Abstract
Histamine-producing bacteria (HPB) such as Photobacterium phosphoreum and Raoultella planticola possess histidine decarboxylase (HDC), which converts histidine into histamine. Histamine fish poisoning (HFP) is attributable to the ingestion of fish containing high levels of histamine produced by HPB. Because freezing greatly decreases the histamine-producing ability of HPB, especially of P. phosphoreum, it has been speculated that HFP is caused by HDC itself from HPB cells autolyzing during frozen storage, even when HPB survive frozen storage. Here we constructed recombinant HDCs of P. phosphoreum, Photobacterium damselae, R. planticola, and Morganella morganii and investigated the ability of HDCs to produce sufficient histamine to cause HFP. To elucidate the character of these HDCs, we examined the specific activity of each recombinant HDC at various temperatures, pH levels, and NaCl concentrations. Further, we also investigated the stability of each HDC under different conditions (in reaction buffer, tuna, and dried saury) at various temperatures. P. damselae HDC readily produced sufficient histamine to cause HFP in fish samples. We consider that if HDC is implicated as an independent cause of HFP in frozen-thawed fish, the most likely causative agent is HDC of P. damselae.
Histamine fish poisoning (HFP) is a food-borne chemical intoxication caused by the ingestion of histidine-rich scombroid fish such as tuna, bonito, and mackerel in which histamine-producing bacteria (HPB) produce a large amount of histamine (1, 22). HPB isolated from fish implicated in actual HFP incidents reported to date include Raoultella planticola, Morganella morganii, Hafnia alvei, and Photobacterium phosphoreum (11, 15). Enteric bacteria such as R. planticola and M. morganii are reported to be the dominant HPB in fish, whereas P. phosphoreum and Photobacterium damselae are frequently isolated from fish subjected to appropriate examination for marine bacteria, such as by the addition of NaCl to the culture medium and avoidance of exposure of the bacterium to high temperatures (11, 16, 17). The former is a psychrotrophic bacterium in the marine environment which has greater histamine-producing activity than enteric bacteria at low temperatures (11). The latter is also a prolific HPB but a mesophilic marine bacterium (13).
It has been shown that accumulation of histamine by HPB occurs after the level of bacterial growth exceeds 107 CFU/ml in culture medium (19). Viable cell counts of P. phosphoreum and R. planticola have been reported to decrease during frozen storage (2, 8). Moreover, the histidine-producing capability of HPB decreases due to injury from freezing, even if the HPB survive frozen storage (6, 21). Tuna, a scombroid fish frequently implicated in HFP, is commonly frozen during fishing and distribution (3). Other scombroid fish are also frequently frozen in current practice. These factors have led to speculation that the accumulation of histamine in thawed fish arises from the release of histidine decarboxylase (HDC) from the autolyzed HPB, which might occur when fish is frozen just before the level of bacterial growth reaches the concentration of formed histamine (8, 15). HDC catalyzes the decarboxylation of histidine to histamine and contains two types of enzyme, a pyridoxal-5′-phosphate (PLP)-dependent enzyme and a pyruvoyl-dependent enzyme. Gram-negative bacteria including enteric bacteria and Photobacterium species have the PLP-dependent enzyme (20).
In this study, we constructed recombinant HDCs of P. phosphoreum, P. damselae, R. planticola, and M. morganii in an Escherichia coli expression system under control of the T7lac promoter and compared HDCs between enteric bacteria and Photobacterium species. We then monitored the activities of HDCs from P. phosphoreum, P. damselae, and R. planticola in tuna and dried saury samples to verify speculation that the accumulation of histamine in frozen-thawed fish results from the HDC itself.
MATERIALS AND METHODS
Bacterial strains used in this study.
We used five bacterial strains in this study, namely, P. phosphoreum NBRC 13896 (NITE Biological Resource Center, Chiba, Japan), P. phosphoreum YS4-7 isolated in our laboratory, P. damselae JCM 8968 (Japan Collection of Microorganisms, Saitama, Japan), R. planticola ATCC 43176 (American Type Culture Collection), and M. morganii JCM 1672.
Sequencing of hdc genes.
We used a modified inverse PCR to determine the complete sequences of the open reading frames (the hdc genes) encoding the HDCs from P. phosphoreum NBRC 13896, P. phosphoreum YS4-7, and P. damselae JCM 8968. Primers LAHDC3 (5′-CTC TGG GCA CAA AAT GAT TGG) and LAHDC4 (5′-GAA GGG CAA GGA AAT ACA AC) were designed on the basis of the hdc gene of Listonella anguillarum (DDBJ, GenBank, and EMBL accession no. AY312585) as common primers among Photobacterium strains. Nested primers PPHDC5 (5′-CCA TGT GGT ATC GTG GTA GC) and PPHDC6 (5′-ACA CCA TGC GTT AAT ACC AG) were designed to prepare a probe in Southern blot hybridization by nested PCR with P. phosphoreum NBRC 13896. Genomic DNAs of P. phosphoreum NBRC 13896 and YS4-7 and P. damselae JCM 8968 were digested with the respective restriction enzyme in the multicloning site of an enforcement cloning vector, pKF3 DNA (Takara Biomedicals, Shiga, Japan). We performed hybridization to detect the smallest digestion fragment among fragments digested by each enzyme which included the hdc genes by using a previously described procedure (12). pKF3 DNA was then double digested by an enzyme detected by hybridization and a second enzyme. The digested genomic DNA fragments from the three strains were each ligated to double-digested pKF3 DNA with a DNA ligation kit, ver.2.1 (Takara Biomedicals). We amplified the hdc genes from the ligated DNAs with two primer pairs, namely, LAHDC3 and pKF3 primer R2, as described in the manufacturer's protocol, and LAHDC4 and pKF3 primer R2. We performed direct sequencing of PCR products with a BigDye terminator v1.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) as described previously (12) and an ABI PRISM 310 genetic analyzer (Applied Biosystems) to determine the complete sequences of hdc genes.
The complete sequence of the hdc gene from M. morganii JCM 1672 was determined by direct sequencing with primers MMHDC-F (5′-GCT CCG CTT CAC AGT CTT CA-3′) and MMHDC-R (5′-AGT GGA TAT TTA CCA CTG CC-3′), which were designed on the basis of upstream and downstream regions of the sequence of hdc from M. morganii ATCC 35200 (DDBJ, GenBank, and EMBL accession no. J02577). We used the sequence of the hdc gene from R. planticola ATCC 43176 already deposited in the DDBJ database (DDBJ, GenBank, and EMBL accession no. M62746) for comparison with the four other hdc genes.
DNA constructs.
We obtained PCR products including the complete sequences of the hdc genes from the five strains with the following primers. The sense primers were composed of a 20-bp sequence from the 5′ end of the hdc genes with the addition of the restriction enzyme recognition site of NcoI in place of the initiation codon, while the antisense primers were composed of a 20- or 22-bp sequence from the 3′ end with the addition of the restriction enzyme recognition site of XhoI in place of the stop codon. PCR products were inserted into pCR4-TOPO with a TOPO TA cloning kit for sequencing (Invitrogen, Carlsbad, CA). The plasmid DNAs were amplified with one-shot TOP 10 chemically competent E. coli (Invitrogen) and purified with a QIAprep spin miniprep kit (QIAGEN Inc., Valencia, CA) after confirmation of the sequences of the inserted hdc genes. The hdc genes in pCR4-TOPO plasmid DNAs were digested with NcoI and XhoI and subcloned into expression vector pET-28b(+) (Merck KGaA, Darmstadt, Germany).
Purification of recombinant HDCs.
The DNA constructs were transformed into Origami2(DE3)pLysS competent cells (Merck KGaA), and then the transformed bacteria were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37°C in LB medium according to the manufacturer's pET system manual. The induced bacteria were transferred to 1.5 ml of reaction buffer (0.1 M potassium phosphate [pH 6.5], 0.1 mM sodium EDTA, 0.01 mM PLP, 0.02 mM dithiothreitol, 0.17 g/liter hydroxypropyl cellulose) (9) and subjected to seven 30-s sonic pulses with a Bioruptor UCD-200T sonicater (Cosmo Bio, Tokyo, Japan). After the cell extracts were obtained by centrifugation and filtration with a 0.22-μm-pore-size filter, recombinant HDCs were purified from the extracts by HisTrap FF columns (GE Healthcare Bio-Sciences Co., Piscataway, NJ). After purification, elution buffer was exchanged with reaction buffer with NAP-10 columns (GE Healthcare Bio-Sciences Co.). The purified proteins were analyzed by 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by Coomassie brilliant blue staining (14).
Determination of HDC activity.
First, we assayed HDC activities under different temperature, pH, and NaCl concentration conditions. A 0.85-ml volume of reaction buffer (pH 6.5) was preincubated for 10 min at 30°C, and then 0.05 ml of HDCs was added and the mixture was preincubated further for 2 min (9). The reactions were started by adding 0.1 ml of a 200 mM solution of l-histidine monohydrochloride (Sigma-Aldrich Co., St. Louis, MO). After 30 min, the mixtures were heated in boiling water for 5 min to stop the reaction. The temperature, pH, and NaCl concentration were adjusted to range from 4 to 50°C, 4.5 to 8.5, and 0 to 10%, respectively. In addition, we also assayed activity at −20°C, wherein the mixtures was prepared on ice and stored in a freezer for a maximum of 3 months, and then the reaction was carried out at 30°C and stopped by the same procedure as for the other temperatures. Histamine levels in the mixtures were measured with a Histamarine enzyme-linked immunosorbent assay kit (Beckman Coulter Inc., Fullerton, CA) (12).
Second, we investigated the stability of HDCs at a temperature range of −20 to 40°C. Mixtures of reaction buffer with HDC and without histidine solution were stored for a maximum of 12 weeks at each temperature, and the reactions were started by adding histidine solution and stopped by boiling after 30 min. For stability at −20 and 4°C, the reaction was performed at 30°C.
Third, we investigated the accumulation of histamine by the HDCs of P. phosphoreum NBRC 13896, P. damselae JCM 8968, and R. planticola ATCC 43176 in tuna (Thunnus albacares) and dried saury (Cololabis saira) samples, since these fish contain high levels of free histidine. The samples were ground, divided into 48-g portions, and mixed with 2 ml of HDC (final concentration, 0.5 μg/g) or reaction buffer without HDC as a control. After stirring, the samples were again divided into 5-g portions and incubated for various times at −20, 4, 20, and 30°C. The reactions were stopped at different times (see Fig. 4 and 5). Additionally, for the −20°C reaction, the samples were taken from the freezer and a further reaction was performed for 2 h at 20°C. After the reaction, 40 ml of purified water was added to each sample and mixed well by shaking, and then a 1-ml portion was used for histamine detection after boiling for 5 min to stop the reaction. Histamine levels in the samples were also measured by Histamarine.
FIG. 4.
Histamine accumulation in tuna and dried saury samples by the HDCs of P. phosphoreum NBRC 13896, P. damselae JCM 8968, and R. planticola ATCC 43176 at 4, 20, and 30°C. Symbols: ○, P. phosphoreum NBRC 13896 in tuna; □, P. damselae JCM 8968 in tuna; ▵, R. planticola ATCC 43176 in tuna; ×, control in tuna; •, P. phosphoreum NBRC 13896 in dried saury; ▪, P. damselae JCM 8968 in dried saury; ▴, R. planticola ATCC 43176 in dried saury; +, control in dried saury.
FIG. 5.
Histamine accumulation in tuna and dried saury samples at −20°C and after incubation for 2 h at 20°C, followed by storage at −20°C by the HDC of P. phosphoreum NBRC 13896, P. damselae JCM 8968, and R. planticola ATCC 43176. ○, P. phosphoreum NBRC 13896 at −20°C; □, P. damselae JCM 8968 at −20°C; ▵, R. planticola ATCC 43176 at −20°C; ×, control at −20°C; •, P. phosphoreum NBRC 13896 after incubation; ▪, P. damselae JCM 8968 after incubation; ▴, R. planticola ATCC 43176 after incubation.
Nucleotide sequence accession numbers.
The hdc sequence data for P. phosphoreum NBRC 13896 and YS4-7, P. damselae JCM 8968, and M. morganii JCM 1672 have been submitted to the DDBJ database and assigned accession no. AB259287 to AB259290 (inclusive).
RESULTS
Sequencing of hdc genes.
We obtained the smallest DNA fragments containing the hdc genes when P. phosphoreum NBRC 13896, P. phosphoreum YS4-7, and P. damselae JCM 8968 were digested with XbaI, KpnI, and AvaII, respectively. We digested pKF3 DNA with XbaI and HindIII for P. phosphoreum NBRC 13896, with KpnI and SacI for P. phosphoreum YS4-7, and with AvaII and NdeI for P. damselae JCM 8968. After the DNA fragments were ligated to pKF3 DNA, we performed amplification by PCR and sequenced the PCR products. However, the hdc gene from P. phosphoreum NBRC 13896 possessed an XbaI site at position 117 and the sequence of the 5′ upstream region from an XbaI site was not identified. We therefore determined this sequence with a primer designed on the basis of the upstream region of the hdc gene from P. phosphoreum YS4-7.
The hdc genes from the two P. phosphoreum strains contained 1,137 bp and encoded 379 amino acid residues, while those from P. damselae JCM 8968, R. planticola ATCC 43176, and M. morganii JCM 1672 contained 1,131 bp and encoded 377 amino acid residues (Fig. 1). The hdc genes from the two P. phosphoreum strains showed 89% sequence identity. Nucleic acid sequence identities among the four HPB species ranged from 67% to 74%. These HDCs showed 76% to 84% homology in amino acid sequence and conservation of the lysine that PLP binds at residue 232 (23).
FIG. 1.
Amino acid sequences of HDCs of P. phosphoreum NBRC 13896 and YS4-7, P. damselae JCM 8969, R. planticola ATCC 43176, and M. morganii JCM 1672. Symbols: *, residues common to P. phosphoreum NBRC 13896; +, the lysine at residue 232 that binds PLP.
HDC activity.
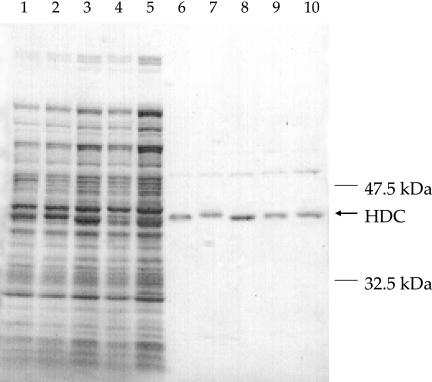
Purity of the recombinant HDCs was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 2). The HDCs of P. phosphoreum NBRC 13896 and YS4-7 had an optimum temperature of 30°C and specific activities of 143 and 144 μmol/min/mg in the reaction buffer. The respective values for P. damselae JCM 8968, R. planticola ATCC 43176, and M. morganii JCM 1672 were 40°C and 147, 136, and 147 μmol/min/mg. To determine the percent change, the specific activity under optimal conditions was taken as 100% (Table 1). In Table 1, P. phosphoreum strains maintained higher HDC activity than the others at low temperatures (4 and 20°C) but had little activity at 50°C. The HDC activities of P. damselae JCM 8968, R. planticola ATCC 43176, and M. morganii JCM 1672 increased temperature dependently up to 40°C, whereas that at 50°C was only half of that at 40°C and no activity was observed at 60°C. No HDC showed activity after 3 months at −20°C. The optimum pH of the HDCs of P. phosphoreum strains was 7.0, and activity was abruptly decreased at pH 5.5. The optimum pH for P. damselae JCM 8968 was 6.0, and activity showed a moderate change over a wide pH range (pH 4.5 to 8.5). The optimum pH for R. planticola ATCC 43176 and M. morganii JCM 1672 was 6.5, with a moderate change between 5.5 and 8.5 and an abrupt decrease at pH 4.5.
FIG. 2.
Results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis of crude and purified preparations of HDC of P. phosphoreum NBRC 13896 (lanes 1 and 6) and YS4-7 (lanes 2 and 7), P. damselae JCM 8968 (lanes 3 and 8), R. planticola ATCC 43176 (lanes 4 and 9), and M. morganii JCM 1672 (lanes 5 and 10). Lanes 1 to 5 represent crude cell extracts from induced cultures, and lanes 6 to 10 represent the purified enzymes.
TABLE 1.
Relative percentages of specific activities of HDC under different conditions of temperature, pH, and NaCl concentration
| Condition | Relative sp act (%) of HDC from:
|
||||
|---|---|---|---|---|---|
| P. phosphoreum NBRC 13896 | P. phosphoreum YS4-7 | P. damselae JCM 8968 | R. planticola ATCC 43176 | M. morganii JCM 1672 | |
| Temp (°C)a | |||||
| 5 | 16 | 16 | 9 | 10 | 10 |
| 20 | 64 | 59 | 27 | 38 | 37 |
| 30 | 100 | 100 | 78 | 78 | 90 |
| 40 | 74 | 84 | 100 | 100 | 100 |
| 50 | 4 | 5 | 55 | 49 | 56 |
| 60 | NDd | ND | ND | 2 | 1 |
| pHb | |||||
| 4.5 | ND | ND | 40 | 17 | 12 |
| 5.5 | 19 | 18 | 75 | 64 | 54 |
| 6.0 | 70 | 78 | 100 | 81 | 88 |
| 6.5 | 90 | 93 | 88 | 100 | 100 |
| 7.0 | 100 | 100 | 86 | 95 | 87 |
| 7.5 | 86 | 98 | 64 | 91 | 75 |
| 8.5 | 76 | 71 | 48 | 71 | 65 |
| NaCl concn (%)c | |||||
| 0 | 100 | 100 | 100 | 100 | 100 |
| 1 | 94 | 79 | 99 | 92 | 99 |
| 3 | 52 | 63 | 98 | 90 | 86 |
| 5 | 27 | 40 | 89 | 95 | 73 |
| 8 | 10 | 16 | 74 | 58 | 41 |
| 10 | 2 | 5 | 50 | 47 | 35 |
At pH 6.5 and 0% NaCl.
At 30°C and 0% NaCl.
At pH 6.5 and 30°C.
ND, not detected (below level of detection).
Regarding the NaCl concentration, an active NaCl concentration-dependent decline was seen for all HDCs. Activity for P. phosphoreum strains was greatly reduced at 3% NaCl. In contrast, that for P. damselae JCM 8968, R. planticola ATCC 43176, and M. morganii 1672 was relatively stable at up to 5% NaCl.
The sequential activity changes in the reaction buffer in a temperature range of −20 to 40°C were exhibited as a percentage, with the initial specific activity without storage regarded as 100% (Fig. 3). The HDCs of R. planticola ATCC 43176 and M. morganii JCM 1672 were more stable than the other HDCs in a temperature range of 4 to 40°C. Among the HDCs of Photobacterium species, that of P. damselae JCM 8968 showed the highest stability during storage at 20 and 30°C. P. phosphoreum YS4-7 was more stable than strain NBRC 13896 and P. damselae JCM 8968 during storage at 40°C. On the other hand, the activity of P. phosphoreum YS4-7 decreased most quickly with storage at 4°C. The HDCs of P. phosphoreum NBRC 13896 and P. damselae JCM 8968 maintained about half of their activity at 8 weeks of storage, while values were 6 weeks for P. phosphoreum YS4-7 and 10 weeks for R. planticola ATCC 43176 and M. morganii JCM 1672. At −20°C, there was no obvious difference in activity among the HDCs and all maintained more than 70% of their initial activity at 12 weeks of storage.
FIG. 3.
Temporal changes in the relative percentages of the specific activities of HDC at −20, 4, 20, 30, and 40°C. Symbols: •, P. phosphoreum NBRC 13896; ▪, P. phosphoreum YS4-7; ▴, P. damselae JCM 8968; ×, R. planticola ATCC 43176; +, M. morganii JCM 1672.
Accumulation of histamine by the HDCs of P. phosphoreum NBRC 13896, P. damselae JCM 8968, and R. planticola ATCC 43176 in tuna and dried saury samples at 4, 20, and 30°C is shown in Fig. 4, and that at −20°C is shown in Fig. 5. The HDC of P. phosphoreum NBRC 13896 produced more than 2,000 mg/kg histamine in tuna at 20°C. In contrast, that of P. phosphoreum NBRC 13896 did not produce much histamine in tuna at the other temperatures or in dried saury at any temperature. In particular, the accumulation of histamine produced by the HDC of P. phosphoreum NBRC 13896 was only 296 mg/kg in dried saury stored for 48 h at 4°C. The HDC of R. planticola ATCC 43176 produced less histamine than that of P. phosphoreum NBRC 13896 in tuna samples at 30°C during the initial period but more than that of P. phosphoreum NBRC 13896 after 8 h. This HDC also showed the same ability to produce histamine at 20°C as at 30°C. The HDC of P. damselae JCM 8968 displayed the highest performance, producing more than 1,000 mg/kg histamine at 4 h and more than 3,000 mg/kg at 24 h in tuna samples stored at 30°C. The accumulation of histamine by the HDCs of P. damselae JCM 8968 was retarded as the temperature decreased. At 4°C, the difference in accumulation between tuna and dried sardine markedly decreased. Accumulation was not increased during storage at −20°C. The HDCs of P. phosphoreum NBRC 13896 and R. planticola ATCC 43176 maintained 42% and 66% of the initial activity in tuna after 12 weeks but lost activity in dried saury after 6 and 8 weeks, respectively. The P. damselae HDC maintain 60% and 50% of the initial activity in tuna and dried saury for up to 12 weeks at −20°C, respectively.
DISCUSSION
A previous study reported the specific activities of the native HDCs of R. planticola and M. morganii as 142 and 150 μmol/min/mg at 37°C (9), which corresponded well to those of the present recombinant HDCs of R. planticola ATCC 43176 and M. morganii JCM 1672 (9). Further, the optimal pHs of our recombinant HDCs agreed with those of the native HDCs (9). HDCs of P. phosphoreum NBRC 13896 and YS4-7, P. damselae JCM 8968, R. planticola ATCC 43176, and M. morganii JCM 1672 had closely similar specific activities under optimum conditions in the reaction buffer used. We expected that the P. phosphoreum HDC would show higher activity at low temperatures, since P. phosphoreum is a psychrotrophic bacterium (4). In our previous study, P. phosphoreum accumulated more than 500 mg/kg histamine in A sardine sample stored for 6 h after P. phosphoreum was induced into the stationary phase at 20°C (11). Likewise, a 0.5-μg/g concentration of the recombinant HDC of P. phosphoreum NBRC 13896 accumulated more than 500 mg/kg in a tuna sample stored for 6 h at 20°C. Consequently, we considered that the 0.5-μg/g concentration of HDC was not less than the actual dose of HDC from the autolyzed cells, because this amount produced the same amount of histamine as the number of active bacteria required to cause HFP.
We previously reported that HFP in Japan is associated mainly with sashimi and fillets of tuna and marlin and that half of the HPB incidents in 2002 occurred in the cooler months of March, October, and December (10). In this previous report, P. phosphoreum was isolated from 6 of 10 fresh tuna samples but from none of 10 other fresh fish. We assumed that P. phosphoreum in fresh fish, particularly tuna, is the most noteworthy factor in recent HPB incidents. On the other hand, tuna is usually frozen during distribution and storage and P. phosphoreum in frozen-thawed fish did not show high activity owing to the bacterial injury incurred during freezing (15). These findings led us to speculate that the HDC of P. phosphoreum is the primary candidate cause of HPB incidents with frozen-thawed fish. Against this, however, the HDC of P. phosphoreum NBRC 13896 showed unstable activity, which was halved in tuna at 4 weeks and inactivated at 6 weeks in dried saury at −20°C. High sensitivity to salt appears to influence the HDC capacity of P. phosphoreum strains in dried fish products. The accumulation of histamine by the HDC of P. phosphoreum NBRC 13896 stopped at a low level because of its instability, except in tuna at 20°C. We therefore consider it unlikely that P. phosphoreum HDC alone causes HFP incidents, even in fish left at a high ambient temperature or kept in long-term storage at a low temperature.
Enteric HPB have temperature limits for growth and histamine formation that are lower than those of P. phosphoreum (2, 11). A large amount of enteric HPB was detected in fish products, not fresh fish (10), suggesting that HFP from fish products is mainly caused by enteric HPB. Here, however, HDC of R. planticola ATCC 43176 was nearly inactivated in dried saury by 8 weeks of storage at −20°C. Moreover, freezing for 4 weeks eliminated P. phosphoreum from modified-atmosphere-packed salmon (6), while it took more than 9 months for species of Enterobacteriaceae to disappear from albacore specimens during frozen storage (3). We therefore consider that frozen storage does not provide sufficient HDC from autolyzed R. planticola cells to cause HFP.
The HDC of P. damselae JCM 8968 produced 649 mg/kg histamine in tuna samples kept for 2 h at 30°C and 654 mg/kg in tuna samples kept for 4 h at 20°C and produced 505 mg/kg in dried saury kept for 4 h at 30°C and 754 mg/kg in dried saury kept for 6 h at 20°C. The Food and Drug Administration has proposed a toxicity level of histamine in tuna of 500 mg/kg (7). Here, the HDC of P. damselae JCM 8968 was markedly stable at an acidic pH and could immediately produce toxic levels of histamine in fish samples. This high ability to produce histamine is reasonable considering reports that tuna has a pH of 5.8 (5) and that other scombroid fish such as sardines and yellowtail have a pH range of 5.5 to 6.5 (18). The viability of P. damselae during frozen storage is similar to that of P. phosphoreum (8). These previous findings and the present findings suggest that P. damselae HDC is independently involved in HFP incidents in fresh fish and dried fish products.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Bartholomew, B. A., P. R. Berry, J. C. Rodhouse, and R. J. Gilbert. 1987. Scombrotoxic fish poisoning in Britain: features of over 250 suspected incidents from 1976 to 1986. Epidemiol. Infect. 99:775-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behling, A. R., and S. L. Taylor. 1982. Bacterial histamine production as a function of temperature and time of incubation. J. Food Sci. 47:1311-1317. [Google Scholar]
- 3.Ben-Gigirey, B., J. M. V. B. de Sousa, T. G. Villa, and J. Barros-Velazquez. 1998. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J. Food Prot. 61:608-615. [DOI] [PubMed] [Google Scholar]
- 4.Emborg, J., and P. Dalgaard. 2006. Formation of histamine and biogenic amines in cold-smoked tuna: an investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. J. Food Prot. 69:897-906. [DOI] [PubMed] [Google Scholar]
- 5.Emborg, J., B. G. Laursen, and P. Dalgaard. 2005. Significant histamine formation in tuna (Thunnus albacares) at 2°C—effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int. J. Food Microbiol. 101:263-279. [DOI] [PubMed] [Google Scholar]
- 6.Emborg, J., B. G. Laursen, T. Rathjen, and P. Dalgaard. 2002. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2°C. J. Appl. Microbiol. 92:790-799. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. 2001. FDA and EPA safety levels in regulations and guidance. Fish and fisheries products hazards and controls guidance, 3rd ed., appendix 5. http://www.cfsan.fda.gov/∼comm/haccp4x5.html.
- 8.Fujii, T., K. Kurihara, and M. Okuzumi. 1994. Viability and histidine decarboxylase activity of halophilic histamine-forming bacteria during frozen storage. J. Food Prot. 57:611-613. [DOI] [PubMed] [Google Scholar]
- 9.Guirard, B. M., and E. E. Snell. 1987. Purification and properties of pyridoxal-5′-phosphate-dependent histidine decarboxylases from Klebsiella planticola and Enterobacter aerogenes. J. Bacteriol. 169:3963-3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanki, M., M. Ishibashi, T. Yoda, and T. Tsukamoto. 2004. Incidence of halophilic and enteric histamine-producing bacteria in fish samples consisting mainly of scombroid fish. Jpn. J. Food Microbiol. 21:216-220. [Google Scholar]
- 11.Kanki, M., T. Yoda, M. Ishibashi, and T. Tsukamoto. 2004. Photobacterium phosphoreum caused a histamine fish poisoning incident. Int. J. Food Microbiol. 92:79-87. [DOI] [PubMed] [Google Scholar]
- 12.Kanki, M., T. Yoda, T. Tsukamoto, and T. Shibata. 2002. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella ornithinolytica strains are histamine producers. Appl. Environ. Microbiol. 68:3462-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura, B., S. Hokimoto, H. Takahashi, and T. Fujii. 2000. Photobacterium histaminum Okuzumi et al. 1994 is a later subjective synonym of Photobacterium damselae subsp. damselae (Love et al. 1981) Smith et al. 1991. Int. J. Syst. Evol. Microbiol. 50:1339-1342. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Lehane, L., and J. Olley. 2000. Histamine fish poisoning revisited. Int. J. Food Microbiol. 58:1-37. [DOI] [PubMed] [Google Scholar]
- 16.Okuzumi, M., A. Hiraishi, T. Kobayashi, and T. Fujii. 1994. Photobacterium histaminum sp. nov., a histamine-producing marine bacterium. Int. J. Syst. Bacteriol. 44:631-636. [Google Scholar]
- 17.Okuzumi, M., S. Okuda, and M. Awano. 1982. Occurrence of psychrophilic and halophilic histamine-forming bacteria (N-group bacteria) on/in red meat fish. Bull. Jpn. Soc. Sci. Fish. 48:799-804. [Google Scholar]
- 18.Simidu, W., and S. Hibiki. 1954. Studies on putrefaction of aquatic products. XIV. Comparison on putrefaction of different kinds of fish (2). Nippon Suisan Gakkaishi 20:302-304. [Google Scholar]
- 19.Takahashi, H., B. Kimura, M. Yoshikawa, and T. Fujii. 2003. Cloning and sequencing of the histidine decarboxylase genes of gram-negative, histidine-producing bacteria and their application in detection and identification of these organisms in fish. Appl. Environ. Microbiol. 69:2568-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanase, S., B. M. Guirard, and E. E. Snell. 1985. Purification and properties of a pyridoxal 5′-phosphate-dependent histidine decarboxylase from Morganella morganii AM-15. J. Biol. Chem. 260:6738-6746. [PubMed] [Google Scholar]
- 21.Taylor, S. L. 1986. Histamine food poisoning: toxicology and clinical aspects. Crit. Rev. Toxicol. 17:91-128. [DOI] [PubMed] [Google Scholar]
- 22.Taylor, S. L., J. E. Stratton, and J. A. Nordlee. 1989. Histamine poisoning (scombroid fish poisoning): an allergy-like intoxication. Clin. Toxicol. 62:225-240. [DOI] [PubMed] [Google Scholar]
- 23.Vaaler, G. L., M. A. Brasch, and E. E. Snell. 1986. Pyridoxal 5′-phosphate-dependent histidine decarboxylase. J. Biol. Chem. 261:11010-11014. [PubMed] [Google Scholar]