Abstract
In August and September 2005, Hurricanes Katrina and Rita caused breeches in the New Orleans, LA, levee system, resulting in catastrophic flooding. The city remained flooded for several weeks, leading to extraordinary mold growth in homes. To characterize the potential risks of mold exposures, we measured airborne molds and markers of molds and bacteria in New Orleans area homes. In October 2005, we collected air samples from 5 mildly water-damaged houses, 15 moderately to heavily water-damaged houses, and 11 outdoor locations. The air filters were analyzed for culturable fungi, spores, (1→3,1→6)-β-d-glucans, and endotoxins. Culturable fungi were significantly higher in the moderately/heavily water-damaged houses (geometric mean = 67,000 CFU/m3) than in the mildly water-damaged houses (geometric mean = 3,700 CFU/m3) (P = 0.02). The predominant molds found were Aspergillus niger, Penicillium spp., Trichoderma, and Paecilomyces. The indoor and outdoor geometric means for endotoxins were 22.3 endotoxin units (EU)/m3 and 10.5 EU/m3, respectively, and for (1→3,1→6)-β-d-glucans were 1.7 μg/m3 and 0.9 μg/m3, respectively. In the moderately/heavily water-damaged houses, the geometric means were 31.3 EU/m3 for endotoxins and 1.8 μg/m3 for (1→3,1→6)-β-d-glucans. Molds, endotoxins, and fungal glucans were detected in the environment after Hurricanes Katrina and Rita in New Orleans at concentrations that have been associated with health effects. The species and concentrations were different from those previously reported for non-water-damaged buildings in the southeastern United States.
On 29 August and 24 September 2005, Hurricanes Katrina and Rita, respectively, made landfall on the Gulf Coast of Louisiana. Hurricane-induced breeches in the New Orleans, LA, levee system resulted in the catastrophic flooding of approximately 80% of the city. The city remained flooded for several weeks, leading to extraordinary mold growth in homes and buildings (Fig. 1). The Centers for Disease Control and Prevention estimated that more than 100,000 homes in the flooded areas had significant mold growth (1). In early October, residents began returning to New Orleans and started cleaning and remediating their flood-damaged homes. Many returning residents did not consistently use appropriate personal protective precautions while in their moldy homes (1).
FIG. 1.
Visible mold and damage in a bedroom (A) and on the ceiling (B) of a representative house in St. Bernard's Parish on 10 October 2005.
Although no exposure standards have been established that relate health effects to specific levels of airborne molds (14), there were concerns from local and federal public health agencies regarding exposure to these remarkable environments (2). Much evidence exists indicating that indoor exposures to molds contribute to occupant respiratory disease and symptoms (6, 18, 20). Increases in mold levels have been documented in homes flooded by inches of water, even after remediation has been completed (3, 5). The data are limited, however, on the types and concentrations of bioaerosols in homes that have been flooded for weeks by several feet of water.
In October 2005, we conducted a survey to quantify levels of airborne microorganisms and indicators of microorganisms in residences with different categories of flood damage and to characterize the microflora in these unusual indoor environments.
MATERIALS AND METHODS
Residence selection.
We categorized a four-parish area (Orleans, Jefferson, Plaquemines, and St. Bernard) into three damage categories (mild, moderate, and heavy) based on Federal Emergency Management Agency flood and damage maps. Geographic information system mapping software was used to select a random number of residences proportionate for each category. The detailed description of the damage categorization, residence selection methodology, and survey results is presented elsewhere (1). We chose a subset of the 112 surveyed residences to conduct air sampling. Residences were selected for sampling based on damage category (i.e., mild, moderate, and heavy), convenience (i.e., the first 3 of the 12 residences assigned daily to each survey team were asked to participate), and accessibility (i.e., the consent of the homeowners and the ability of the industrial hygienist to enter the residence safely).
Air sampling.
We collected indoor and outdoor air samples between 22 and 27 October 2005. Indoor samples were taken from the “moldiest” room of the residence, as visually assessed by the industrial hygienist. The “moldiest” room was an issue in multilevel residences where flooding did not reach the upper floors (i.e., we sampled downstairs but not upstairs). Outdoor samples were taken from the front yards of the residences. We used 0.4-μm-pore-size, 37-mm closed-faced, pyrogen-free, polycarbonate filters housed in three-piece cassettes (SKC, Eighty Four, PA), sampling at a rate of 3 liters per minute. The sampling time ranged from 36 to 144 min. In addition, we measured air temperature and relative humidity with an HHPC-6 (ARTI, Inc., Grants Pass, OR).
Sample extraction and analysis.
The filter cassettes were transported via overnight mail to the analytical laboratory and stored at −20°C until extraction and analysis. Filters were extracted from 5-ml pyrogen-free water with 0.05% Tween 20 in endotoxin-free screw-cap borosilicate glass tubes (16 by 100 mm). Extraction entailed shaking tubes on a platform shaker at room temperature for 1 h. Four 1-ml aliquots of the mixture were placed into individual sterile polyethylene plastic cryogenic tubes, one of which was reserved and stored at −80°C for future analysis.
The filter and the remaining 1-ml aliquot in each borosilicate glass tube were analyzed for (1→3,1→6)-β-d-glucans using a newly developed method (10a). To extract glucans, 0.1 ml of 10× phosphate-buffered saline was added to the aliquot and filter, which were shaken for 1 h at room temperature, autoclaved for 1 h at 120°C, shaken for 15 min, and centrifuged at 600 × g for 20 min at 4°C. The amount of fungal glucan in the supernatant was quantified by a sandwich enzyme-linked immunosorbent assay using custom mouse anti-(1→3,1→6)-β-d-glucan monoclonal antibody as the capture antibody and rabbit antiscleroglucan polyclonal antibody as the second antibody.
One aliquot was analyzed for endotoxins by a Limulus amebocyte lysate kinetic QCL test kit according to the manufacturer's instructions (Cambrex, Walkersville, MD). Twofold serial dilutions of endotoxin standards were prepared in clean, endotoxin-free borosilicate glass tubes using pyrogen-free water containing 0.05% Tween 20. A 12-point calibration curve ranging from 0.05 to 100 endotoxin units (EU)/ml gave a correlation of 0.997 to 0.998 based on the maximum change of optical density over time (19).
One aliquot was analyzed for culturable fungi by serially diluting (full strength, 1:10 and 1:100) the aliquot in phosphate-buffered saline with 0.05% Tween 20, plating the aliquot onto replicate 1.275% malt extract agar (MEA) plates with chloramphenicol (Remel Inc., Lenexa, KS), and incubating one replicate plate at room temperature (approximately 20°C) on a bench top and the other at 37°C in an incubator. The plates were incubated until colonies could be enumerated, analyzed under microscopy, and identified to genus (approximately 7 days). Members of the genus Aspergillus isolated on MEA were identified to species while Penicillium colonies were isolated, subcultured onto MEA (incubated at room temperature) and Czapek Dox yeast agar (incubated at room temperature, 5°C, and 37°C), and identified to species according to Pitt (13).
One aliquot was analyzed for fungal spores (enumeration and genus/taxon identification) by fixing with formaldehyde (adding 57 μl of 37% formaldehyde to a 1-ml aliquot for a 2% solution), filtering the solution onto a mixed cellulose ester filter, clearing the filter with triacetin (Sigma-Aldrich, St. Louis, MO), and staining with aniline blue in lactic acid. Approximately 50 random fields per filter were examined under direct microscopy at ×400 magnification.
Data analysis.
Statistical analyses were conducted using JMP (version 5.01) and SAS (version 9.12) software packages (SAS Institute, Cary, NC). For culture analysis, one sample below the limit of detection was assigned a value of 0.5 CFU. Overgrown plates (three samples) were assigned a value of the maximum number of colonies counted on any one plate plus 1 (i.e., 63 CFU).
We expressed concentrations in terms of geometric mean and geometric standard deviation. For statistical analyses, we grouped together the residences in the moderately and heavily water-damaged areas since levels in the moderately water-damaged houses were within the range of levels found in heavily water-damaged houses. Since the data were log-normally distributed (skewed to the right), we used nonparametric statistical tests. We calculated Spearman's ρ correlation coefficients to compare the environmental measurements. We calculated indoor-to-outdoor ratios by dividing the indoor concentration by the outdoor concentration. We performed a one-sided Wilcoxon two-sample exact test (PROC NPAR1WAY) to assess differences between damage categories and between indoor and outdoor concentrations.
RESULTS
We sampled inside 20 of the 112 residences that participated in the survey and outdoors at 11 of these 20 residences approximately 2 months after the initial levee breach after Hurricane Katrina. Of the 20 residences sampled, 5 were in mildly, 2 were in moderately, and 13 were in heavily water-damaged areas. The average temperature and relative humidity inside the residences were 23.2°C and 45%, while those outside were 22.7°C and 40%.
All measures of microorganisms were higher in the moderately/heavily water-damaged residences (n = 15) than in the mildly water-damaged residences (n = 5); only culturable fungi were significantly different (Table 1). The concentrations of spores and culturable fungi incubated at room temperature and at 37°C were strongly correlated (ρ values from 0.74 to 0.88, with P values of <0.001). The concentration of glucans was significantly correlated with that of spores (ρ = 0.48, P = 0.006) and weakly correlated with that of culturable fungi incubated at room temperature and 37°C (ρ = 0.35 [P = 0.06] and ρ = 0.28 [P = 0.12], respectively).
TABLE 1.
Concentrations of airborne mold and microbial constituents by damage categories and by indoor/outdoor samples
| Sampling site (n) | Endotoxin
|
Glucansa
|
Spores
|
Cultured fungi at:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Geometric mean concn, EU/m3 (GSDc) | P valueb | Geometric mean concn, μg/m3 (GSD) | P value | Geometric mean concn, no./m3 (GSD) | P value | Room temp
|
4°C
|
|||
| Geometric mean concn, CFU/m3 (GSD) | P value | Geometric mean concn, CFU/m3 (GSD) | P value | |||||||
| All houses | 23.3 (5.6) | NAd | 1.6 (4.4) | NA | 280,000 (13.7) | NA | 32,700 (13.1) | NA | 7,300 (12.7) | NA |
| By damage category | ||||||||||
| Mild (5) | 9.7 (2.0) | 1.1 (5.3) | 60,000 (5.8) | 3,700 (9.1) | 630 (3.1) | |||||
| Moderate/heavy (15) | 31.3 (6.5) | 0.08 | 1.8 (4.3) | 0.28 | 460,000 (14.9) | 0.10 | 67,000 (10.1) | 0.02 | 16,000 (10.5) | 0.002 |
| By paired indoor/outdoor | ||||||||||
| Indoor (11) | 22.3 (6.0) | 1.7 (4.6) | 356,000 (18.3) | 6,950 (15.6) | 38,000 (14.2) | |||||
| Outdoor (11) | 10.5 (2.5) | 0.12 | 0.9 (2.0) | 0.3 | 43,000 (5.0) | 0.03 | 980 (3.7) | 0.02 | 4,700 (2.8) | 0.03 |
(1→3,1→6)-β-d-Glucans.
P values were assessed by the nonparametric Wilcoxon two-sample exact test for pairwise comparisons.
GSD, geometric standard deviation.
NA, not applicable.
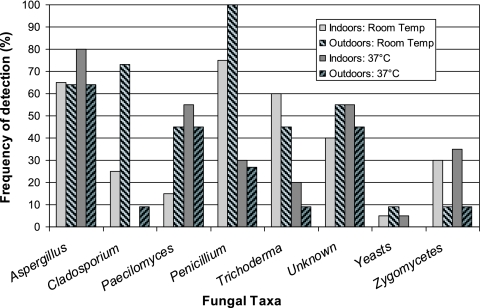
Penicillium and Aspergillus were the most commonly cultured fungal genera found indoors. We identified six Aspergillus species (A. niger, A. versicolor, A. flavus, A. terreus, A. ustus, and A. fumigatus). In the outdoor samples, we also detected Eurotium species (i.e., the A. glaucus group). A. niger was the predominant indoor Aspergillus species, while A. versicolor was the most predominant outdoor species. For samples incubated at 37°C, we detected A. fumigatus in 5 out of 20 indoor samples and 4 out of 11 outdoor samples. We isolated 68 Penicillium colonies and were able to identify 12 species. Many of the isolates (33 of 68) were in the Biverticillium subgenus, with P. variabile and P. purpurogenum being the most frequent and abundant. P. oxalicum was found in the most locations, although typically in low concentrations. Penicillium, Paecilomyces, and Cladosporium were more frequently outdoors than indoors in samples incubated at room temperature, while zygomycetes were more frequently found indoors regardless of incubation temperature (Fig. 2). The thermotolerant zygomycetes were Syncephalastrum species. For fungal spores, we identified 19 different taxa indoors and 18 outdoors (Table 2).
FIG. 2.
Frequency of cultured fungal taxa detected indoors (n = 20) and outdoors (n = 11) and incubated at room temperature (Temp) and 37°C. Curvularia is not shown in the graph since it was detected only once in an outdoor sample.
TABLE 2.
Frequencies of fungal spore taxa detecteda
| Taxon | Frequency (%) in:
|
|
|---|---|---|
| Indoor samples (n = 20) | Outdoor samples (n = 11) | |
| Penicillium/Aspergillus/Paecilomyces-likeb | 100 | 100 |
| Unidentifiable | 100 | 91 |
| Aspergillus niger | 95 | 55 |
| Cladosporium | 80 | 100 |
| Basidiospores | 65 | 64 |
| Chaetomium | 50 | 45 |
| Ascospores | 35 | 27 |
| Zygomycetes | 35 | 0 |
| Stachybotrys | 25 | 27 |
| Trichoderma | 25 | 0 |
| Alternaria | 20 | 9 |
| Curvularia | 20 | 45 |
| Smut | 20 | 64 |
| Scopulariopsis | 15 | 0 |
| Epicoccum nigrum | 10 | 0 |
| Memnoniella | 10 | 0 |
| Torula | 10 | 9 |
| Beltrania | 5 | 0 |
| Cercospora | 5 | 9 |
| Drechslera/Bipolaris | 0 | 27 |
| Fusarium | 0 | 9 |
| Nigrospora | 0 | 9 |
| Rust | 0 | 9 |
| Spegazzinia | 0 | 9 |
Identified by direct microscopy. The frequency value indicates the percentage of samples with at least one spore of a given fungal taxon.
Spores of these taxa were grouped since they are indistinguishable from one another, except for Aspergillus niger, which is listed separately.
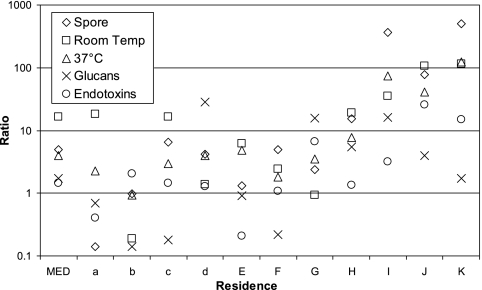
We collected both indoor and outdoor measurements for 11 residences: 4 in mildly water-damaged areas and 7 in moderately/heavily water-damaged areas. The majority of indoor/outdoor ratios were greater than unity even in the mildly water-damaged residences (Fig. 3). The geometric means of all the measured analytes were higher indoors than outdoors, with only culturable fungi and fungal spores being statistically significantly different (Table 1). The indoor and outdoor concentrations were significantly and strongly correlated for fungi cultured at 37°C (ρ = 0.89, P = 0.0002), spores (ρ = 0.85, P = 0.001), and endotoxins (ρ = 0.61, P = 0.047).
FIG. 3.
Indoor-to-outdoor concentration ratios. Residences a, b, c, and d were in the mildly water-damaged areas, while the other residences (in uppercase) were in the moderately to heavily water-damaged areas. The medians (MED) are of the 11 residences. Temp, temperature.
DISCUSSION
The concentrations of microorganisms were higher in the areas that were moderately to heavily water damaged following Hurricanes Katrina and Rita than in the areas that were mildly water damaged. There was large variability in the concentrations, even within the mildly water-damaged areas. The concentrations in the more severely damaged houses were at least an order of magnitude higher than previously reported for non-water-damaged buildings in other subtropical geographical areas of the United States (8, 17). Many of the isolated Penicillium species were in the subgenus Biverticillium, which includes mostly hydrophilic or weakly xerophilic species. These taxa are not typical of those in normal indoor environments but probably reflect the extremely wet conditions in these homes.
Endotoxins are constituents of gram-negative bacterial cell membranes that have inflammatory and toxic properties (19). The concentrations of airborne endotoxins in indoor environments tend to be low (usually less than 2 EU/m3) and similar to outdoor levels while often exceeding 45 EU/m3 in occupational and agricultural settings (9, 12, 16, 21). Five of the residences that we sampled, all in the moderately to heavily water-damaged areas, had endotoxin concentrations greater than 45 EU/m3.
Glucans are a component of fungal cell walls and have been used as an indicator of total fungal biomass (4, 15). We found that glucan levels correlated significantly with airborne spore counts but not culturable fungal levels, possibly because measures of culturable fungi underestimate the airborne fungal load (10). In addition, different fungal species have different concentrations of (1→3,1→6)-β-d-glucans (11).
Indoor and outdoor concentrations of molds were correlated. In non-water-damaged indoor environments, the outdoor air is usually the major source of indoor airborne microorganisms (17). New Orleans was experiencing relatively dry and mild weather in late October 2005 compared to the weather in September 2005, when temperature and relative humidity averaged 28.4°C and ∼80%, respectively (http://www.noaa.gov). Many of the residences in all areas had their doors and windows open during the sampling period. The outdoor environment in the flooded areas had considerable residual organic debris from the flooding (e.g., mud, sewage, and marsh grass). In addition, homeowners were beginning to clean out their residences, leaving water-damaged household goods and furnishings curbside. Thus, in the flooded areas of New Orleans, we do not know whether the outdoor environment contributed to indoor concentrations or vice versa, considering that the outdoor environment was also extensively contaminated. Nonetheless, we found differences between indoor and outdoor concentrations and detected fungal taxa (Tables 1 and 2) which indicate that the indoor environments were contaminated with fungi different from those found outdoors.
Our study is subject to several limitations. We sampled approximately 2 months after Hurricane Katrina and 1 month after Hurricane Rita, both of which caused widespread flooding in New Orleans due to levee breaches. The city had been dewatered between the two hurricanes. We do not know the effect of repeated flooding on the composition of the microflora in this environment. Also, our sampling captured the fungal genera/taxa only at a single time point and under a single environmental condition. Molds with different water activity requirements may grow at different rates depending upon the water available in the building materials (7). For example, we might expect to see more hydrophilic molds immediately after dewatering while more xerotolerant molds might be detected as the building materials dry out.
The Federal Emergency Management Agency damage categories were based on general area estimates on the level of flooding rather than on actual observed flooding and damage levels for each individual house that was sampled. Misclassification of damage levels could have occurred. In addition, the residences in the moderately/heavily damaged areas were in different stages of remediation during our sampling, including no activity, cleaning activity, active demolition/remediation, and recent renovation. These factors may account for the large variability in the concentrations we found within the categories.
We selected houses to sample based on whether someone was home, convenience, and assent of the resident. In these homes, sampling location selection was biased towards the worst-case exposure scenario since we selected the moldiest rooms to sample. These sampled rooms may not represent where the residents spent the greater part of their time when inside their homes. The majority of residents in the moderately to heavily water-damaged areas, however, were not currently living in their homes. We did not sample in hazardous geographical areas, such as the Ninth Ward, where residents were not allowed reentry. Therefore, our sampling results may not be representative of the worst-hit areas, nor of the homes of residents without the means to return to New Orleans.
Several caveats should be considered when our results are compared to those of other studies. We used long-term filter sampling since airborne fungi have high temporal variability and traditional short-term “grab” samples (i.e., less than 5 min) do not characterize well airborne fungi over the course of the day (10). In addition, differences in analytical methods may affect comparability across studies. We washed the filters which may not have been efficient in removing the fungal spores from the filter (possibly leading to underestimation of counts) but which would have broken up any collected spore clusters (possibly leading to overestimation of spore counts in comparison to direct impaction methods). In addition, since spore clusters often aid in taxon identification, breaking up clusters may have affected the reported fungal spore taxon distribution. Although this may affect comparisons with previously published studies using “grab” samples, this would not affect our intrastudy comparisons. In addition, we used several different analytical methods to quantify and evaluate airborne microorganisms, which better characterizes the microbial ecology in these environments.
We had some indication that the indoor microflora was different after the hurricanes. For example, we found thermotolerant Syncephalastrum, a zygomycete rarely isolated in air samples, in 35% of our indoor air samples. However, the question of whether the composition of microflora in New Orleans and inside these flooded buildings was significantly altered by the hurricanes or the subsequent flooding remains unanswered. The data are limited regarding microbial air spora in New Orleans residences prior to the hurricanes. Nevertheless, we found high concentrations of molds, glucans, and endotoxins in New Orleans after Hurricanes Katrina and Rita. The health effects from exposure to these highly contaminated environments are still undetermined.
Acknowledgments
This work was done as part of routine public health investigations conducted by the Centers for Disease Control and Prevention. In addition to CDC funding, G. L. Chew (culturable fungal analysis), M. L. Muilenberg (spore analysis and fungal species identification), and P. S. Thorne (endotoxin and glucan analysis) were partially supported by NIEHS (P30ES05605, P30ES00000243, and P30ES009089).
We are indebted to Kristin Cummings (EIS officer, CDC) and Raoul Ratard (Louisiana Department of Health and Hospitals) for supporting the investigation, to the Office of the Inspector General of the U.S. Department of Health and Human Services for ensuring the safety of the sampling teams, and to Pamela Failing, Dewayne Knott, and Christopher Montoya (Air National Guard) for assisting with field sampling.
The use of product names does not imply their endorsement by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2006. Health concerns associated with mold in water-damaged homes after Hurricanes Katrina and Rita—New Orleans area, Louisiana, October 2005. Morb. Mortal. Wkly. Rep. 55:41-44. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2006. Mold prevention strategies and possible health effects in the aftermath of hurricanes and major floods. Morb. Mortal. Wkly. Rep. 55(RR-8):1-27. [PubMed] [Google Scholar]
- 3.Curtis, L., M. Ross, V. Persky, P. Scheff, R. Wadden, V. Ramakrisnan, and D. Hryhorczuk. 2000. Bioaerosol concentrations in the quad cities 1 year after the 1993 Mississippi river floods. Indoor Built Environ. 9:35-43. [Google Scholar]
- 4.Douwes, J., G. Doekes, R. Montijn, D. Heederik, and B. Brunekreef. 1996. Measurement of β(1→3)-glucans in occupational and home environments with an inhibition enzyme immunoassay. Appl. Environ. Microbiol. 62:3176-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabian, M. P., S. L. Miller, T. Reponen, and M. T. Hernandez. 2005. Ambient bioaerosol indices for indoor air quality assessments of flood reclamation. J. Aerosol Sci. 36:763-783. [Google Scholar]
- 6.Gent, J. F., P. Ren, K. Belanger, E. Triche, M. B. Bracken, T. R. Holford, and B. P. Leaderer. 2002. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 110:A781-A786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant, C., C. A. Hunter, B. Flannigan, and A. F. Bravery. 1989. The moisture requirements of moulds isolated from domestic dwellings. Int. Biodeterior. 25:259-284. [Google Scholar]
- 8.Horner, W. E., A. G. Worthan, and P. R. Morey. 2004. Air- and dustborne mycoflora in houses free of water damage and fungal growth. Appl. Environ. Microbiol. 70:6394-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs, R. R. 1997. Endotoxins in the environment. Int. J. Occup. Med. Environ. Health 3:S3-S5. [Google Scholar]
- 10.Martinez, K. F., C. Y. Rao, and N. C. Burton. 2004. Exposure assessment and analysis for biological agents. Grana 43:193-208. [Google Scholar]
- 10a.Metwali, N., P. S. Thorne, K. Kolegraf, and M. E. O'Neill. 2005. Abstr. 100th Am. Thorac. Soc. Int. Conf., abstr. C94.
- 11.Milton, D. K., K. U. Alwis, L. Fisette, and M. Muilenberg. 2001. Enzyme-linked immunosorbent assay specific for (1→6) branched, (1→3)-β-d-glucan detection in environmental samples. Appl. Environ. Microbiol. 67:5420-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olenchock, S. A., D. C. Christiani, J. C. Mull, T. T. Ye, and P. L. Lu. 1990. Airborne endotoxin concentrations in various work areas within two cotton textile mills in the People's Republic of China. Biomed. Environ. Sci. 3:443-451. [PubMed] [Google Scholar]
- 13.Pitt, J. 1979. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, New York, NY.
- 14.Rao, C. Y., H. A. Burge, and J. C. Chang. 1996. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 46:899-908. [DOI] [PubMed] [Google Scholar]
- 15.Rao, C. Y., J. M. Cox-Ganser, G. L. Chew, G. Doekes, and S. White. 2005. Use of surrogate markers of biological agents in air and settled dust samples to evaluate a water-damaged hospital. Indoor Air 15:89-97. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds, S. J., D. W. Black, S. S. Borin, G. Breuer, L. F. Burmeister, L. J. Fuortes, T. F. Smith, M. A. Stein, P. Subramanian, P. S. Thorne, and P. Whitten. 2001. Indoor environmental quality in six commercial office buildings in the midwest United States. Appl. Occup. Environ. Hyg. 16:1065-1077. [DOI] [PubMed] [Google Scholar]
- 17.Shelton, B. G., K. H. Kirkland, W. D. Flanders, and G. K. Morris. 2002. Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68:1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorn, J., and R. Rylander. 1998. Airways inflammation and glucan in a rowhouse area. Am. J. Respir. Crit. Care Med. 157:1798-1803. [DOI] [PubMed] [Google Scholar]
- 19.Thorne, P. S. 2000. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology 152:13-23. [DOI] [PubMed] [Google Scholar]
- 20.Verhoeff, A. P., and H. A. Burge. 1997. Health risk assessment of fungi in home environments. Ann. Allergy Asthma Immunol. 78:544-554. [DOI] [PubMed] [Google Scholar]
- 21.Wan, G. H., and C. S. Li. 1999. Indoor endotoxin and glucan in association with airway inflammation and systemic symptoms. Arch. Environ. Health 54:172-179. [DOI] [PubMed] [Google Scholar]