Abstract
Maize is subject to ear rot caused by toxigenic Aspergillus and Fusarium species, resulting in contamination with aflatoxins, fumonisins, trichothecenes, and zearalenone (ZEN). The trichothecene group and ZEN mycotoxins are produced by the cereal pathogen Fusarium graminearum. A transgenic detoxification system for the elimination of ZEN was previously developed using an egfp::zhd101 gene (gfzhd101), encoding an enhanced green fluorescent protein fused to a ZEN-degrading enzyme. In this study, we produced a transgenic maize line expressing an intact copy of gfzhd101 and examined the feasibility of transgene-mediated detoxification in the kernels. ZEN-degrading activity has been detected in transgenic kernels during seed maturation (for a period of 6 weeks after pollination). The level of detoxification activity was unaltered after an additional storage period of 16 weeks at 6°C. When the seeds were artificially contaminated by immersion in a ZEN solution for 48 h at 28°C, the total amount of the mycotoxin in the transgenic seeds was uniformly reduced to less than 1/10 of that in the wild type. The ZEN in the transgenic maize kernels was also efficiently decontaminated under conditions of lower water activity (aw) and temperature; e.g., 16.9 μg of ZEN was removed per gram of seed within 48 h at an aw of 0.90 at 20°C. F. graminearum infection assays demonstrated an absence of ZEN in the transgenic maize seeds, while the mycotoxin accumulated in wild-type kernels under the same conditions. Transgene-mediated detoxification may offer simple solutions to the problems of mycotoxin contamination in maize.
Maize is one of three major cereal crops that dominate world agriculture. Maize kernels are processed primarily for livestock feed (78%) and, to some extent, for human consumption (13%) (28). In substantial proportions of maize-producing areas worldwide, the crops are subject to contamination from mycotoxins (22). Although numerous toxic fungal metabolites may be found in maize kernels, studies have focused on four major mycotoxins: aflatoxins associated with Aspergillus ear rot and fumonisins, trichothecenes, and a myco-estrogen zearalenone (ZEN) associated with Fusarium/Gibberella ear rot (16, 27). The risk of contamination with these mycotoxins has been recognized for decades, but preventive measures remain costly and inadequate.
Recent advances in the study of plant pathology have opened the way to achieving transgenic resistance to fungal pathogens, including toxigenic fungi and their toxins (2). However, transgenic strategies have not been successful in achieving a convincing and practical level of resistance to fungi in the field (4). An alternative way to address mycotoxin contamination could be transgene-mediated detoxification in plants by the deployment of anti-mycotoxin antibodies (plantibodies) (35) or mycotoxin-degrading enzymes (12). The latter idea is being tested with genetically modified (GM) maize engineered so that fumonisin B1 is eliminated through the expression of the fumonisin esterase and amine oxidase genes of Exophiala spinifera (4). However, there have been no published data that support the practicality of mycotoxin-degrading maize.
Fusarium graminearum is a broad-range fungal pathogen that infects a number of important crops worldwide (7). It is a causal agent of Gibberella ear rot in maize and Fusarium head blight in wheat and barley, diseases by which grains become contaminated with trichothecene group and ZEN mycotoxins. The fungus enters maize ears down the silk or through insect wounds to the kernels and cob. Under field conditions, the time courses of the fungal growth and mycotoxin level vary depending on the environmental conditions (25, 26), but the mycotoxin appears to increase toward the late milk stage. As to the removal of ZEN, a gene for detoxification (designated zhd101) encoding an alkaline lactonohydrolase was cloned and characterized (29). In this study, we established a homozygous maize line stably expressing zhd101 and conducted laboratory scale experiments to see whether transgenic detoxification is feasible or not for mitigation of the mycotoxin problems caused by F. graminearum.
MATERIALS AND METHODS
Plant and plasmid materials.
The Zea mays inbred line A 188 (JP no. 808) was supplied by the National Institute of Agrobiological Biosciences (NIAS) GenBank-Plant. Plants were grown in a greenhouse under conditions of a 12-h photoperiod (400 W metal halide lamp; Toshiba) at temperatures of 25°C in the day and 20°C in the night.
For easy monitoring of the ZHD101 enzyme's distribution in various tissues, we previously constructed an egfp::zhd101 gene (hereafter designated gfzhd101) that encodes an enhanced green fluorescent protein (EGFP) N-terminally fused to ZHD101 (30). In this study, we constructed the plasmid pEU-Ωgfzhd101 containing gfzhd101 under the control of the Act1 promoter (20) and an Ω translation enhancer (32).
Transformation of maize.
The culture and transformation of maize were done following the method reported by Frame et al. (5) with modifications. Briefly, immature embryos (approximately 1.5 mm) were placed embryo axis-side down on D1.5 medium (Murashige minimal organic medium [Invitrogen, Carlsbad, CA] supplemented with 1.5 mg/liter 2,4-dichlorophenoxyacetic acid, 0.5 mg/liter nicotinic acid, 0.5 mg/liter pyridoxine HCl, 700 mg/liter l-proline, 1.7 mg/liter silver nitrate, 20 g/liter sucrose, and 8 g/liter agar) so that calluses could form. The plates (90 by 20 mm; Eiken Co., Tokyo, Japan) containing the plant materials were sealed with surgical tape (3M Health Care, Borken, Germany) and incubated at 28°C in the dark.
After 4 to 7 days, embryos producing calluses were transferred to D2Man medium (Murashige minimal organic medium supplemented with 2.0 mg/liter 2,4-dichlorophenoxyacetic acid, 30 g/liter sucrose, 72.8 g/liter mannitol, and 2.5 g/liter gellun gum) with the scutellum side up. The embryos were cobombarded with plasmids pDM302 (3) and pEUΩ-gfzhd101 (2 μg each) at 650 lb/in2 at a target distance of 9 cm using a PDS-1000/He biolistic system (Bio-Rad, Hercules, CA). After an overnight culture, the bombarded embryos were transferred to a selection medium (D1.5 medium without l-proline, supplemented with 2.5 mg/liter bialaphos) and subcultured every 2 weeks.
Selected calluses showing expression of EGFP and resistance to bialaphos were transferred to SE medium (Murashige minimal organic medium supplemented with 0.5 mg/liter nicotinic acid, 0.5 mg/liter pyridoxine HCl, 0.5 mg/liter bialaphos, 60 g/liter sucrose, and 1.5 g/liter gellun gum) for somatic embryogenesis and cultured at 25°C under 24-h illumination. The somatic embryos were transferred to RE medium (1/2 Murashige minimal organic medium supplemented with 0.5 mg/liter nicotinic acid, 0.5 mg/liter pyridoxine HCl, 30 g/liter sucrose, and 8 g/liter agar) for plant regeneration.
Southern and Western blot analyses.
Genomic DNA was isolated from mature leaves using a Nucleon PhytoPure plant and fungal DNA extraction kit (Amersham Biosciences, Piscataway, NJ). For Southern blot analysis, a digoxigenin-labeled probe was prepared by using a PCR digoxigenin probe synthesis kit (Roche Applied Science GmbH, Manheim, Germany). The probe consists of the entire coding region of gfzhd101 amplified with the following primers: 5′-ATGGTGAGCAAGGGCGAGGAGCTGTT-3′ and 5′-TCAAAGATGCTTCTGCGTAGTTTCCA-3′. After agarose gel electrophoresis, DNA was transferred to a Nytran N membrane (Schleicher and Schell, Dassel, Germany). Standard hybridization techniques recommended by the manufacturer were used.
Crude protein was extracted from mature leaves (50 mg) of nontransgenic and transgenic plants by grinding in a mortar with 200 to 500 μl of extraction buffer (50 mM Tris-HCl, 10 mM 2-mercaptoethanol, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5% [vol/vol] glycerol, 2% [wt/vol] polyvinylpyrrolidone K25, and 1.5% [wt/vol] poly[vinylpolypyrrolidone], pH 8.0). Twenty micrograms of denatured protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 12.5% polyacrylamide gel and electroblotted to a polyvinylidene difluoride membrane (Bio-Rad). The membrane was first incubated with a polyclonal antibody that recognizes GFPs (living colors full-length A.v. polyclonal antibody; Takara BIO Inc., Ohtsu, Japan) and then with a biotinylated goat anti-rabbit immunoglobulin G (heavy plus light chains) (Bio-Rad) as a secondary antibody. Detection was achieved using an AP conjugate substrate kit (Bio-Rad).
Statistical analyses.
Observed segregation ratios were compared to the Mendelian 3:1, 15:1, or 63:1 ratio using the chi-square test (χ2, >3.84; P, <0.05).
In vitro ZEN degradation assay using maize kernels.
The wild-type and homozygous T3 seeds (self-pollinated progenies derived from line N11-2-19 or N11-2-21) were harvested after seed maturation (6 weeks after pollination) and then stored for 16 weeks at 6°C. The moisture content was determined by heating the grains at 80°C for 16 h. The value of the water activity (aw) was measured using a water activity meter PawKit (Decagon Devices Inc., Pullman, WA) calibrated with the salt solutions.
For assaying the degradation of ZEN in vitro, seeds were collected at 2 weeks (moisture content, 78%; aw, 0.99), 4 weeks (moisture content, 45%; aw, 0.98), and 6 weeks (moisture content, 14%; aw, 0.82) after pollination, and some seeds collected at 6 weeks after pollination were stored for 16 weeks at 6°C (a total of 22 weeks after pollination). Seeds were ground in a chilled mortar and the crude protein was extracted with buffer A (50 mM Tris-HCl, pH 7.0, 10 mM EDTA, and 5% glycerol). The protein concentration of this crude protein extract was adjusted to 0.2 mg/ml with buffer A. The enzymatic reaction was initiated by adding 5 μl of ZEN stock solution (5 mg/ml in dimethyl sulfoxide) to 495 μl of crude protein extract in a siliconized microtube. After 4 or 24 h of incubation at 30°C, a portion of the reaction mixture (50 μl) was mixed with methanol (450 μl) and the resulting sample was passed through a 0.2-μm Millex-LG filter (Millipore, Billerica, MA). An aliquot (10 μl) was analyzed using a high-performance liquid chromatography (HPLC) system (SCL-10A; Shimadzu) equipped with a PEGASIL octyldecyl silane column (diameter, 4.6 mm; length, 250 mm; Senshu Scientific Co., Tokyo, Japan). Samples were eluted with 0.1% trifluoroacetic acid-acetonitrile (4:6) at a flow rate of 1.0 ml/min at 40°C. ZEN and its degradation product, 1-(3,5-dihydroxyphenyl)-10′-hydroxy-1′-undecen-6′-1 (DPHU) (11, 29), were monitored at 254 nm. The experiments were carried out in triplicate for both the wild-type and T3 seeds.
In vivo ZEN degradation assay using maize kernels.
Four-week-old wild-type and homozygous T3 seeds (self-pollinated progenies of N11-2-9) were surface sterilized with 80% ethanol, rinsed with sterilized water, and placed in a siliconized polypropylene centrifuge tube (50 ml; BD Falcon, Franklin Lakes, NJ). An equal amount of ZEN solution (50 μg/ml) was added to each tube and the kernels were allowed to absorb ZEN at 28°C for 48 h. After the incubation, the seeds were lightly tapped against the inner wall of the tube to remove excess liquid on the surface and then dissected into three parts: embryos, endosperms, and pericaps. After each tissue was ground in a chilled mortar, the sample was transferred to a 50-ml tube and mixed with 80% acetonitrile (4 to 10 times the amount of each tissue). Each sample was homogenized using a handy micro homogenizer (Physcotron; Microtech Co., Ltd., Chiba, Japan) and ZEN was extracted by gently shaking the mixture for 2 h. The concentration of ZEN was determined by HPLC based on the fluorescence of ZEN (excitation, 278 nm; emission, 460 nm).
Influence of water activity (aw) and temperature on ZEN degradation.
To evaluate the influence of environmental conditions on the ZEN-degrading activity of transgenic kernels, the in vitro and in vivo ZEN degradation assays were also carried out under conditions in which the aw was adjusted to 1.00 (at 30°C), 0.97 (at 25°C), and 0.90 (at 20°C) by adding sodium chloride. The in vitro and in vivo ZEN-degrading activities of transgenic kernels were measured as described above, except that 80% methanol was used for the ZEN extraction in the in vivo experiment (normalized to the efficiency of 80% acetonitrile extraction for comparison). These experiments were carried out in triplicate.
Inoculation of maize kernels with F. graminearum.
Four-week-old maize seeds (moisture content, 45%; aw, 0.98) surface-sterilized as described above were placed in a sterilized 100-ml Erlenmyer flask and mixed with a mycelial plug of F. graminearum ZEA-1 (33). Each flask contained a mixture of six wild-type and six homozygous T3 seeds (self-pollinated progenies of N11-2-9). The flasks were placed in a sealed plastic box containing water (equilibrium relative humidity, 100%) and incubated for 7 to 8 days at 25°C. After the incubation, the mycelia on the surface of the kernels were lightly scrubbed with paper, and then the seeds were lightly rinsed with water. The seeds were divided into wild-type and transgenic groups, and each group was separately analyzed by HPLC for the amount of ZEN as described above. The experiment was independently carried out 5 times.
RESULTS
Transformation of maize with pEU-Ωgfzhd101.
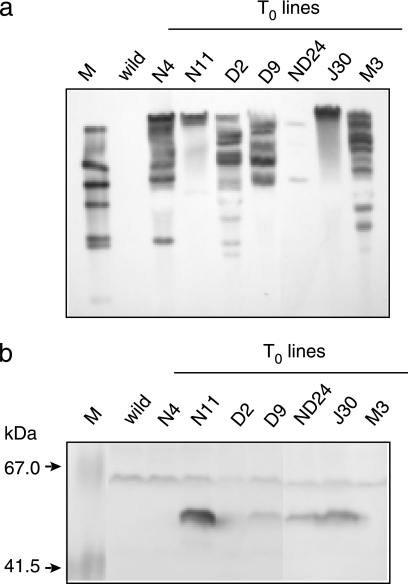
Maize embryos cobombarded with pEU-Ωgfzhd101 and pDM302 were selected with bialaphos, and the calluses with EGFP fluorescence were regenerated into plantlets as described in Materials and Methods. Seven independent T0 lines were obtained from the approximately 1,500 embryos bombarded. The genomic DNA of these lines was digested with XbaI, which does not cut the vector, and examined by Southern blotting using gfzhd101 as a probe. As shown in Fig. 1a, many lines carried multiple copies of the transgene in the genome, which is often the case with the biolistic method. Epifluoresence stereomicroscopic observation of mature T0 leaves revealed that three transgenic lines, N4, D2, and M3, which carry multiple copies of the transgene, did not show EGFP fluorescence at this developmental stage, presumably due to gene silencing. Consistent with this observation, Western blot analysis also demonstrated that the fluorescent ZEN-degrading enzyme was produced only in lines N11, D9, ND24, and J30 (Fig. 1b).
FIG. 1.
Analyses of T0 transgenic lines of maize expressing gfzhd101. (a) Southern blot analysis of DNA from wild-type and transgenic lines. Genomic DNA was digested with XbaI, which does not cut the probe DNA. M, λ HindIII marker DNA. (b) Western blot analysis of total leaf protein extracted from wild-type and transgenic lines. The sizes of biotinylated marker proteins (M) are shown by arrows.
Inheritance of the transgene.
The EGFP-positive transgenic lines were self-crossed to obtain T1 seeds, which were used for self-crosses in successive generations to achieve homozygosity for the transgene. While lines N11, D9, and J30 were fertile and produced viable T1 seeds, ND24 failed to produce anther dusts and T1 seeds were not obtained. Out of 301 and 473 T1 seeds originating from T0 lines D9 and J30, 262 and 311 seeds, respectively, showed EGFP fluorescence. These expression patterns of the transgene differed significantly from a single- or multiple-site integration of the transgene as assessed with a chi-square test. As to progenies of N11, 52 of 71 T1 seeds showed EGFP fluorescence, which did not differ significantly from a 3:1 Mendelian segregation for a single insertion site (χ2, 0.117; P, <3.84). For the subsequent characterization of transgenic plants, we used progenies of line N11.
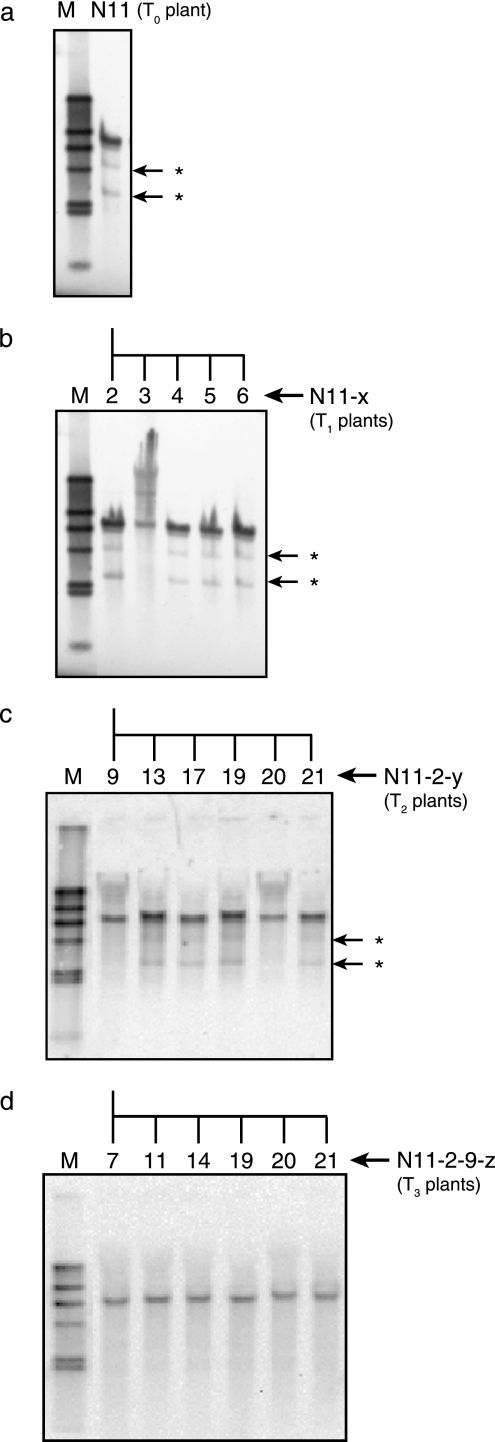
Figure 2 traces the integration patterns of gfzhd101 in self-pollinated progenies of line N11. When DNA was digested with ClaI, which does not cut the probe, one intense and two faint bands were detected from the T0 and T1 progenies, except for the line N11-3 T1 progenies (Fig. 2a and b). Lines N11-5 and N11-6 failed to produce seeds, and lines N11-3 and N11-4 were heterozygous, as suggested by the segregation pattern of the EGFP-fluorescence in the self-pollinated T2 generation. Among these T1 progenies, only line N11-2 produced 100% fluorescent T2 seeds expressing gfzhd101. The T2 plants N11-2-9 and N11-2-20 lost the two faint bands that were observed on the Southern blot of the parent N11-2, and only a single ClaI fragment was detected (Fig. 2c). When the T3 progenies derived from N11-2-9 were analyzed by Southern blotting, they were found to have similarly inherited this single banding pattern (Fig. 2d). These T3 progenies and their T2 parent N11-2-9 revealed a single band with another no-cut enzyme, BglII, and double bands with a single-cut enzyme, Bsp1047I (data not shown), suggesting that they are homozygous carrying a single copy of the transgene. These self-pollinated progenies of N11-2-9 were used for in vivo ZEN detoxification and Fusarium infection assays.
FIG. 2.
Inheritance of gfzhd101 in progenies of line N11. The extended names joined by hyphens represent the self-crossed next generation derived from the original parent (e.g., “N11-2-9-z” represents T3 progeny “z” descended from the T2 parent “N11-2-9”). The arrows with asterisks indicate faint ClaI bands that segregated from the main single band in the two progenies in the T2 generation. M, λ HindIII marker DNA.
In vitro ZEN-degrading activities of transgenic seeds.
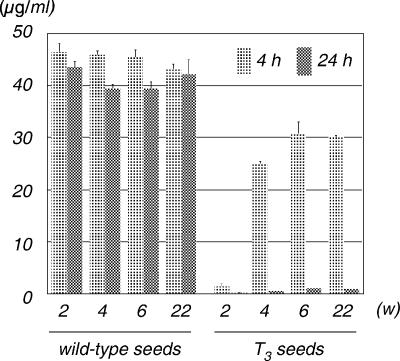
To evaluate the ZEN-degrading activities of the transgenic maize kernels, crude protein extracts were prepared from the T3 seeds and used for the in vitro detoxification assay. Compared to a blank control (data not shown), no decontamination was observed with the protein extract prepared from the wild-type kernels during seed maturation (Fig. 3). In contrast, the amount of ZEN decreased significantly in the transgenic reaction mixture compared to the amount in the control reaction mixture with heat-denatured transgenic seed extract (data not shown) or the amount in the wild-type seed extract. With 4 h of incubation, the detoxifying activity was most evident in developing seeds at 2 weeks after self-pollination. The enzyme activities were decreased in seeds at 4 weeks, 6 weeks, and 22 weeks (6 weeks on the cob with an additional 16 weeks of storage at 6°C). When the incubation time was increased to 24 h, nearly all the ZEN in the reaction mixture was eliminated by the transgenic protein at all stages of seed maturation. The cleavage product of ZEN, DPHU, was also detected in the reaction mixture of the transgenic seeds by the HPLC analysis (data not shown). These results indicate that the EGFP-tagged ZHD101 enzyme is produced in an active form in the transgenic maize kernels.
FIG. 3.
Degradation of ZEN by crude proteins extracted from T3 seeds at 2, 4, 6, and 22 weeks (developing on the cob for 6 weeks and then harvested and stored for 16 weeks at 6°C) after pollination. The enzymatic reaction was carried out for 4 h or 24 h at 30°C. The vertical axis represents the concentration of ZEN in the reaction mixture.
In vivo ZEN-degrading activities of transgenic seeds.
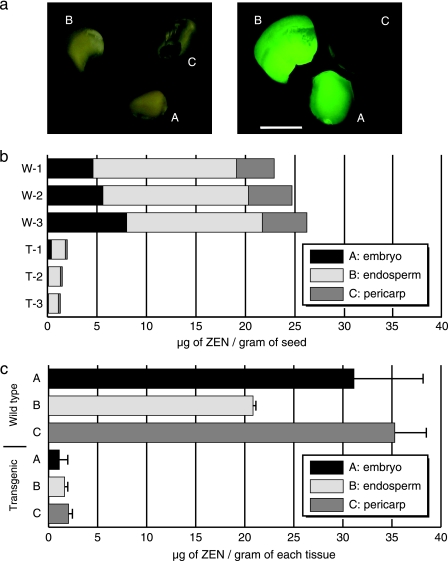
T3 homozygous N11-2-9-z seeds (4 weeks after self-pollination) were immersed in a 50-μg/ml ZEN solution and allowed to absorb the mycotoxin for 48 h. After removal of the ZEN solution on the surface, the ZEN-soaked seeds were dissected into embryos, endosperms, and pericarps and then observed under an epifluorescence microscope (MZFLIII; Leica Microsystems GmbH, Wetzlar, Germany). The EGFP-tagged enzyme was found mostly in the embryos and endosperms, and to a lesser extent in the pericarp fraction (Fig. 4a). This demonstrates that the enzyme can be expressed at a certain level in maize kernels and distributed throughout the kernel tissues.
FIG. 4.
Degradation of ZEN in vivo by mature T3 seeds. (a) Dissection of maize kernels into three parts: embryos (A), endosperms (B), and pericarps (C). Photographs of dissected parts are shown in the left panel. An epifluorescent stereomicroscopic image of transgenic seeds is shown in the right panel (the bar represents 5 mm). (b) Reduced amounts of ZEN in transgenic seeds soaked in the mycotoxin solution (50 μg/ml of ZEN) at 28°C. W and T represent experiments with wild-type and transgenic seeds, respectively, which were independently carried out three times (distinguished by a number after a hyphen). The stacked bars represent the amount of ZEN (μg) detected per gram of seed in each experiment. (c) Reduced amounts of ZEN in transgenic embryos, endosperms, and pericarps soaked with the mycotoxin solution (50 μg/ml of ZEN) at 28°C. The bars represent the amount of ZEN (μg) per gram of each tissue; the error bars represent standard deviations (n = 3).
The amount of ZEN in the supernatant and each of the above three fractions was quantified by HPLC. As shown in Fig. 4b, the wild-type seeds contained a total of 24.6 ± 1.7 μg of ZEN per gram of seed, which is far above the usual levels of ZEN found in the field (1/2,000 to 1/500 the level of contamination compared to the level found in this experiment). The concentration of ZEN in the supernatant solution was reduced to 0.83 μg/ml, suggesting that most of the mycotoxin in the solution was absorbed by the seeds. Although slightly less ZEN tended to accumulate in the endosperms than in the embryos and pericarps, the distribution patterns of this mycotoxin were not remarkably different in the wild-type seeds (Fig. 4c).
In spite of the assay conditions that result in such high accumulation levels of ZEN, the transgenic seeds contained only 1.6 ± 0.4 μg of ZEN per gram of seed, and the amount of ZEN in the supernatant solution was reduced to 0.16 μg/ml. The reduction in the amount of ZEN in the seeds is attributed to degradation by the enzyme, since a large fluorescent peak of DPHU was observed in the HPLC chromatogram of the reaction mixture (data not shown). Again, there were no remarkable differences in the residual amounts of ZEN in the embryos, endosperms, and pericarps of the transgenic seeds (Fig. 4c). Therefore, the ZEN-degrading enzyme and ZEN are similarly distributed within the maize kernels, suggesting the feasibility of the transgene-mediated detoxification strategy.
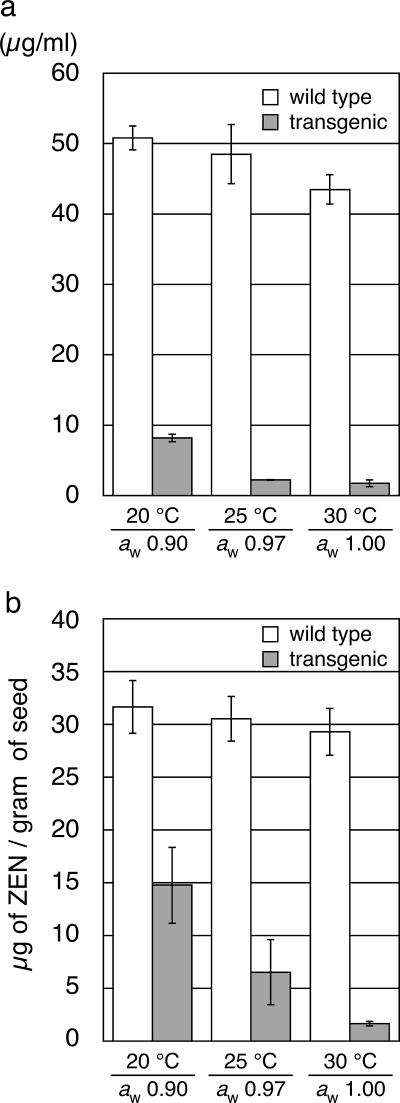
Degradation of ZEN in transgenic maize kernels under conditions of lower water activity and temperature.
To assess the ability of the recombinant enzyme to decontaminate the mycotoxin under different conditions that are less preferable for enzymes in general, we examined the influence of water activity and temperature on ZEN degradation. Since the toxigenic Fusarium no longer produces ZEN when the aw is reduced to 0.90 (21), the effect of the water activity on ZEN-degrading activity was examined at an aw above this value. Experiments with purified recombinant ZHD101 (30) showed that the enzyme activity when the aw was 0.90 was at least 64% of that when the aw was 1.00 when compared at the same temperature (in the range of 15 to 30°C) and that, at an aw of 0.90 at 15°C, the enzyme still retained 33% of its activity compared to that when the aw was 1.00 at 30°C (data not shown).
The ZEN-degrading activities of transgenic kernels were assayed by an in vitro experiment with an incubation period of 24 h. Consistent with the relative insensitivity of purified ZHD101 to lower water activity and temperature, the crude cell extract of the transgenic seeds could remove 84% of ZEN in the reaction mixture at an aw of 0.90 at 20°C (Fig. 5a). This in vitro experiment also demonstrated that as much as 95% of ZEN in the reaction mixture was degraded when the aw was 0.97 at 25°C, one of the conditions under which the fungus is known to produce its highest yield of ZEN (10, 21). The activity under these conditions was substantially equal to that at an aw of 1.00 at 30°C.
FIG. 5.
Degradation of ZEN under conditions of different water activities and temperatures (aw of 0.90 at 20°C; aw of 0.97 at 25°C; and aw of 1.00 at 30°C). (a) Degradation of ZEN by crude proteins extracted from T3 seeds at 4 weeks after pollination. The enzymatic reaction was carried out for 24 h. The vertical axis represents concentrations of ZEN in the reaction mixture. (b) Degradation of ZEN in vivo by mature T3 seeds at 4 weeks after pollination. Maize kernels artificially contaminated with ZEN as described in the Fig. 4 legend (placed under the conditions for 48 h) were analyzed as described in Materials and Methods. The vertical axis represents the amount of ZEN (μg) per gram of seed.
The in vivo ZEN degradation assay also demonstrated that the transgenic kernels could degrade significant amount of ZEN under the above conditions. As shown in Fig. 5b, the residual amounts of ZEN in the artificially contaminated transgenic seeds were significantly reduced compared to the amount in the wild-type control during an incubation period of 48 h. In terms of the decontamination efficiency, 16.9 and 24.0 μg of ZEN were removed per gram of seed at an aw of 0.90 at 20°C and at an aw of 0.97 at 25°C, respectively (cf. 27.6 μg removed per gram of seed at an aw of 1.00 at 30°C).
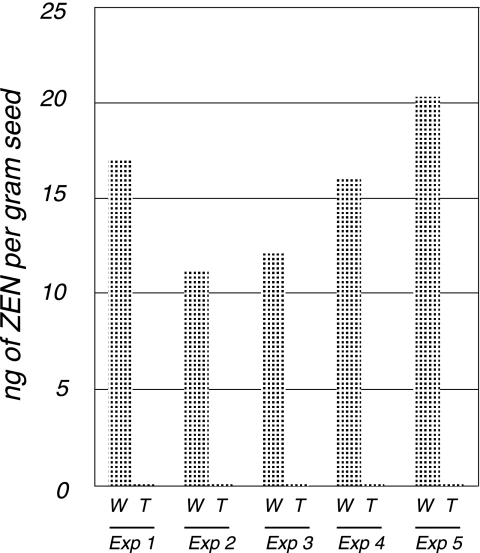
Detoxification of ZEN in Fusarium-infected transgenic kernels.
After the surfaces of the Fusarium-infected seeds were washed, the amounts of ZEN were quantified. The ZEN-producing conditions for F. graminearum in the wild-type and transgenic maize kernels should be equal in each experiment since the fungal inoculation of both types of kernels was carried out in a single Erlenmyer flask. The wild-type and transgenic seeds were easily distinguished by the EGFP-tag attached to the detoxification enzyme. As shown in Fig. 6, the wild type contained 15.4 ± 3.7 ng of ZEN per gram of seed. This level of contamination is comparable to the concentration of ZEN reported to occur naturally in infected maize kernels (e.g., 4 to 388 ng per gram of seed in the results reported in reference 13 and 14 to 169 ng per gram of seed in the results reported in reference 17). In contrast, noninoculated seeds (data not shown) and the T3 transgenic seeds inoculated with the fungus contained no ZEN.
FIG. 6.
Detoxification of transgenic seeds infected with F. graminearum. The mature T3 seeds, with wild-type (W) and transgenic (T) seeds mixed, were inoculated with the fungus and incubated for 7 to 8 days at 25°C under humid conditions (equilibrium relative humidity, 100%). After removal of the mycelia on the surface of the infected grain, the wild-type and transgenic seeds were separated and examined under the epifluorescence stereomicroscope. ZEN was extracted from the seeds and quantified by HPLC. The experiment (Exp) was carried out independently five times.
DISCUSSION
Although reducing fungal infections is the most desirable method of eliminating mycotoxins from maize, cultural practices and genetic approaches have achieved limited success in disease control in terms of effectiveness and cost. In addition, efforts to produce GM crops with enhanced disease resistance, such as the introduction of insecticidal genes (22), overexpression of antifungal proteins (1), engineering of novel secondary metabolites (8), and transgene-mediated enhancement of plant defense pathways (18), have not been successful on a practical level. As an alternative solution, transgene-mediated detoxification of mycotoxins has been proposed (12). A prerequisite for this is the availability of genes encoding enzymes with detoxification activities, and with regard to ZEN, we have previously isolated zhd101 (29). This detoxification gene was useful for transgenic decontamination of ZEN when expressed in baker's yeasts after the codon optimization (31); in addition, the active enzyme proved to be expressed in the grains of model cereal rice plants transformed with gfzhd101 (9). Since ZEN causes a major problem in maize intended for use in stockbreeding, we have established a homozygous transgenic maize line stably expressing gfzhd101. Promisingly, the transgenic maize kernels showed sufficient ZEN-degrading activity throughout seed maturation and after storage as assessed by the in vitro enzyme assay. These results warrant the availability of active ZHD101 enzyme when and where it is needed in transgenic maize.
Unlike the situation in vitro, the transgenic detoxification of mycotoxins in maize kernels was postulated to be problematic with regard to (i) the distribution of the enzyme in relation to the mycotoxin, and (ii) the activity of the enzyme under physiological conditions in maize tissue (4). In the case of fumonisin B1, the establishment of a system of transgene-mediated detoxification has not yet been reported, presumably due to such problems. Therefore, laboratory-directed enzyme evolution was conducted to improve transgenic detoxification (34). In contrast, detoxification of ZEN in vivo was successful regardless of the conditions listed above. Indeed, the EGFP-tagged enzyme was present in all three fractions of the seeds, embryos, endosperms, and pericarps (Fig. 4a), and the amount of ZEN in the transgenic kernels was markedly reduced in each tissue compared to that in the nontransgenic control (Fig. 4b and c). ZEN′s occurrence seems not to be confined to (i) specific tissues or (ii) extracellular spaces as postulated for fumonisin B1, because (i) exogenously absorbed ZEN was detected equally in the above three fractions of the wild-type kernels and (ii) the secretion signal was not necessary for the in vivo detoxification. Therefore, the enzyme's distribution and stability appear not to be technical problems that need to be resolved in the case of ZEN-related decontamination.
The fungus produces ZEN under various conditions, including those of lower water activities and temperatures which are less favorable for the function of enzymes. We therefore evaluated the ZEN-detoxifying activities of transgenic maize under the conditions in which F. graminearum produces the highest yield of ZEN (an aw of 0.97 at 25°C) and in which it can no longer afford to produce this secondary metabolite (an aw of 0.90 at 20°C). Consistent with the relative insensitivity of the ZHD101 enzyme to decreases in water activity and temperature, the transgenic maize kernels showed significant ZEN-detoxifying activity under these conditions; e.g., the in vivo experiments demonstrated that 16.9 μg of ZEN was removed per gram of seed within 48 h when the aw was 0.90 at 20°C (Fig. 5b). In this way, transgenic detoxification of ZEN in maize kernels was indicated to be useful to solve the problem of ZEN contamination under conditions similar to field situations.
Aside from the efficiency of ZEN′s degradation to DPHU, its further metabolism in maize plants (e.g., conjugation and degradation) must be examined under various abiotic and biotic stress conditions in the field. As to the potential toxicity of DPHU, in vitro assays with human MCF-7 breast cancer cells demonstrated that it shows no estrogenicity (11). The toxicity of DPHU other than estrogenicity, if any, also needs to be evaluated by intensive in vivo studies, including large-scale ingestion experiments with several different animal systems. In addition, the potentially negative impacts of mycotoxin-degrading GM crops, such as effects on nutritional properties and the risk from allergens, also need to be assessed in the future.
F. graminearum concomitantly produces structurally unrelated mycotoxin trichothecenes, e.g., deoxynivalenol (DON), in addition to ZEN in infected grains. Therefore, DON detoxification should also be considered together with the transgenic strategies of ZEN decontamination. Although genes for irreversible detoxification of trichothecenes are not currently available, numerous efforts are being made to solve the problems of DON contamination (6, 14, 15, 19, 23, 24). In combination with such progress in biotechnological detoxification of DON, the transgenic system of detoxification of ZEN demonstrated in this paper will contribute significantly to solving the mycotoxin problems in maize and other cereals.
Acknowledgments
We thank K. Kadokura for technical assistance in the transformation of maize. We also thank T. Nakajima and M. Yoshida for the gift of F. graminearum strains.
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Anand, A., T. Zhou, H. N. Trick, B. S. Gill, W. W. Bockus, and S. Muthukrishnan. 2003. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 54:1101-1111. [DOI] [PubMed] [Google Scholar]
- 2.Ayliffe, M. A., and E. S. Lagudah. 2004. Molecular genetics of disease resistance in cereals. Ann. Bot. 94:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, J., X. L. Duan, D. McElroy, and R. Wu. 1992. Regeneration of herbicide resistant transgenic rice plants following microprojectile-mediated transformation of suspension-culture cells. Plant Cell Rep. 11:586-591. [DOI] [PubMed] [Google Scholar]
- 4.Duvick, J. 2001. Prospects for reducing fumonisin contamination of maize through genetic modification. Environ. Health Perspect. 109 (Suppl. 2):337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frame, B. R., H. Y. Zhang, S. M. Cocciolone, L. V. Sidorenko, C. R. Dietrich, S. E. Pegg, S. F. Zhen, P. S. Schnable, and K. Wang. 2000. Production of transgenic maize from bombarded type II callus: effect of gold particle size and callus morphology on transformation efficiency. In Vitro Cell. Dev. Biol. Plant 36:21-29. [Google Scholar]
- 6.Fuchs, E., E. M. Binder, D. Heidler, and R. Krska. 2002. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 19:379-386. [DOI] [PubMed] [Google Scholar]
- 7.Goswami, R. S., and H. C. Kistler. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5:515-525. [DOI] [PubMed] [Google Scholar]
- 8.Hain, R., H. J. Reif, E. Krause, R. Langebartels, H. Kindl, B. Vornam, W. Wiese, E. Schmelzer, P. H. Schreier, R. H. Stocker, et al. 1993. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 361:153-156. [DOI] [PubMed] [Google Scholar]
- 9.Higa-Nishiyama, A., N. Takahashi-Ando, T. Shimizu, T. Kudo, I. Yamaguchi, and M. Kimura. 2005. A model transgenic cereal plant with detoxification activity for the estrogenic mycotoxin zearalenone. Transgenic Res. 14:713-717. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez, M., M. Manez, and E. Hernandez. 1996. Influence of water activity and temperature on the production of zearalenone in corn by three Fusarium species. Int. J. Food Microbiol. 29:417-421. [DOI] [PubMed] [Google Scholar]
- 11.Kakeya, H., N. Takahashi-Ando, M. Kimura, R. Onose, I. Yamaguchi, and H. Osada. 2002. Biotransformation of the mycotoxin, zearalenone, to a non-estrogenic compound by a fungal strain of Clonostachys sp. Biosci. Biotechnol. Biochem. 66:2723-2726. [DOI] [PubMed] [Google Scholar]
- 12.Karlovsky, P. 1999. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 7:1-23. [DOI] [PubMed] [Google Scholar]
- 13.Kim, J. C., H. J. Kang, D. H. Lee, Y. W. Lee, and T. Yoshizawa. 1993. Natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in barley and corn in Korea. Appl. Environ. Microbiol. 59:3798-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura, M., I. Kaneko, M. Komiyama, A. Takatsuki, H. Koshino, K. Yoneyama, and I. Yamaguchi. 1998. Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J. Biol. Chem. 273:1654-1661. [DOI] [PubMed] [Google Scholar]
- 15.Kimura, M., T. Tokai, G. Matsumoto, M. Fujimura, H. Hamamoto, K. Yoneyama, T. Shibata, and I. Yamaguchi. 2003. Trichothecene nonproducer Gibberella species have both functional and nonfunctional 3-O-acetyltransferase genes. Genetics 163:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, F. Q., T. Yoshizawa, O. Kawamura, X. Y. Luo, and Y. W. Li. 2001. Aflatoxins and fumonisins in corn from the high-incidence area for human hepatocellular carcinoma in Guangxi, China. J. Agric. Food Chem. 49:4122-4126. [DOI] [PubMed] [Google Scholar]
- 17.Luo, Y., T. Yoshizawa, and T. Katayama. 1990. Comparative study on the natural occurrence of Fusarium mycotoxins (trichothecenes and zearalenone) in corn and wheat from high- and low-risk areas for human esophageal cancer in China. Appl. Environ. Microbiol. 56:3723-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makandar, R., J. S. Essig, M. A. Schapaugh, H. N. Trick, and J. Shah. 2006. Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant-Microbe Interact. 19:123-129. [DOI] [PubMed] [Google Scholar]
- 19.Manoharan, M., L. S. Dahleen, T. M. Hohn, S. M. Neate, X.-H. Yu, N. J. Alexander, S. P. McCormick, P. Bregitzer, P. B. Schwarz, and R. D. Horsley. 2007. Expression of 3-OH trichothecene acetyltransferase in barley (Hordeum vulgare L.) and effects on deoxynivalenol. Plant Sci. 171:699-706. [Google Scholar]
- 20.McElroy, D., A. D. Blowers, B. Jenes, and R. Wu. 1991. Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol. Gen. Genet. 231:150-160. [DOI] [PubMed] [Google Scholar]
- 21.Montani, M. L., G. Vaamonde, S. L. Resnik, and P. Buera. 1988. Influence of water activity and temperature on the accumulation of zearalenone in corn. Int. J. Food Microbiol. 6:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Munkvold, G. P. 2003. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 41:99-116. [DOI] [PubMed] [Google Scholar]
- 23.Ohsato, S., T. Ochiai-Fukuda, N. Takahashi-Ando, T. Nishiuchi, S. Koizumi, H. Hamamoto, T. Kudo, I. Yamaguchi, and M. Kimura. 11 October 2006, posting date. Transgenic rice plants expressing trichothecene 3-O-acetyltransferase show resistance to the Fusarium phytotoxin deoxynivalenol. Plant Cell Rep. [Online.] doi: 10.1007/s00299-006-0251-1. [DOI] [PubMed]
- 24.Okubara, P. A., A. E. Blechl, S. P. McCormick, N. J. Alexander, R. Dill-Macky, and T. M. Hohn. 2002. Engineering deoxynivalenol metabolism in wheat through the expression of a fungal trichothecene acetyltransferase gene. Theor. Appl. Genet. 106:74-83. [DOI] [PubMed] [Google Scholar]
- 25.Reid, L. M., R. W. Nicol, T. Ouellet, M. Savard, J. D. Miller, J. C. Young, D. W. Stewart, and A. W. Schaafsma. 1999. Interaction of Fusarium graminearum and F. moniliforme in maize ears: disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 89:1028-1037. [DOI] [PubMed] [Google Scholar]
- 26.Reid, L. M., T. Woldemariam, X. Zhu, D. W. Stewart, and A. W. Schaafsma. 2002. Effect of inoculation time and point of entry on disease severity in Fusarium graminearum, Fusarium verticillioides, or Fusarium subglutinans inoculated maize ears. Can. J. Plant Pathol. 24:162-167. [Google Scholar]
- 27.Schollenberger, M., H. M. Muller, M. Rufle, S. Suchy, S. Plank, and W. Drochner. 2006. Natural occurrence of 16 fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 161:43-52. [DOI] [PubMed] [Google Scholar]
- 28.Taba, S., D. van Ginkel, D. Hoisington, and D. Poland. 2004. Wellhausen-Anderson plant genetic resources center operations manual 2004. International Maize and Wheat Improvement Center (CIMMYT), El Batan, Mexico.
- 29.Takahashi-Ando, N., M. Kimura, H. Kakeya, H. Osada, and I. Yamaguchi. 2002. A novel lactonohydrolase responsible for the detoxification of zearalenone: enzyme purification and gene cloning. Biochem. J. 365:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi-Ando, N., S. Ohsato, T. Shibata, H. Hamamoto, I. Yamaguchi, and M. Kimura. 2004. Metabolism of zearalenone by genetically modified organisms expressing the detoxification gene from Clonostachys rosea. Appl. Environ. Microbiol. 70:3239-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi-Ando, N., T. Tokai, H. Hamamoto, I. Yamaguchi, and M. Kimura. 2005. Efficient decontamination of zearalenone, the mycotoxin of cereal pathogen, by transgenic yeasts through the expression of a synthetic lactonohydrolase gene. Appl. Microbiol. Biotechnol. 67:838-844. [DOI] [PubMed] [Google Scholar]
- 32.Tyc, K., M. Konarska, H. J. Gross, and W. Filipowicz. 1984. Multiple ribosome binding to the 5′-terminal leader sequence of tobacco mosaic virus RNA. Assembly of an 80S ribosome X mRNA complex at the AUU codon. Eur. J. Biochem. 140:503-511. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, M., T. Nakajima, M. Arai, and F. Suzuki. 2004. Mycotoxin productivity of Fusarium graminearum distributed in Western part of Japan and pathogenicity of the nivalenol-producing strains. Jpn. J. Phytopathol. 70:27. [Google Scholar]
- 34.Yuan, L., I. Kurek, J. English, and R. Keenan. 2005. Laboratory-directed protein evolution. Microbiol. Mol. Biol. Rev. 69:373-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan, Q., W. Hu, J. J. Pestka, S. Y. He, and L. P. Hart. 2000. Expression of a functional antizearalenone single-chain Fv antibody in transgenic Arabidopsis plants. Appl. Environ. Microbiol. 66:3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]