutrients, temperature, and pH (6). Recent research also has indicated that campylobacters may survive within vacuoles of protozoans in environmental waters (2, 44). Additionally, Campylobacter spp. may enter a viable but nonculturable state (where organisms are not able to grow in culture but remain metabolically active), which was first described by Rollins and Colwell (38). The viable but nonculturable state has been considered to play a role in prolonging Campylobacter spp. survival in the environment (8, 32). The common agricultural practice of spreading livestock manure on land is an obvious and well-described vector for the contamination of crops and water resources at the farm level if the wastes contain pathogenic microorganisms (17). The most common use of poultry litter (manure mixed with bedding material) is application to cropland as fertilizer on fields close to poultry houses. Campylobacter spp. have been isolated from the feces of bovine animals (19, 20, 24, 45) and poultry (18, 25, 29); seasonal shedding has been reported for both cattle (45) and poultry (49).
Mixed-use watersheds supporting agriculture production, human populations, and wildlife may be at risk for contamination by campylobacters. Determining environmental factors that contribute to both loading and persistence of campylobacters is important for controlling potential waterborne transmission. Here we describe the distribution of thermotolerant campylobacters in an agrarian watershed in southeast Georgia (United States) with a consistently high rate of campylobacter cases (17.0 cases per 100,000 people in Coffee County, compared with a state-wide average of 6.8 cases per 100,000 people in 2003 [35]) and a multivariable environmental model that begins to describe the seasonality of campylobacters in this area.
MATERIALS AND METHODS
Field study. (i) Sampling area.
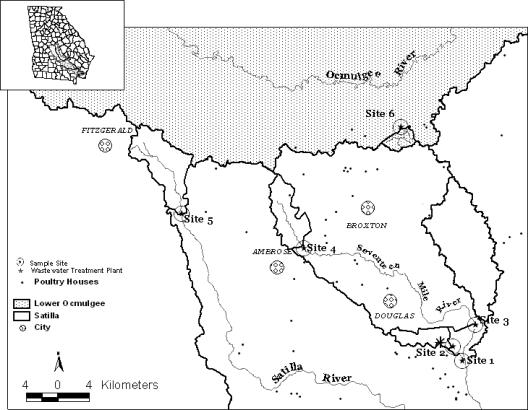
Samples were collected in the Satilla River basin, which drains 10,204 km2 in southeast Georgia (United States). This area is flanked by the Altamaha River basin to the north and the Suwannee River and St. Mary's River basins to the south. Field sampling was focused in the Seventeen Mile River (four of six stations), which is located in the headwaters of the Satilla River basin near Douglas, Broxton, and Ambrose, GA (Coffee County) (Fig. 1); the area of the watershed is approximately 764 km2. An upstream site was located near the headwaters of the Satilla River (site 5). A control station (site 6) was located in the Lower Ocmulgee watershed in the Broxton Rocks Preserve. Stations 1 to 4 (along the Seventeen Mile River) were designated downstream (site 1) to upstream (site 4) (Fig. 1). Site 2, on a tributary of Seventeen Mile River, was immediately downstream from the municipal wastewater treatment plant (WWTP) for the city of Douglas, which handles both human and poultry slaughterhouse waste. Approximately 50% of the influent that the WWTP receives comes from poultry-processing facilities in Douglas. The other three stations along the Seventeen Mile River were primarily influenced by agriculture (Table 1). Site 5 was located on the Satilla River (Fig. 1) and was not only the most upstream of all the stations sampled but also had the highest percentage of agricultural land use (Table 1). Site 1, the most downstream of all stations, had slightly less agricultural influence (26% in its immediate area), but it received flow from sites 2, 3, and 4. Poultry houses, including 20 poultry houses in the drainage area of site 1 and 20 poultry houses in the drainage area of site 3, were located throughout the Satilla River watershed (Fig. 1 and Table 1). No poultry houses were located in the immediate drainage area of the control station (site 6) or sites 2 and 5 (Table 1). Poultry litter application was assumed to occur in the immediate areas surrounding the houses; however, poultry litter was also applied to cropland throughout the watershed, including areas where no poultry houses were present (B. Bannister, USDA Natural Resources Conservation Service, personal communication). Within the Satilla River watershed, the amount of poultry litter applied was considered to be proportional to the amount of cropland in a watershed (Bannister, personal communication).
FIG. 1.
Sampling locations in southeastern Georgia (United States).
TABLE 1.
Description of the sample sites in the Satilla River watershed
| Site | Drainage area (km2) | No. of poultry houses | Land use (%)
|
|||
|---|---|---|---|---|---|---|
| Row crops | Pasture | Foresta | Otherb | |||
| 1 | 462 | 20 | 26 | 6 | 51 | 17 |
| 2 | 14 | 0 | 17 | 5 | 47 | 32 |
| 3 | 434 | 20 | 27 | 6 | 50 | 17 |
| 4 | 28 | 1 | 34 | 3 | 50 | 14 |
| 5 | 45 | 0 | 51 | 1 | 31 | 18 |
| 6c | 7.25 | 0 | 6 | 1 | 77 | 16 |
Includes deciduous forest, evergreen forest, mixed forest, and forested wetland.
Includes open water, transportation, utility swaths, urban, clear-cut or sparse vegetation, and golf courses.
Control site, located in the Lower Ocmulgee watershed.
(ii) Sample collection.
Samples were collected biweekly from June through August 2003 and monthly from September 2003 through May 2004; all samples were collected between 8:00 a.m. and 10:00 a.m. Water was analyzed to determine its conductivity (mS cm−1), temperature (oC), pH, dissolved oxygen concentration (mg liter−1), turbidity (nephlometric turbidity units), oxidation reduction potential (ORP) (mV), fluorescence, and chlorophyll a concentration (mg liter−1) with a YSI 6600 multiparameter Sonde (Yellow Springs Instruments, Yellow Springs, OH). The probe was placed into the deepest part of the stream channel and used to record instantaneous values after stabilization. Water used for nutrient and microbial analyses was collected in sterile 1-liter polypropylene bottles as discrete surface grabs and transported on ice to the USDA Agricultural Research Services (USDA-ARS) Southeast Watershed Research Laboratory in Tifton, GA.
Nutrient analyses.
Water samples were filtered through Whatman 934 AH filters to determine the amount of suspended sediment using standard methods (7). The filtrate was analyzed to determine nitrate-N and ammonium-N concentrations using Environmental Protection Agency-approved colorimetric techniques (7) with a Lachat flow injection analyzer. Samples were analyzed to determine potassium contents using standard methods with a Perkin-Elmer atomic absorption spectrophotometer (7).
Microbiological analyses.
Samples were processed for microbial analysis within 4 h after the first sample was taken. Water samples were screened for fecal coliform bacteria by membrane filtration and growth on mFC agar using standard methods (12). Plates were incubated for 24 ± 2 h in a 44.5°C water bath. All blue colonies were considered fecal coliform bacteria and enumerated, and the results were expressed as CFU per 100 ml.
Thermotolerant campylobacters were detected and enumerated using a modified direct-plating method, without preenrichment. Rosef et al. (39) previously reported that preenrichment did not significantly increase the percentage of campylobacters detected in environmental samples. Furthermore, Atabay and Corry (1) suggested that this step may selectively enrich Arcobacter spp. over Campylobacter spp. Briefly, duplicate 100-μl aliquots of water from each sample were directly spread onto modified charcoal cefoperazone deoxycholate agar (Oxoid, Lenexa, KS). Petri dishes were then placed in nonvented BBL (Cockeysville, MD) GasPak jar systems, and microaerobic conditions were created and maintained using BBL (Sparks, MD) CampyPak Plus microaerophilic system envelopes with a palladium catalyst (H2 plus CO2). Plates were incubated for 48 ± 1 h at 42°C, and gray, mucoid colonies were counted as presumptive campylobacters. The identities of colonies were confirmed by observation of gram-negative spiral bacteria, darting motility, a positive oxidase reaction, a positive catalase reaction, and growth only in a microaerobic atmosphere (34, 40). The detection techniques used here confirmed the presence of campylobacters and campylobacter-like organisms (including Campylobacter and Acrobacter spp. [9]), without regard to species (34).
Other data.
Basin-wide rainfall was estimated using data from a National Climatic Data Center weather station in Douglas, GA (COOPS 092783) (Fig. 1). In order to examine the potential impact of precipitation on other study variables, several measures of precipitation were compiled for analyses, including daily rainfall, rainfall on the day before sample collection, total rainfall in the 7 days before sample collection, total rainfall in the 10 days before sample collection, total and average daily rainfall in the 30 days before sample collection, and maximum daily rainfall values for the 7-, 10-, and 30-day periods before sample collection.
Monthly data reports on flow and treatment standards were obtained from the WWTP upstream of site 2. The treatment plant's capacity is 6.00 × 106 gallons per day, and during times of increased rainfall the influent flow may exceed this (D. Wilcox, Douglas Wastewater Department, personal communication).
Statistical analyses.
Microbiological data were not normally distributed, even following log transformation; therefore, nontransformed counts were used in nonparametric statistical tests. For seasonal analyses, seasons were defined as follows: summer, June to August; fall, September to November; winter, December to February; and spring, March to May. Kruskal-Wallis tests were used to examine differences in both water quality (e.g., fecal coliform density, pH, NO3−-N content, etc.) and campylobacter density between study sites and seasons. Spearman's rank correlations were used to examine potential associations among water quality variables and between water quality variables and campylobacter densities. All associations were considered significant at a P value of ≤0.05.
A generalized estimating equation model (PROC GENMOD, SAS 9.1) was used to construct a multivariable predictive model for waterborne campylobacters to accommodate the distribution structure of count data (CFU ml−1) likely to exhibit multiple levels of correlation (i.e., correlations in observed campylobacter densities were expected for samples collected in the same month and for samples collected at the same study site) (3). Initially, all variables significantly associated with campylobacter concentrations at a P value of ≤0.10 were entered into a saturated model using a Poisson distribution. Counts were recorded as zero when values were below the detection limit. When all variables were entered into the model, overdispersion of the model was assessed. Variables were eliminated from the model in a backward stepwise manner to obtain the most predictive model. Variables were retained in the model if Wald P values were <0.05 or if removal of a variable resulted in a significant likelihood ratio test.
RESULTS
Differences in water quality among study sites.
Fourteen samples were collected from each of the six sites; however, campylobacter plate counts were determined for only 12 samples from sites 1, 3, 4, and 6 and for 13 samples from sites 2 and 5 due to the presence of swarming colonies that prohibited accurate reading of the plates. Although campylobacters were detected at all sites sampled, the mean concentrations were lowest at the control site (site 6; 2 CFU ml−1) and highest downstream from the municipal WWTP (site 2; 158 CFU ml−1) (Table 2). The highest concentration of campylobacters detected at any sampling time was also at site 2 (595 CFU ml−1 in August 2003). All 13 of the samples from this site were positive as determined by culture analysis, whereas only 17% (2/12) of the samples from the control site were positive. While the campylobacter levels at site 2 were significantly higher than the levels at all of the upstream sites (sites 3 to 6; P < 0.05), they did not differ significantly from the levels at site 1, immediately downstream (Table 2). Upstream of the WWTP (sites 3, 4, 5, and 6) the percentage of positive samples corresponded with the percentage of land used for agriculture (i.e., the higher percentage of agricultural land, the higher the percentage of samples positive for campylobacters) (Table 2).
TABLE 2.
Agricultural land use and summary statistics for campylobacters by site
| Site | Agricultural land use (%)a | Mean concn (CFU ml−1) (minimum, maximum)b | % Positive for campylobacter (n) |
|---|---|---|---|
| 1 | 32 (+ WWTP) | 65 (ND, 325) AB | 75 (12) |
| 2 | 22 (+ WWTP) | 158 (ND, 595) A | 100 (13) |
| 3 | 33 | 9 (ND, 60) B | 33 (12) |
| 4 | 37 | 30 (ND, 115) B | 58 (12) |
| 5 | 52 | 10 (ND, 50) B | 69 (13) |
| 6 | 7 | 2 (ND, 10) B | 17 (12) |
Includes row crops and pasture land. Only sites 1 and 2 were influenced by or downstream of the municipal WWTP.
ND, not detected. The limit of detection was 10 CFU ml−1. Mean values followed by the same letter are not significantly different at the 95% confidence level (Kruskal-Wallis test).
The mean conductivity (0.61 mS cm−1), NO3-N concentration (2.6 mg liter−1), PO43− concentration (2.8 mg liter−1), and NH3-N concentration (0.3 mg liter−1) at site 2 (WWTP) were also significantly higher than the values at all other sites (P < 0.05) (Table 3). In contrast, the potassium concentration (0.3 mg liter−1) and ORP (131 mV) were significantly lower at this station (P < 0.05). The average pH at site 6 (control) (pH 6.5) was significantly lower than the pH values all other sites, and the ORP (227 mV) was highest at this station.
TABLE 3.
Measured water quality and nutrient parameters at each site in the Satilla River watersheda
| Site(s) | Fecal coliform concn (CFU 100 ml−1)b | pH | ORP (mV) | Concn (mg liter−1) of:
|
|||
|---|---|---|---|---|---|---|---|
| NO3−-Nb | PO43−b | NH3-Nb | K | ||||
| 1 | 173 (15, 570) | 7.2 (6.6, 7.6) | 147 (63, 356) | 0.21 (ND, 0.84) | 0.17 (0.01, 1.30) | 0.023 (ND, 0.06) | 6.7 (1.4, 23.8) |
| 2 | 698 (ND, 5,000) | 7.2 (6.9, 7.5) | 131 (54, 310) | 8.06 (0.90, 7.14)c | 56.75 (0.23, 6.61)c | 2.78 (0.02, 1.48)c | 0.3 (4.4, 33.8)c |
| 3 | 121 (ND, 315) | 7.0 (6.2, 7.8) | 164 (81, 296) | 0.16 (0.01, 0.86) | 0.10 (ND, 0.99) | 0.04 (0.01, 0.20) | 3.2 (2.2, 4.4) |
| 4 | 186 (20, 680) | 7.1 (6.5, 7.8) | 217 (97, 459) | 0.20 (ND, 1.00) | 0.02 (ND, 0.06) | 0.07 (0.01, 0.21) | 2.3 (1, 3) |
| 5 | 248 (120, 490) | 7.2 (6.6, 7.5) | 226 (80, 471) | 0.19 (ND, 0.87) | 0.01 (ND, 0.06) | 0.07 (0.01, 0.33) | 2.8 (0.4, 3.6) |
| 6 | 55 (ND, 250) | 6.5 (5.7, 7.4)c | 227 (102, 423) | 0.11 (ND, 0.90) | 0.03 (ND, 0.98) | 0.02 (0.01, 0.04) | 1.4 (0.8, 1.8) |
| All | 249 (ND, 5,000) | 7.0 (5.7, 7.8) | 183 (54, 471) | 0.600 (ND, 7.14) | 0.54 (ND, 6.61) | 0.09 (ND, 1.48) | 6.2 (0.4, 33.8) |
The values are the means (minimum, maximum).
ND, not detected. The limit of detection for fecal coliforms was 20 CFU 100 ml−1; the limit of detection for NO3−-N was 0.05 mg liter−1; the limit of detection for PO43− was 0.005 mg liter−1; and the limit of detection for NH3-N was 0.005 mg liter−1.
The value is significantly different at the 0.05 (95%) level from other values for individual sites in the same column.
The fecal coliform concentration did not differ significantly by site; however, higher concentrations were detected most frequently at site 2, immediately downstream from the WWTP (mean, 698 CFU 100 ml−1) (Table 3). The concentrations were lowest at the control site, site 6 (mean, 55 CFU 100 ml−1).
Basin-wide campylobacter concentrations were significantly correlated with the fecal coliform levels, conductivity, chlorophyll a concentration, nutrient levels (NO3−-N, Cl, PO43−, and K), and dissolved inorganic carbon concentration (P < 0.05) (Table 4). However, since many of these water quality parameters were also significantly associated with site 2, we also calculated correlations excluding this site as a possible confounding variable. When site 2 was excluded from the analysis, campylobacters were significantly correlated only with fecal coliform bacteria (r = 0.37, P = 0.003).
TABLE 4.
Spearman's rank correlations between water quality variables and campylobacter for all sites (only significant correlations; P < 0.05)
| Parameter | Spearman r | P |
|---|---|---|
| Fecal coliform bacteria (CFU 100 ml−1) | 0.36 | 0.0016 |
| Temp (oC) | 0.45 | <0.0001 |
| Conductivity (mS cm−1) | 0.42 | 0.0002 |
| Chlorophyll a concn (mg liter−1) | 0.24 | 0.0433 |
| NO3−-N concn (mg liter−1) | 0.34 | 0.0034 |
| PO43− concn (mg liter−1) | 0.4 | 0.0004 |
| K concn (mg liter−1) | 0.34 | 0.0033 |
Seasonal and climatic influences on water quality.
The water temperature ranged from 4.8°C in January to 27.9°C in July. As expected, the average water temperatures were significantly different in different seasons (P < 0.0001). In the summer the average water temperature was 24.5°C, compared to 16.7°C for the fall, 7.9°C for the winter, and 17.9°C for the spring. The dissolved oxygen levels also varied seasonally (P < 0.0001) and correlated inversely with the water temperature (r = −0.66, P < 0.001). Nitrate levels, chlorophyll a levels, pH, and turbidity also showed significant seasonal variation (P < 0.05). The dissolved oxygen content and pH were highest during the winter (11.06 mg liter−1 and pH 7.40, respectively). The nitrate concentration peaked in the fall (0.9 μg liter−1), and the chlorophyll a concentration was highest in the spring (35.4 μg liter−1). The turbidity was highest during the summer (4.4 nephlometric turbidity units).
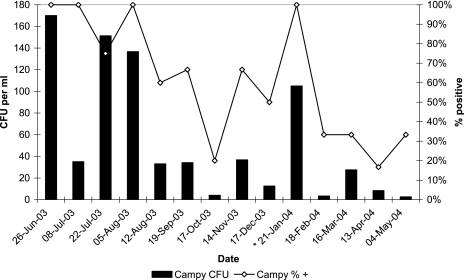
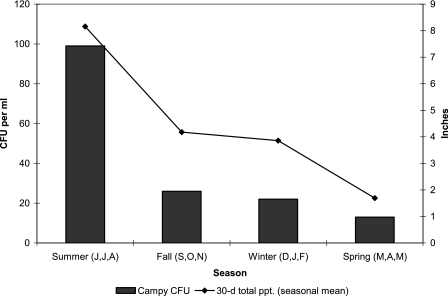
The highest concentrations of campylobacters were observed at all sites between July and August 2003 (Fig. 2). The mean levels were significantly lower during the spring (13 CFU ml−1) and highest during the summer (99 CFU ml−1) (P = 0.003) (Fig. 3). At the control site (site 6) campylobacters were detected only in the summer months. Consequently, campylobacter counts were positively correlated with temperature (r = 0.45, P < 0.0001). Additionally, the observed campylobacter concentrations showed a significant association with three closely related rainfall variables: average rainfall during the 30 days before sample collection (r = 0.36, P = 0.0015), the maximum daily rainfall in the 30 days before sample collection (r = 0.36, P = 0.0017), and the total rainfall in the 30 days before sample collection (r = 0.36, P = 0.0015) (Fig. 3). While fecal coliform concentrations did not vary significantly by season, the highest concentrations were also detected in the summer (mean, 459 CFU 100 ml−1). The mean fecal coliform concentrations were 162 CFU 100 ml−1 in the fall, 138 CFU 100 ml−1 in the winter, and 121 CFU 100 ml−1 in the spring.
FIG. 2.
Mean campylobacter concentrations and prevalence on different sampling dates for all sites. Data could be obtained for only two samples (from sites 2 and 5) on 21 January 2004 due to the presence of swarmers. Campy, campylobacter; Campy % +, percentage of samples that were campylobacter positive.
FIG. 3.
Seasonal campylobacter concentrations and seasonal mean monthly (30-day) rainfall totals for all sites. J,J,A, June, July, and August; S,O,N, September, October, and November; D,J,F, December, January, and February; M,A,M, March, April, and May; Campy, campylobacter; 30-d total ppt., 30-day total precipitation.
Influence of wastewater treatment and discharge on campylobacter levels.
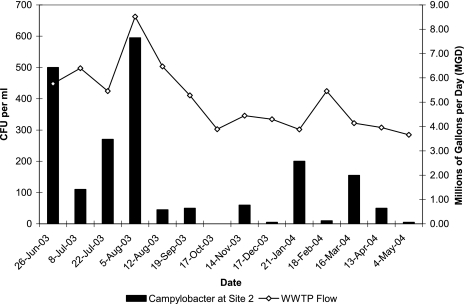
Over the period of study, the mean influent flow at the wastewater treatment plant was 5.12 × 106 ± 1.4 × 106 gallons per day. The campylobacter levels specifically at site 2 were not significantly correlated with WWTP flow on the day of sampling or with any climate or water quality variable; nonetheless, the influent flow at the treatment plant was greatest during the summer, when the mean flow was 6.43 × 106 gallons per day. When the highest concentration of campylobacters was observed (5 August 2003; 595 CFU ml−1), the influent flow was also at its peak (8.52 × 106 gallons per day) (Fig. 4).
FIG. 4.
Campylobacter concentrations and WWTP flow for site 2 on different sampling dates.
Multivariable model of campylobacters.
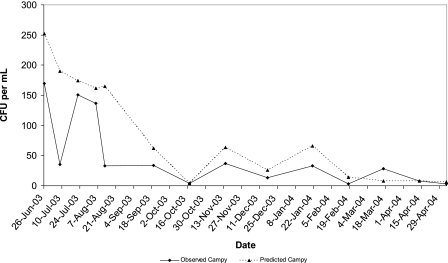
Overdispersion of the data was evident when all variables were fitted in the Poisson model. Consequently, the model was refitted using a negative binomial distribution. The independent variables entered into the full model included the random effects of temperature, total rainfall in the month (30 days) before sample collection, and fecal coliform counts and the fixed effects of season and site. Dummy variables for each season were also examined, using winter as the referent category. Models controlled for sampling variability within a sampling round. Significant interactions between temperature and season and between rainfall and season were observed in reduced models; however, the best-fit, most predictive model included the seasons summer and spring, total rainfall in the previous month (30 days), site, and interactions between total rainfall and two of the seasons, summer and spring (Table 5). The significant predictors of measured campylobacter concentrations in water retained in the final multivariate model included total precipitation in the month (30 days) prior to sample collection, the site of sample collection, and season (Table 5). Compared to the background site (site 6), significantly higher campylobacter counts were obtained for all sampling sites when climatic and sampling effects were controlled in the model. Downstream of the WWTP, the concentration of campylobacter was highest (∼5.5 times the concentrations observed at the control site) in the multivariable model (β = 5.55; 95% confidence interval, 4.17 to 6.90). Compared to the winter and fall, the observed campylobacter concentrations were nearly four times higher in the summer months (β = 3.83; 95% confidence interval, 2.60 to 5.06), and the counts increased further slightly with each additional inch of rainfall during the previous 30 days. For the spring months the model suggested that the campylobacter counts were also approximately threefold higher than the counts for the referent fall and winter months (β = 2.91; 95% confidence interval, 1.95 to 3.88), but reduced total rainfall in the spring resulted in lower observed campylobacter counts; this was adequately accounted for when interaction terms, including season and rainfall, were included in the multivariate model (Table 5 and Fig. 5).
TABLE 5.
Generalized estimating equation parameter estimates and significance values for variables significantly associated with campylobacter concentrations in surface waters in a multivariate model
| Independent variable | Parameter estimate (β) | SE | P |
|---|---|---|---|
| Season | |||
| Summer | 3.83 | 0.63 | <0.0001 |
| Spring | 2.91 | 0.49 | 0.0298 |
| Winter and fall | Reference | ||
| Site | |||
| Site 1 | 4.40 | 0.74 | <0.0001 |
| Site 2 | 5.54 | 0.70 | <0.0001 |
| Site 3 | 1.99 | 0.64 | 0.016 |
| Site 4 | 3.25 | 0.78 | <0.0001 |
| Site 5 | 2.21 | 0.63 | <0.0001 |
| Site 6 | Reference | ||
| Total rainfall (inches) 30 days before sample collection | 0.065 | 0.0978 | <0.0001 |
| Interactions | |||
| Summer + 30-day total rainfall | 0.1089 | 0.0427 | 0.01 |
| Spring + 30-day total rainfall | −0.7252 | 0.0864 | <0.0001 |
FIG. 5.
Observed and predicted mean campylobacter concentrations for all study sites based on the final multivariable model including season, total 30-day rainfall, and interactions between rainfall and season for summer and spring. Campy, campylobacter.
DISCUSSION
Our objectives in this study were to determine the prevalence of campylobacters in a mixed-use watershed and to relate the prevalence to season and other environmental parameters. Coffee County, GA, which includes the Seventeen Mile River, and other adjacent counties in south Georgia have some of the highest case rates of campylobacteriosis in the state (e.g., 17.0 to 40.6 cases per 100,000 people, compared with a state-wide average of 6.8 cases per 100,000 people in 2003) (35), yet there is little information on probable exposure routes in this largely agrarian area.
We used traditional culture-based detection methods to identify campylobacters directly from environmental waters (39). The bacteria were detected at all sites during this study, and their identities were confirmed by simple biochemical tests (catalase and oxidase tests), growth in a microaerobic environment, and a motility test (34, 40). Testing of waters for fecal indicator bacteria is commonly used as a proxy for the presence of enteric pathogens; however, many studies have demonstrated that indicators are poorly correlated with many pathogens, including Campylobacter spp. (42, 51). In the present study, campylobacters were significantly correlated with fecal coliform bacteria using Spearman rank correlations (r = 0.36, P < 0.05) (Table 4); however, when other predictive variables, such as sampling site, rainfall, and summer season, were included in the multivariate model, fecal coliform bacteria counts were not associated with campylobacter counts. This suggests that fecal coliforms might be influenced by the same environmental variables as campylobacters and may be a useful tool in predicting the presence of Campylobacter in environmental waters.
In the watershed studied, the highest concentrations of campylobacters were found immediately downstream of a municipal wastewater treatment plant. The influent received by this WWTP is split evenly between human waste and poultry-processing waste; approximately 33% of the poultry waste originates from slaughterhouses (Wilcox, personal communication). Sewage and sewage sludge have been shown to contain campylobacters at concentrations of 102 to 105 and 101 to 103 CFU 100 ml−1, respectively (15, 46). Furthermore, studies have shown that Campylobacter spp. can survive typical wastewater treatment and can persist in sewage effluent (4, 46). Both Campylobacter and Arcobacter have been detected in contaminated river water and wastewater samples by culture and molecular direct detection methods (29). Although the numbers detected in the present study are higher than the numbers reported in other surveys, the large loads from anthropogenic and agricultural inputs into the WWTP from poultry processing are probably factors driving these high numbers. The treatment plant's capacity is 6.00 × 106 gallons per day, but this is sometimes exceeded during large rainfall events (Wilcox, personal communication).
In this study campylobacters were isolated more frequently and larger numbers were present during the summer months, which is consistent with the trends for clinical cases of campylobacteriosis (48) but did not correspond with the low survival rates reported for summer temperatures in vitro (23). In this region of Georgia, the maximum number of clinical cases of campylobacteriosis also occurs in the summer (35), when precipitation tends to be high. These results differ from the results of other environmental studies, in which detection of campylobacter peaked in late autumn and winter months (4, 22). Given the low survival rate of Campylobacter at warm temperatures in vitro (23), we hypothesized that in this watershed the environmental loading observed in the summer months is mediated by rainfall and runoff and the input is sufficiently high to offset the decrease in survival due to the high seasonal temperatures. Indeed, carriage rates for animals are known to increase in the summer months (30), and our findings are similar to the seasonal pattern of campylobacter carriage found in chickens and sewage sludge (21, 36, 52). An additional source of contamination may be land-applied poultry litter (18, 27, 31), which is widespread in the agricultural portions of the watershed (Bannister, personal communication). Therefore, increased rainfall may lead to higher levels of runoff carrying animal manure and poultry litter into the receiving streams. Studies have shown that nutrients and coliform bacteria can be leached from surface-applied poultry litter by heavy rainfall and transported from the field in surface runoff (11, 37). Finally, given that there are similar summer peaks in clinical cases and that in-stream counts were greatest downstream of a WWTP (26, 33), human sewage may also contribute to the high numbers of campylobacters found in this study.
Conclusion.
Campylobacters were frequently present in surface waters receiving effluent from a WWTP and in areas receiving agricultural runoff. In the present study, the highest concentration of campylobacters, 595 CFU ml−1, was detected directly downstream from a wastewater treatment plant that processes both human and poultry slaughterhouse waste. While wastewater drives a significant portion of the campylobacter loading in the watershed, effects in the larger watershed and in upstream watersheds are more likely related to rainfall and runoff. The highest culturable counts of campylobacters were detected during the summer, consistent with increases in precipitation and also paralleling typical clinical patterns in the high-case-rate county studied. This work should contribute to hierarchical models that relate human cases of campylobacter infection to environmental drivers and watershed contamination.
Acknowledgments
This work was funded by grant NA03OAR4310160 from the National Oceanic and Atmospheric Administration (NOAA) (with NSF, EPA, and EPRI) Joint Program on Climate Variability and Human Health. Student support was provided in part by the USDA Agricultural Research Service (USDA-ARS) Student Career Employment Program.
This research was not subjected to NOAA or USDA-ARS review and therefore does not necessarily reflect the views of these agencies.
We thank Patricia Smith and Theng-Theng Fong for help with sample processing, Chris Clegg and Leila Hargett for nutrient analyses, and Susan Crow for Geographical Information System assistance and spatial map generation.
Footnotes
Published ahead of print on 15 December 2006.
REFERENCES
- 1.Atabay, H. I., and J. E. L. Corry. 1997. The prevalence of campylobacters and arcobacters in broiler chickens. J. Appl. Microbiol. 83:619-626. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson-Olsson, D., J. Waldenström, T. Broman, B. Olsen, and M. Holmberg. 2005. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl. Environ. Microbiol. 71:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballinger, G. A. 2004. Using generalized estimating equations for longitudinal data analysis. Organ. Res. Methods 7:127-150. [Google Scholar]
- 4.Bolton, F. J., D. Coates, D. N. Hutchinson, and A. F. Godfree. 1987. A study of thermophilic campylobacters in a river system. J. Appl. Bacteriol. 62:167-176. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2004. 2003 final FoodNet surveillance report. Centers for Disease Control and Prevention, Atlanta, GA.
- 6.Chaveerach, P., A. A. ter Huurne, L. J. Lipman, and F. van Knapen. 2003. Survival and resuscitation of ten strains of Campylobacter jejuni and Campylobacter coli under acid conditions. Appl. Environ. Microbiol. 69:711-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clesceri, L. S., A. E. Greenburg, and D. A. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 8.Daczkowska-Kozon, E., and J. Brzostek-Nowakowska. 2001. Campylobacter spp. in waters of three main Western Pomerania water bodies. Int. J. Hyg. Environ. Health 203:435-443. [DOI] [PubMed] [Google Scholar]
- 9.Diergaardt, S. M., S. N. Venter, A. Spreeth, J. Theron, and V. S. Brozel. 2004. The occurrence of campylobacters in water sources in South Africa. Water Res. 38:2589-2595. [DOI] [PubMed] [Google Scholar]
- 10.Engberg, J., S. L. W. On, C. S. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giddens, J., and A. P. Barnett. 1980. Soil loss and microbiological quality of runoff from land treated with poultry litter. J. Environ. Qual. 9:518-520. [Google Scholar]
- 12.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, Washington, DC.
- 13.Hänninen, M. L., H. Haajanen, T. Pummi, K. Wermundsen, M. L. Katila, H. Sarkkinen, I. Miettinen, and H. Rautelin. 2003. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 69:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiett, K. L., N. J. Stern, P. Fedorka-Cray, N. A. Cox, M. T. Musgrove, and S. Ladely. 2002. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 68:6220-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höller, C., and V. Schomakers-Revaka. 1994. A note: comparison of different homogenization procedures for detecting Campylobacter spp. in sewage sludges. J. Appl. Bacteriol. 77:591-596. [DOI] [PubMed] [Google Scholar]
- 16.Hörman, A., R. Rimhanen-Finne, L. Maunula, C. H. von Bonsdorff, N. Torvela, A. Heikinheimo, and M. L Hänninen. 2004. Campylobacter spp., Giardia spp., Cryptosporidium spp., noroviruses, and indicator organisms in surface water in southwestern Finland, 2000-2001. Appl. Environ. Microbiol. 70:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchison, M. L., L. D. Walters, S. M. Avery, B. A. Synge, and A. Moore. 2004. Levels of zoonotic agents in British livestock manures. Lett. Appl. Microbiol. 39:207-214. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison, M. L., L. D. Walters, S. M. Avery, F. Munro, and A. Moore. 2005. Analyses of livestock production, waste storage, and pathogen levels and prevalences in farm manures. Appl. Environ. Microbiol. 71:1231-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inglis, G. D., and L. D. Kalischuk. 2003. Use of PCR for direct detection of Campylobacter species in bovine feces. Appl. Environ. Microbiol. 69:3435-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inglis, G. D., L. D. Kalischuk, H. W. Busz, and J. P. Kastelic. 2005. Colonization of cattle intestines by Campylobacter jejuni and Campylobacter lanienae. Appl. Environ. Microbiol. 71:5145-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, K., M. Betaieb, and D. R. Telford. 1990. Correlation between environmental monitoring of thermophilic campylobacters in sewage effluent and the incidence of Campylobacter infection in the community. J. Appl. Bacteriol. 69:235-240. [DOI] [PubMed] [Google Scholar]
- 22.Jones, K. 2001. Campylobacters in water, sewage and the environment. J. Appl. Microbiol. 90:68S-79S. [DOI] [PubMed] [Google Scholar]
- 23.Korhonen, L. K., and P. J. Martikainen. 1991. Comparison of survival of Campylobacter jejuni and Campylobacter coli in culturable form in surface water. Can. J. Microbiol. 37:530-533. [DOI] [PubMed] [Google Scholar]
- 24.Leatherbarrow, A. J. H., C. A. Hart, R. Kemp, N. J. Williams, A. Ridley, M. Sharma, P. J. Diggle, E. J. Wright, J. Sutherst, and N. P. French. 2004. Genotypic and antibiotic susceptibility characteristics of a Campylobacter coli population isolated from dairy farmland in the United Kingdom. Appl. Environ. Microbiol. 70:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner, A., T. Tasara, and R. Stephan. 2005. Relevant aspects of Arcobacter spp. as potential foodborne pathogen. Int. J. Food Microbiol. 102:127-135. [DOI] [PubMed] [Google Scholar]
- 26.Louis, V. R., I. A. Gillespie, S. J. O'Brien, E. Russek-Cohen, A. D. Pearson, and R. R. Colwell. 2005. Temperature-driven Campylobacter seasonality in England and Wales. Appl. Environ. Microbiol. 71:85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund, M., S. Nordentoft, K. Pedersen, and M. Madsen. 2004. Detection of Campylobacter spp. in chicken fecal samples by real-time PCR. J. Clin. Microbiol. 42:5125-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melby, K., B. Gondrosen, S. Gregusson, H. Ribe, and P. P. Dahl. 1991. Waterborne campylobacteriosis in northern Norway. Int. J. Food Microbiol. 12:151-156. [DOI] [PubMed] [Google Scholar]
- 29.Moreno, Y., S. Botella, J. L. Alonso, M. A. Ferrus, M. Hernandez, and J. Hernandez. 2003. Specific detection of Arcobacter and Campylobacter strains in water and sewage by PCR and fluorescent in situ hybridization. Appl. Environ. Microbiol. 69:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newell, D. G. 2002. The ecology of Campylobacter jejuni in avian and human hosts and in the environment. Int. J. Infect. Dis. 6:3S16-3S21. [DOI] [PubMed] [Google Scholar]
- 31.Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343-4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogva, H. K., A. Bergh, A. Holck, and K. Rudi. 2000. Application of the 5′-nuclease PCR assay in evaluation and development of methods for quantitative detection of Campylobacter jejuni. Appl. Environ. Microbiol. 66:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nylen, G., F. Dunstan, S. R. Palmer, Y. Anderson, F. Bager, J. Cowden, G. Feierl, Y. Galloway, G. Kapperud, F. Megraud, K. Molbak, L. R. Petersen, and P. Ruutu. 2002. The seasonal distribution of Campylobacter infection in nine European countries and New Zealand. Epidemol. Infect. 128:383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.On, S. L. W. 1996. Identification methods for campylobacters, helicobacters, and related organisms. Clin. Microbiol. Rev. 9:405-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onifade, T. J. 2005. Campylobacteriosis in Georgia: demographics, geography, land-use and weather. M.S. thesis. University of Georgia, Athens.
- 36.Patrick, M. E., L. E. Christiansen, M. Wainø, S. Ethelberg, H. Madsen, and H. C. Wegener. 2004. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol. 70:7474-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pote, D. H., W. L. Kingery, G. E. Aiken, F. X. Han, P. A. Moore, Jr., and K. Buddington. 2003. Water-quality effects of incorporating poultry litter into perennial grassland soils. J. Environ. Qual. 32:2392-2398. [DOI] [PubMed] [Google Scholar]
- 38.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosef, O., G. Kapperud, and E. Skjerve. 1987. Comparison of media and filtration procedures for qualitative recovery of thermotolerant Campylobacter spp. from naturally contaminated surface water. Int. J. Food Microbiol. 5:29-39. [Google Scholar]
- 40.Sails, A. D., A. J. Fox, F. J. Bolton, D. R. A. Wareing, D. L. A. Greenway, and R. Borrow. 2001. Development of a PCR ELISA assay for the identification of Campylobacter jejuni and Campylobacter coli. Mol. Cell. Probes 15:291-300. [DOI] [PubMed] [Google Scholar]
- 41.Sails, A. D., F. J. Bolton, A. J. Fox, D. R. A. Wareing, and D. L. A. Greenway. 2002. Detection of Campylobacter jejuni and Campylobacter coli in environmental waters by PCR enzyme-linked immunosorbent assay. Appl. Environ. Microbiol. 68:1319-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savill, M. G., J. A. Hudson, A. Ball, J. D. Klena, P. Scholes, R. J. Whyte, R. E. McCormick, and D. Jankovic. 2001. Enumeration of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 91:38-46. [DOI] [PubMed] [Google Scholar]
- 43.Skirrow, M. B., and M. J. Blaser. 1992. Clinical and epidemiologic considerations, p. 3-8. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 44.Snelling, W. J., J. P. McKenna, D. M. Lecky, and J. S. G. Dooley. 2005. Survival of Campylobacter jejuni in waterborne protozoa. Appl. Environ. Microbiol. 71:5560-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480. [DOI] [PubMed] [Google Scholar]
- 46.Stelzer, W., J. Jacob, and E. Schulze. 1991. Environmental aspects of Campy-lobacter infections. Zentbl. Mikrobiol. 146:3-15. [PubMed] [Google Scholar]
- 47.Tauxe, R. 1992. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 9-19. In I. Nachamkin, M. J. Blaser, and L. S. Tompkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 48.Tauxe, R. V., N. Hargrett-Bean, C. M. Patton, and I. K. Wachsmuth. 1988. Campylobacter isolates in the United States, 1982-1986. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 37:1-13. [PubMed] [Google Scholar]
- 49.Taylor, D. N., J. A. Kiehlbauch, W. Tee, C. Pitarangsi, and P. Echeverria. 1991. Isolation of group 2 aerotolerant Campylobacter species from Thai children with diarrhea. J. Infect. Dis. 163:1062-1067. [DOI] [PubMed] [Google Scholar]
- 50.Vandamme, P., and J. De Ley. 1991. Proposal for a new family, Campylobacteraceae. Int. J. Syst. Bacteriol. 41:451-455. [Google Scholar]
- 51.Waage, A. S., T. Varund, V. Lund, and G. Kapperud. 1999. Detection of small numbers of Campylobacter jejuni and Campylobacter coli cells in environmental water, sewage, and food samples by a seminested PCR assay. Appl. Environ. Microbiol. 66:1636-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace, J. S., K. N. Stanley, J. E. Currie, P. J. Diggle, and K. Jones. 1997. Seasonality of thermophilic Campylobacter populations in chickens. J. Appl. Microbiol. 82:219-224. [PubMed] [Google Scholar]
- 53.Wasseenaar, T., and D. G. Newell. 1999. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]