Abstract
Biofilm formation by Bacillus cereus was assessed using 56 strains of B. cereus, including the two sequenced strains, ATCC 14579 and ATCC 10987. Biofilm production in microtiter plates was found to be strongly dependent on incubation time, temperature, and medium, as well as the strain used, with some strains showing biofilm formation within 24 h and subsequent dispersion within the next 24 h. A selection of strains was used for quantitative analysis of biofilm formation on stainless steel coupons. Thick biofilms of B. cereus developed at the air-liquid interface, while the amount of biofilm formed was much lower in submerged systems. This suggests that B. cereus biofilms may develop particularly in industrial storage and piping systems that are partly filled during operation or where residual liquid has remained after a production cycle. Moreover, depending on the strain and culture conditions, spores constituted up to 90% of the total biofilm counts. This indicates that B. cereus biofilms can act as a nidus for spore formation and subsequently can release their spores into food production environments.
Bacillus cereus is a gram-positive, spore-forming bacterium. It causes two types of food poisoning: the diarrheal type by enterotoxin production in the small intestine and the emetic type by toxin which is formed in food (18). B. cereus is also implicated in food spoilage by vegetative growth, especially in dairy products (1). Since B. cereus is ubiquitously present in nature, it is easily spread through food production systems, and contamination with B. cereus is almost inevitable. B. cereus spores are both highly resistant to a large number of stresses and very hydrophobic, which causes them to adhere easily to food processing equipment (9, 43). Besides the above-mentioned problems, B. cereus can also cause problems in food industry because of its capacity to form biofilms on several substrata (2, 26, 28, 34). B. cereus has been found to account for 12.4% of the constitutive microflora, growing in biofilms, in a commercial dairy plant (36).
A biofilm is a multicellular complex, formed of microorganisms that are attached to a surface and embedded in a matrix, consisting of exopolymeric substances (EPS). Cells within a biofilm are surrounded by the EPS matrix and cells in the outer layers of the biofilm, protecting them from harsh influences from the environment, thereby making them more resistant to cleaning agents and other antimicrobial substances (28). In addition to the role of the matrix, the low growth rate of cells and biofilm-specific gene expression patterns can also increase the resistance of biofilm cells (38). Biofilm formation can therefore cause economic loss by equipment failure and shorten the time between cleaning. Contamination of products via biofilm cells can cause subsequent limitation of shelf life and raise safety concerns (10, 23).
Biofilm formation occurs in distinct phases (12, 38, 42). First, cells come into contact with a surface, after which they will be irreversibly bound to that surface. The attached cells will produce EPS and multiply, thereby forming a so-called microcolony. From this microcolony, a mature biofilm can arise. Within this biofilm, cells can be physiologically differentiated due to the wide range of conditions they encounter, since diffusion of nutrients and oxygen is limited within a biofilm (12, 38, 42). In the final stage of biofilm formation, cells may also detach from a biofilm. This can occur in a passive way because of extrinsic factors, such as shear, sloughing, and erosion (33); changes in nutrient availability (35); or a sudden stop of flow (40). Cellular detachment from a biofilm can also be caused by mechanisms employed by the biofilm cells themselves. In Pseudomonas aeruginosa, production of rhamnolipids has been shown to cause detachment of cells (3). For Shewanella oneidensis and other bacteria, the level of cyclic di-GMP regulates attachment and detachment of cells from a biofilm (31, 39).
It has been suggested by Fux et al. (11) that no bacterial strain can represent the whole species, because of the extraordinary plasticity of bacterial genomes. Studies of different isolates of Listeria species have shown that within this species there is a lot of variation in phenotypic properties, including biofilm formation (4, 24). A comparison of the genomes of B. cereus ATCC 14579 and ATCC 10987 indicates that there are many differences between these strains (30). Features that could be important for biofilm formation include differences in genes for polysaccharide capsule synthesis and genes possibly involved in glycosylation of flagella and flagellins (30). Besides the genomes of ATCC 14579 and ATCC 10987 (17, 30), more B. cereus genomes are available (14, 15). Comparison of these strains shows even more differences (14, 29), emphasizing the importance of including several strains in this research. Research performed so far on biofilm formation by B. cereus has always focused on a single strain and did not take diversity among strains into account (26, 28). Gaining more knowledge on diversity within B. cereus biofilms is important to improve prediction and prevention of biofilm formation and can be used to set up representative test panels for sanitation procedures.
Using various assays and culture conditions and a range of strains, we have studied the roles of strain diversity and environmental conditions in biofilm formation by B. cereus. A selection of strains was used to gather quantitative data on biofilm formation, using assays that allowed for development of submerged and/or air-liquid interface biofilms. Substantial differences in biofilm formation using these two assays were found and revealed B. cereus to form biofilms preferentially at air-liquid interfaces.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. cereus strains from various origins, including clinical and food isolates, were used in this study. Strains used are listed in Table 1. Stock cultures were stored at −80°C in 20% glycerol. Working cultures were maintained on brain heart infusion (Becton Dickinson, France) plates. Broth cultures were inoculated from single colonies and incubated overnight. Media used for cultures were Luria-Bertani broth (LB), and Y1, a defined medium for B. cereus (7), which contained the following components (final concentrations): d-glucose (10 mM), l-glutamic acid (20 mM), l-leucine (6 mM), l-valine (2.6 mM), l-threonine (1.4 mM), l-methionine (0.47 mM), l-histidine (0.32 mM), sodium-dl-lactate (5 mM), acetic acid (1 mM), FeCl3 (50 μM), CuCl2 (2.5 μM), ZnCl2 (12.5 μM), MnSO4 (66 μM), MgCl2 (1 mM), (NH4)2SO4 (5 mM), Na2MoO4 (2.5 μM), CoCl2 (2.5 μM), and Ca(NO3)2 (1 mM). The medium was buffered at pH 7.4 with 100 mM potassium phosphate buffer.
TABLE 1.
Overview of biofilm-forming capacities of all 56 B. cereus strains tested using the microtiter plate assay
| B. cereus straina | Biofilm-forming capacityb of strain under the following conditions:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 30°C
|
20°C
|
|||||||
| LB
|
Y1
|
LB
|
Y1
|
|||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| B434 | − | − | + | + | − | − | + | + |
| B435 | − | − | + | − | − | − | + | + |
| B436† | − | − | + | + | − | − | + | + |
| B437 | − | − | + | + | − | − | + | + |
| B439 | − | + | + | + | − | − | + | + |
| B443† | − | − | − | − | − | − | + | − |
| 75-95 | − | + | + | + | − | − | + | + |
| 391-98 | − | + | − | − | − | − | − | − |
| ATCC 14579 | − | − | + | − | − | − | + | − |
| PAL2 | − | − | + | + | − | − | + | + |
| PAL3† | − | − | + | − | − | + | + | − |
| PAL5 | − | − | + | − | − | − | + | − |
| PAL7† | + | − | + | + | − | − | + | + |
| PAL17† | + | − | + | + | − | − | + | + |
| PAL18† | − | − | + | + | − | + | + | − |
| PAL20† | − | − | − | + | − | + | + | − |
| PAL22† | + | + | + | + | − | + | + | + |
| PAL25† | + | + | + | − | − | − | + | − |
| PAL26 | − | − | + | − | − | − | + | − |
| PAL27 | − | + | + | − | − | − | + | + |
| PAL28 | − | + | + | + | − | − | + | + |
| 17† | − | − | + | + | − | − | + | + |
| 55† | − | − | + | − | − | − | + | + |
| 59† | − | − | + | + | − | − | + | + |
| 61† | − | − | + | − | − | − | + | + |
| 72† | − | − | + | + | − | − | + | + |
| 132† | − | − | + | + | − | − | − | − |
| 43-92† | − | − | − | − | − | − | + | − |
| 401-92† | − | − | + | + | − | − | + | + |
| 453-92† | − | − | + | − | + | − | + | − |
| 67-498† | − | − | + | + | − | − | + | + |
| 1230-88 | − | − | + | + | − | − | + | + |
| F450183† | − | − | + | + | − | − | − | + |
| DSM11821† | − | + | + | + | − | − | − | + |
| KW85 | − | − | + | + | − | − | + | + |
| VC7401 | − | + | + | + | − | − | − | + |
| F5581 | + | + | + | + | − | − | − | + |
| TZ415 | − | − | + | + | − | − | − | + |
| TZ426 | − | − | + | + | − | − | + | + |
| TZ427 | − | − | + | + | − | − | + | + |
| TZ428 | − | − | + | + | − | − | + | + |
| Z4234 | − | − | + | + | − | − | + | + |
| P215 | − | − | + | − | − | − | + | + |
| Z421 | + | + | + | + | − | − | + | + |
| F3752A/86 | + | − | + | + | − | − | + | + |
| B6/Ac | − | − | + | − | − | − | + | + |
| F2797/87 | + | − | + | + | − | − | + | + |
| F3351/87 | − | − | + | + | − | − | + | + |
| F3748/75 | + | + | + | + | − | − | + | + |
| F4635A/90 | − | − | − | − | − | − | − | − |
| F4632/90 | − | − | + | − | − | − | + | + |
| F4628/90 | − | − | + | − | − | − | + | + |
| F4626/90 | − | − | − | − | − | − | + | − |
| F4620/90 | − | − | + | + | − | − | + | + |
| F4623/90 | − | − | + | − | − | − | + | + |
| ATCC 10987 | − | + | + | + | − | − | + | + |
| Total no. of positive strains | 9 | 13 | 50 | 36 | 1 | 4 | 48 | 42 |
The selection of 10 strains used in quantitative and stainless steel experiments are shown in bold type. Psychrotolerant (growth at temperatures of ≤8°C) B. cereus strains are indicated by daggers.
Symbols: −, does not form a biofilm; +, forms a biofilm.
Spore suspensions were produced as described previously (6) and obtained from Y. P. de Vries. Prior to use, spores were washed one time in 10 mM potassium phosphate buffer (pH 7.4) with 0.1% Tween 80. Spores were subsequently resuspended in 1 mM potassium phosphate buffer (pH 7.4) with 0.1% Tween 80 to a concentration of 109 spores per ml and heated for 10 min at 80°C.
Microtiter plate biofilm formation assay.
Biofilm formation on polystyrene was measured as described previously (8) with some modifications. In short, polystyrene microtiter plates (Greiner Bio-one, Germany) were filled with 200 μl LB or Y1 medium, and for each strain, six wells were inoculated with 1.5% (vol/vol) overnight cultures, grown in LB or Y1, respectively. The plates were incubated at 20 and 30°C. When plates were incubated for a total of 48 h, the medium was removed after 24 h by gentle pipetting, and fresh medium was added to support biofilm development with new nutrients. After incubation for either 24 or 48 h, wells were gently washed three times with 200 μl of phosphate-buffered saline, and subsequently biofilm cells were stained with 200 μl of 0.1% (wt/vol) crystal violet for 30 min. After these 30 min, the wells were washed twice with 200 μl sterile deionized water to remove unbound crystal violet. The remaining crystal violet was dissolved in 200 μl 96% ethanol, and the absorbance was measured at 595 nm (μQuant, Bio-Tek Instruments). The maximum absorbance that could be read was 3.0. Strains with optical density at 595 nm (OD595) values of 0.4, which is about two times the background signal, or higher were considered positive for biofilm formation. The average biofilm formation and standard deviation for six wells were calculated for each strain in the conditions tested.
Preparation of stainless steel coupons.
Stainless steel type 304L (Goodfellow, United Kingdom) was used to prepare coupons with a size of 10 by 30 by 0.5 mm. The coupons were soaked for 15 min at 50°C in tubes with a 2% RBS 35 detergent solution (Fluka, Switzerland) and then rinsed five times with deionized water, autoclaved, and dried overnight at 50°C. Fouled coupons were obtained by submerging them in low-fat, ultrahigh-temperature-sterilized milk for 1 h at 10°C and subsequently rinsing them in deionized water before immediate use.
Adhesion of spores to stainless steel coupons.
Adhesion of spores to stainless steel was performed by adding 100 μl spore suspension (109 spores per ml) to polystyrene tubes containing a stainless steel coupon in 10 ml 0.85% NaCl. The tubes were incubated for 1 h at room temperature or at 10°C in a test tube rotator (model LD-79; Labinco) at 12 rpm. The coupons were taken out of the tube and rinsed three times in tubes with 10 ml deionized water. The coupons were then used for either the enumeration of spores or for subsequent biofilm formation.
To determine the number of adhering spores, the stainless steel coupons were transferred to tubes with 10 mM phosphate buffer (pH 7.4) with 0.5% Tween 80 and subjected to ultrasonication for 5 min at room temperature in an ultrasonic water bath (40 to 60 KHz). The coupons were immediately removed from the tube, and the tubes were put on ice. Dilutions were made using 10 mM phosphate buffer (pH 7.4) containing 0.1% Tween 80. Aliquots were plated in duplicate on 20% nutrient broth (NB) (Oxoid, United Kingdom) plates to determine the number of adherent spores. Agar plates were incubated for 24 to 48 h at 30°C. Adhesion of spores was tested in triplicate for each strain.
Biofilm formation on submerged stainless steel coupons.
The coupons with adhered spores were placed in tubes with LB and incubated for 48 h at 30°C or for 96 h at 10°C in a test-tube rotator at 12 rpm. The medium was refreshed after 24 or 48 h, respectively. After incubation, coupons were taken out of the tube and rinsed three times in tubes with 10 ml sterile deionized water. The coupons were transferred to tubes with peptone physiological salt (PPS) with 0.5% Tween 80, and the cells were rinsed off with a cotton swab for 1 min at room temperature. The coupons were immediately removed from the tube, and the tubes were put on ice. Dilutions were made using PPS. Aliquots were plated in duplicate on 20% NB plates to determine the number of cells present in the biofilm. Agar plates were incubated for 24 to 48 h at 30°C. Biofilm formation was tested in triplicate for each strain.
Air-liquid interface biofilm formation.
Stainless steel coupons were bent into a U shape and placed in the wells of a polystyrene 24-well plate (Falcon, Becton Dickinson, France). The wells were half filled with 1.5 ml LB medium, inoculated with 1.5% (vol/vol) of overnight culture of B. cereus, and incubated for 48 h at 30°C. The medium was refreshed after 24 h of incubation. The biofilm formed was visualized by staining the biofilm cells with crystal violet, as described for the microtiter plate assay. The amount of biofilm on the coupons was quantified as described in the previous paragraph. Biofilm formation was tested in triplicate for each strain.
Polystyrene 24-well plates were filled with 2 ml LB or Y1 medium, inoculated with 1.5% (vol/vol) of overnight culture of B. cereus, and incubated for 24 or 48 h at 30°C. The surface area of the wells was 7.7 cm2. In the 48-h incubations, the medium was refreshed after 24 h of incubation. Samples from the suspension phase were taken, after which the well was washed with phosphate-buffered saline. After the addition of 2 ml PPS with 0.5% Tween 80, biofilm cells were removed from the surface by swabbing the well with a cotton swab for 1 min. Spore counts were obtained by heating the samples for 10 min at 80°C. Total CFU and spore counts of suspension and biofilm were determined by plating on 20% NB plates. Agar plates were incubated for 24 to 48 h at 30°C. Sporulation in biofilm and suspension was determined in duplicate for each strain tested.
Statistical analysis.
To determine whether adhesion of spores or biofilm formation was significantly higher for one condition compared to another condition, a one-sided t test was used for the replicate values, using a P of 5% for statistical significance.
RESULTS
Biofilm formation and sporulation in microtiter plates.
To obtain an overview of the biofilm-forming capacities of the B. cereus species, the capacity of 56 strains to form biofilms was tested using a microtiter plate assay. In this screening, the effect of medium composition, temperature, and incubation time was investigated. While all strains displayed similar growth performances in the culture conditions chosen (data not shown), the degree of biofilm formation varied considerably. In Table 1, an overview of the biofilm-forming capacities for all strains under the conditions tested is shown. In the microtiter plate assays, biofilm formation took place preferentially at the side of the well, at the point of the interface between the liquid and air, as can be seen in Fig. 1A. In LB medium after 24 h, only one of the 56 strains formed a biofilm at 20°C and nine strains formed a biofilm at 30°C, which had dispersed again after 48 h for one and four strains, respectively. After 48 h of incubation, four additional strains formed biofilms at 20°C and eight additional strains formed biofilms at 30°C that did not form a biofilm after 24 h. The five strains that formed biofilms at 20°C in LB all belong to the psychrotolerant group of B. cereus strains. In total, 21 of the 56 strains were able to form a biofilm in LB in one of the conditions tested. In Y1 medium, many more strains were capable of biofilm formation: after 24 h, 48 strains showed biofilm formation at 20°C and 50 strains showed biofilm formation at 30°C. With 11 and 15 of these strains, dispersion of the biofilm was observed after 48 h at 20 and 30°C, respectively. Five strains at 20°C and one strain at 30°C showed biofilm formation after 48 h, but not after 24 h. In Y1, 54 of the 56 strains tested were able to form a biofilm in one of the conditions tested. An overview of these data can be seen in Table 2. There was only one strain tested, B. cereus F4635A/90, that did not form a biofilm in any of the conditions tested.
FIG. 1.
Visualization of biofilms formed by B. cereus on LB at 30°C for 48 h at air-liquid interfaces. (A) Biofilms formed in a microtiter plate by B. cereus PAL22 (lane 1), B. cereus PAL27 (lane 2), B. cereus ATCC 14579 (lane 3), B. cereus 72 (lane 4), and B. cereus ATCC 10987 (lane 5). (B) Biofilms formed on stainless steel coupons by B. cereus 72 (lane 1), B. cereus PAL25 (lane 2), and B. cereus ATCC 10987 (lane 3).
TABLE 2.
Summary of biofilm-forming capacities of all 56 B. cereus strains tested using the microtiter plate assay
| Time | No. of strains that formed biofilm under the following conditions:
|
|||
|---|---|---|---|---|
| LB
|
Y1
|
|||
| 20°C | 30°C | 20°C | 30°C | |
| After 24 h only | 1 | 4 | 11 | 15 |
| After 48 h only | 4 | 8 | 5 | 1 |
| After both 24 and 48 h | 0 | 5 | 37 | 35 |
| Total no. of positive strains | 5 | 17 | 53 | 51 |
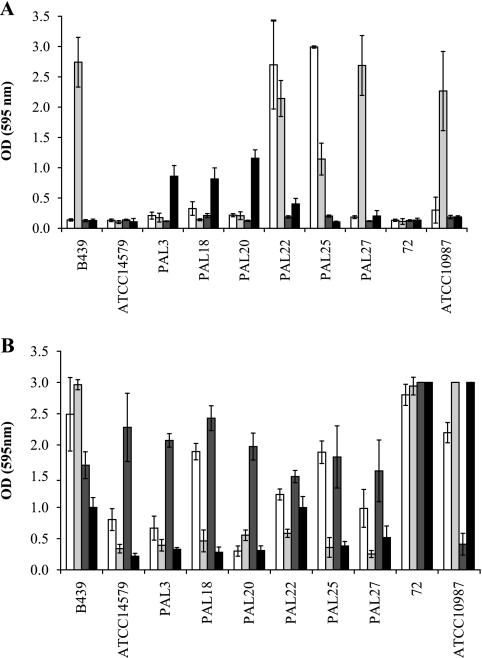
The amount of biofilm formed in the microtiter plate assay is shown for a selection of 10 strains in Fig. 2. These strains include the two sequenced strains, ATCC 14579, which formed biofilm only in Y1 medium after 24 h, and ATCC 10987, which forms biofilm in LB after 48 h at 30°C and in Y1 after both 24 and 48 h at 20 and 30°C; strains PAL3, PAL18, and PAL20, which formed biofilms in LB at 20°C after 48 h; strain 72 that formed biofilms on Y1 but not on LB; and strains PAL22, PAL25, PAL27, and B439 that formed large amounts of biofilm on LB at 30°C. Especially after 24 h, the amount of biofilm formed in Y1 is higher than in LB (Fig. 2). In LB, large amounts of biofilm, with an OD595 value above 1.5, were observed for four of the 10 strains formed and only at 30°C (Fig. 2A). In Y1, large amounts of biofilms can be observed for all strains (Fig. 2B).
FIG. 2.
Biofilm formation by B. cereus on LB (A) and Y1 medium (B) incubated at 30°C for 24 (white bars) and 48 h (light gray bars) and at 20°C for 24 (dark gray bars) and 48 h (black bars). Bars represent average OD595 values plus standard errors (error bars).
Sporulation of B. cereus ATCC 14579, ATCC 10987, and PAL25 was determined in 24-well plates for both the suspension and biofilm phases. Notably, a higher degree of sporulation was seen in the biofilm phase than in suspension under almost all conditions tested (Table 3). Strikingly, with strain B. cereus ATCC 10987, spores were virtually absent in suspension, except for 1.1% spores in Y1 medium after 24 h, and spores, up to 40% of the total counts, were found only in the biofilm. With B. cereus ATCC 14579 and PAL25, high numbers of spores were also found in the suspension, especially in Y1 after 48 h. In this case, the possibility that some of these spores were produced in the biofilm and subsequently released into the suspension cannot be excluded.
TABLE 3.
Percentages of spores in the total counts of suspension and biofilm cells of B. cereus ATCC 14579, ATCC 10987, and PAL25 grown in 24-well plates at 30°C
| B. cereus strain | Phase | Value under the following conditions:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| LB and 24 h
|
Y1 and 24 h
|
LB and 48 h
|
Y1 and 48 h
|
||||||
| Total counta | % Spores | Total count | % Spores | Total count | % Spores | Total count | % Spores | ||
| ATCC 14579 | Suspension | 5.0 × 107 | 0.00034 | 7.9 × 107 | 0.000050 | 2.6 × 107 | 0.037 | 4.2 × 107 | 56 |
| Biofilm | 7.8 × 105 | 5.7 | 3.2 × 106 | 5.6 | 5.2 × 105 | 3.2 | 1.4 × 106 | 30 | |
| ATCC 10987 | Suspension | 4.8 × 107 | 0.0053 | 1.7 × 107 | 1.1 | 6.8 × 107 | 0.090 | 1.2 × 107 | 0.00084 |
| Biofilm | 1.6 × 105 | 4.2 | 4.6 × 107 | 3.2 | 1.1 × 106 | 9.9 | 1.3 × 108 | 39 | |
| PAL25 | Suspension | 3.1 × 107 | 0.0046 | 2.3 × 107 | 72 | 8.2 × 107 | 5.0 | 7.2 × 107 | 30 |
| Biofilm | 6.0 × 105 | 0.63 | 3.1 × 107 | 67 | 1.8 × 106 | 22 | 3.1 × 107 | 91 | |
Total count of cells in suspension is given in CFU/ml. Total count of biofilm cells in given in CFU/cm2. The total surface area of a well is 7.7 cm2.
Adherence of spores.
Subsequently, biofilm formation was studied with stainless steel as a substratum, since this is the most commonly used material in processing equipment of the food industry. In these experiments, spores of the selected strains were used for inoculation, since B. cereus spores are able to survive cleaning and pasteurization processes and have been suggested to adhere to a large variety of surfaces (9, 32). Germination and subsequent outgrowth of spores may result in the development of biofilms. On the basis of the microtiter plate assay results, the selection of 10 strains as discussed above was used to study biofilm formation by B. cereus in more detail. Using spores of B. cereus PAL20, the effects of temperature, time, and spore concentration on the adherence of spores to stainless steel were tested. The adherence of spores was similar at incubation temperatures of 4, 20, or 50°C and incubation times of 15, 30, 60, or 120 min (data not shown). The number of adherent spores increased with the density of the spore suspension, although the increase was not proportional (data not shown). This indicated the surface was not yet completely saturated with spores. In subsequent experiments, 7 log spores per ml, which results in 5.6 log adherent spores per cm2, were added to 10 ml buffer containing submerged stainless steel (304L) coupons.
Submerged biofilm formation.
The number of spores adhering to the stainless steel coupons and the biofilm formed on these coupons at 30°C is shown in Fig. 3. Spores of all strains adhered to the coupons with an average of 5.4 log spores per cm2. Subsequent biofilm formation after germination of the spores resulted in low numbers of biofilm cells, with an average of 2.6 log CFU per cm2. When the coupons were incubated at 10°C, spores adhered with an average of 5.7 log spores per cm2, which was higher than the number of adherent spores at 30°C (P = 0.013). Biofilm was formed with an average of 2.6 log CFU per cm2, which was not significantly different from the results at 30°C (P = 0.48) (Fig. 4A). To investigate the impact of fouling of the coupons on biofilm formation, experiments were performed at 10°C, using coupons preincubated with milk (Fig. 4B). When the coupons were fouled with milk, the number of adherent spores was 5.0 log CFU per cm2 on average, which is lower than the number of spores adhering to clean coupons at 10°C (P = 0.0045). The fouled coupons either did not allow biofilm development or only at low numbers with a maximum of 2.7 log CFU per cm2 and an average of 1.8 log CFU per cm2, indicating that fouling of the coupons significantly decreased the amount of biofilm formed (P = 0.043).
FIG. 3.
Number of adherent B. cereus spores (light gray bars) to stainless steel coupons, after the addition of 7 log spores per ml, and subsequent biofilm (dark gray bars) formed on LB medium at 30°C after 48 h.
FIG. 4.
Number of adherent B. cereus spores (light gray bars) at 10°C to clean stainless steel coupons (A) or stainless steel coupons with milk pretreatment (B) after the addition of 7 log spores per ml and subsequent biofilm formed (dark gray bars) on LB medium at 10°C after 96 h.
Air-liquid interface biofilm formation.
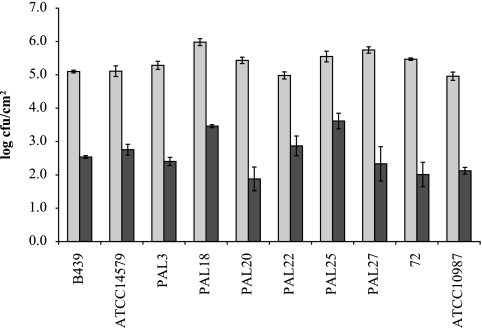
Since in the microtiter plate assay we found biofilms to be formed preferentially at the air-liquid interface, we also wanted to determine the capacity of the selected B. cereus strains to form biofilms at the air-liquid interface of stainless steel coupons. The coupons were placed in a 24-well plate, in which they were partly above the liquid level. This gave rise to biofilms at the air-liquid interface of the coupon, which were clearly visible after staining with crystal violet (Fig. 1B). An overview of the amount of biofilm formed either at the air-liquid interface of the coupons or on the submerged coupons is shown in Fig. 5. For 9 of the 10 strains tested, the amount of biofilm formed was between 6.1 and 7.1 log cells per coupon, while for B. cereus PAL3, the amount of biofilm was 4.2 log cells per coupon. For all strains, the average number of cells in the air-liquid interface biofilms, 5.4 log CFU per cm2, was higher than that in the submerged assay, 2.6 log CFU per cm2 (P = 1.9 × 10−8).
FIG. 5.
Biofilm formation by B. cereus at air-liquid interfaces (light gray bars) and under submerged conditions (dark gray bars) on stainless steel coupons on LB medium at 30°C after 48 h. The left y axis indicates the total log CFU per cm2 of stainless steel coupon; the right y axis indicates the total log CFU per stainless steel coupon.
DISCUSSION
B. cereus has previously been reported to be capable of biofilm formation (9, 26, 28), which might cause problems for the food industry, since biofilm cells of B. cereus have been reported to be more resistant to cleaning procedures than planktonic cells were (28, 34). However, these studies did not investigate diversity in biofilm-forming capacities, both semiquantitative and quantitative, among B. cereus isolates, or the types of biofilm that B. cereus can form. We have used an experimental approach in which we first looked from a broad perspective at the biofilm-forming capacities of the food pathogen B. cereus and subsequently did more-detailed, quantitative studies on a selection of 10 strains. We have shown that the use of submerged or air-liquid interface assays influences the amount of biofilm formed, as well as the location where the biofilm is formed. To our knowledge, this is the first time a food pathogen like B. cereus is described to form biofilms preferentially at the air-liquid interface. The strain and incubation time, as well as environmental conditions, such as the composition of medium and incubation temperature, were also found to be important determinants in B. cereus biofilm formation.
We have shown, using a microtiter plate assay and assays on stainless steel coupons, which are either completely submerged or partly above the fluid level, that B. cereus preferentially forms biofilms at the interface between the air and the medium. Cell counts in air-liquid interface biofilms on stainless steel coupons ranged from 5.3 to 6.3 log CFU per cm2 for 9 of the 10 strains tested, which is much higher than the maximum value of 4.2 log CFU per cm2 obtained in submerged conditions. However, in the air-liquid interface assay, the vast majority of cells is located in the area of this interface, whereas in the submerged assay the cells are spread over the coupon. Taking that into account, it means that the local number of biofilm cells at the air-liquid interface biofilm will be even higher, notably up to 8 log cells per cm2. Factors contributing to the formation of biofilms at the air-liquid interface may involve oxygen availability at the surface, causing aerotaxis of B. cereus towards oxygen (19). In P. fluorescens, reduced oxygen availability is reported to negatively influence the development of a pellicle at the air-liquid interface (37). A recent study in our lab showed that a mutant with reduced motility is severely affected in its capacity to form biofilms at the air-liquid interface (data not shown). Also, biosurfactants, such as surfactin, have been shown to increase biofilm formation by B. cereus and might, together with other physical and chemical parameters, influence air-liquid interface biofilm formation (5, 16, 25).
The potential of B. cereus to form biofilms from spores was tested using submerged stainless steel coupons, since this is the most commonly used material for equipment, such as pipes and tanks, in the food industry. A similar adherence of spores to these coupons was observed for the strains tested. The amount of biofilm that is subsequently formed from these adherent spores is relatively low, with a maximum of 3.6 log CFU per cm2. Lindsay et al. (20) and Peng et al. (28) reported the amount of biofilm formed by B. cereus on stainless steel to be up to 7 log CFU per cm2. However, the experimental setup of these studies was different compared to ours, using shaking flasks, longer incubation times, and different medium compositions, which could be an explanation for the differences found. Another, recent study of Lindsay et al. (21) showed that B. cereus DL5 is able to make biofilms from spores on glass wool but only under nutrient-rich conditions. To test whether the presence of a fouling substance on the stainless steel coupons would influence biofilm formation, the coupons were immersed in milk. Pretreatment of the stainless steel coupons with milk resulted in a lower adherence of spores and subsequently in a smaller amount of biofilm formed for the majority of strains tested. Other studies have found both positive and negative effects of the presence of milk on biofilm formation and attachment. Wong (44) found that the presence of milk and milk proteins reduced the attachment of Listeria monocytogenes on stainless steel by 1.5 log CFU per cm2, while no effect of lactose was seen. A similar effect was seen for attachment to buna-n rubber. Flint et al. (10) showed that attachment of bacteria can be inhibited by whole milk but can also be enhanced by the presence of lactose and noncasein protein solutions. Milk proteins have been found to decrease attachment of both vegetative cells and spores of thermophilic bacilli (27). Our study revealed small amounts of submerged B. cereus biofilms. On the basis of our observation that B. cereus preferentially forms biofilms at the air-liquid interface, it is conceivable that biofilm formation from spores would have been more extensive under these conditions.
The results of the microtiter plate assay indicate differences in biofilm-forming capacity among the B. cereus strains tested. For example, in LB, the two sequenced strains, ATCC 14579 and ATCC 10987, show very distinct behaviors: B. cereus ATCC 10987 is able to make thick biofilms at 30°C after 48 h, while B. cereus ATCC 14579 does not produce biofilms under these conditions. In Y1 medium, both strains form biofilms after 24 h at 20 and 30°C, but after 48 h the biofilm formed by B. cereus ATCC 14579 had dispersed, whereas that of B. cereus ATCC 10987 was maintained. Since 55 out of 56 strains were able to form a biofilm in at least one of the conditions tested, we can conclude that within the B. cereus species the biofilm way of life is widely spread. In both LB and Y1, differences between biofilm formation after 24 and 48 h can be observed. For example, at 30°C in LB, there were eight strains that needed a time period of 48 h to establish a biofilm, while four strains show biofilm formation only after 24 h. This phenomenon is also observed in Y1 at 30°C. Obviously, B. cereus can exhibit significant biofilm formation within 24 h, followed by dispersion resulting in the absence of biofilms after 48 h. Biofilms have been suggested to be a nidus for the spread of large numbers of cells to the environment (13). We have shown that within 24 h efficient sporulation already occurs in B. cereus biofilms. Recently, sporulation in biofilms was also reported in other studies on B. cereus (21, 34) and Bacillus subtilis biofilms (22, 41). However, Lindsay et al. (21) reported only limited sporulation of B. cereus and spore percentages did not reach values higher than 0.01% after 24 and 48 h, and Ryu and Beuchat (34) used incubation times of 6 to 12 days and observed a maximum of 10% of spores of total B. cereus biofilm counts. Our study already shows sporulation in biofilms after 24 h and much higher numbers of spores are reached, with up to 5.7% on LB and up to 67% spores on Y1. Notably, after 48 h, spore percentages of biofilm counts even increase to 22% and 91% on LB and Y1, respectively. Spores already occur in biofilms after 24 h, indicating that dispersion of these biofilms will result in the spread of both cells and highly resistant spores. Since industrial cleaning-in-place practices often have cycles of 24 h, this can result in B. cereus biofilms formation in food production systems within this time period. More-efficient sporulation was seen in the biofilm phase than in the suspension phase, suggesting that B. cereus biofilms function as a nidus for sporulation, increasing the spore content of the food production environment by dispersion.
Mechanisms involved in dispersion of cells from a biofilm involve oxygen and/or nutrient limitations, as shown in studies using P. aeruginosa and S. oneidensis (35, 40). The biofilm assays used in this study are static batch cultures, so conditions change during time, which may have activated dispersion mechanisms. Recent studies with P. aeruginosa revealed production of rhamnolipids to cause detachment of biofilm cells (3). For S. oneidensis, the level of cyclic di-GMP has been shown to regulate attachment to surfaces and also the detachment of cells from a biofilm (39). Mechanisms involved in dispersion of B. cereus cells and spores from biofilms remain to be elucidated. However, our studies identified conditions and strains that show this behavior, allowing more-detailed analysis of this phenomenon.
When studying diversity in biofilm formation by B. cereus, it is important to bear in mind that environmental conditions have a large impact on the results. The type of assay used to study biofilm formation is also of great importance, since B. cereus shows a preference to form biofilms at an air-liquid interface. Using submerged assays might lead to an underestimation of the possible number of biofilm cells in a system. We have seen great variation in biofilm formation capacities among the isolates tested. Our results show that in the majority of conditions we tested, the two sequenced B. cereus strains, ATCC 14579 and ATCC 10987, cover a sufficient range of properties to be part of test panels for industrial cleaning procedures. At low temperatures in LB, the use of a psychrotolerant strain will provide additional information. A major advantage of using sequenced strains for characterization of biofilm formation is that phenotypes found can be linked to features on the genome. Future research will involve the use of random and directed mutagenesis and transcriptome analysis using whole-genome DNA microarrays to identify genes involved in different steps of biofilm formation in both B. cereus ATCC 14579 and ATCC 10987. Together with the results of this study, this may lead to the identification of factors contributing to the multiple pathways of B. cereus biofilm formation and dispersion.
In conclusion, we have found that B. cereus biofilm formation is highly dependent on the strain, the assay used, and environmental conditions, such as medium and temperature. Both with polystyrene and stainless steel, thick biofilms were formed at the air-liquid interface, while the amount of biofilm formed was much lower in submerged systems. These results suggest that biofilms may particularly develop in industrial storage and piping systems that are only partly filled during operation or where some residual fluid has remained after a production cycle. Moreover, B. cereus biofilms can act as a nidus for spore formation and subsequently release their spores into food production environments. This can cause recontamination of products and equipment failure, thereby affecting food quality and safety.
Footnotes
Published ahead of print on 5 January 2007.
REFERENCES
- 1.Andersson, A., U. Ronner, and P. E. Granum. 1995. What problems does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? Int. J. Food Microbiol. 28:145-155. [DOI] [PubMed] [Google Scholar]
- 2.Auger, S., E. Krin, S. Aymerich, and M. Gohar. 2006. Autoinducer 2 affects biofilm formation by Bacillus cereus. Appl. Environ. Microbiol. 72:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boles, B. R., M. Thoendel, and P. K. Singh. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 57:1210-1223. [DOI] [PubMed] [Google Scholar]
- 4.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 98:11621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries, Y. P. 2006. Bacillus cereus spore formation, structure and germination. Thesis. Wageningen University and Research Centre, Wageningen, The Netherlands.
- 7.de Vries, Y. P., L. M. Hornstra, W. M. de Vos, and T. Abee. 2004. Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl. Environ. Microbiol. 70:2514-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faille, C., C. Jullien, F. Fontaine, M. N. Bellon-Fontaine, C. Slomianny, and T. Benezech. 2002. Adhesion of Bacillus spores and Escherichia coli cells to inert surfaces: role of surface hydrophobicity. Can. J. Microbiol. 48:728-738. [DOI] [PubMed] [Google Scholar]
- 10.Flint, S. H., P. J. Bremer, and J. D. Brooks. 1997. Biofilms in dairy manufacturing plant—description, current concerns and methods of control. Biofouling 11:81-97. [Google Scholar]
- 11.Fux, C. A., M. Shirtliff, P. Stoodley, and J. W. Costerton. 2005. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 13:58-63. [DOI] [PubMed] [Google Scholar]
- 12.Ghigo, J. M. 2003. Are there biofilm-specific physiological pathways beyond a reasonable doubt? Res. Microbiol. 154:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Hall-Stoodley, L., and P. Stoodley. 2005. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 13:7-10. [DOI] [PubMed] [Google Scholar]
- 14.Han, C. S., G. Xie, J. F. Challacombe, M. R. Altherr, S. S. Bhotika, D. Bruce, C. S. Campbell, M. L. Campbell, J. Chen, O. Chertkov, C. Cleland, M. Dimitrijevic, N. A. Doggett, J. J. Fawcett, T. Glavina, L. A. Goodwin, K. K. Hill, P. Hitchcock, P. J. Jackson, P. Keim, A. R. Kewalramani, J. Longmire, S. Lucas, S. Malfatti, K. McMurry, L. J. Meincke, M. Misra, B. L. Moseman, M. Mundt, A. C. Munk, R. T. Okinaka, B. Parson-Quintana, L. P. Reilly, P. Richardson, D. L. Robinson, E. Rubin, E. Saunders, R. Tapia, J. G. Tesmer, N. Thayer, L. S. Thompson, H. Tice, L. O. Ticknor, P. L. Wills, T. S. Brettin, and P. Gilna. 2006. Pathogenomic sequence analysis of Bacillus cereus and Bacillus thuringiensis isolates closely related to Bacillus anthracis. J. Bacteriol. 188:3382-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmaster, A. R., J. Ravel, D. A. Rasko, G. D. Chapman, M. D. Chute, C. K. Marston, B. K. De, C. T. Sacchi, C. Fitzgerald, L. W. Mayer, M. C. Maiden, F. G. Priest, M. Barker, L. Jiang, R. Z. Cer, J. Rilstone, S. N. Peterson, R. S. Weyant, D. R. Galloway, T. D. Read, T. Popovic, and C. M. Fraser. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. USA 101:8449-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh, Y. H., E. B. Somers, D. Lereclus, and A. C. Wong. 2006. Biofilm formation by Bacillus cereus is influenced by PlcR, a pleiotropic regulator. Appl. Environ. Microbiol. 72:5089-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 18.Kotiranta, A., K. Lounatmaa, and M. Haapasalo. 2000. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2:189-198. [DOI] [PubMed] [Google Scholar]
- 19.Laszlo, D. J., M. Niwano, W. W. Goral, and B. L. Taylor. 1984. Bacillus cereus electron transport and proton motive force during aerotaxis. J. Bacteriol. 159:820-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay, D., V. S. Brozel, J. F. Mostert, and A. von Holy. 2000. Physiology of dairy-associated Bacillus spp. over a wide pH range. Int. J. Food Microbiol. 54:49-62. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay, D., V. S. Brozel, and A. Von Holy. 2006. Biofilm-spore response in Bacillus cereus and Bacillus subtilis during nutrient limitation. J. Food Prot. 69:1168-1172. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay, D., V. S. Brozel, and A. von Holy. 2005. Spore formation in Bacillus subtilis biofilms. J. Food Prot. 68:860-865. [DOI] [PubMed] [Google Scholar]
- 23.Mittelman, M. W. 1998. Structure and functional characteristics of bacterial biofilms in fluid processing operations. J. Dairy Sci. 81:2760-2764. [DOI] [PubMed] [Google Scholar]
- 24.Moltz, A. G., and S. E. Martin. 2005. Formation of biofilms by Listeria monocytogenes under various growth conditions. J. Food Prot. 68:92-97. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa, M. 2006. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J. Biosci. Bioeng. 101:1-8. [DOI] [PubMed] [Google Scholar]
- 26.Oosthuizen, M. C., B. Steyn, D. Lindsay, V. S. Brozel, and A. von Holy. 2001. Novel method for the proteomic investigation of a dairy-associated Bacillus cereus biofilm. FEMS Microbiol. Lett. 194:47-51. [DOI] [PubMed] [Google Scholar]
- 27.Parkar, S. G., S. H. Flint, J. S. Palmer, and J. D. Brooks. 2001. Factors influencing attachment of thermophilic bacilli to stainless steel. J. Appl. Microbiol. 90:901-908. [DOI] [PubMed] [Google Scholar]
- 28.Peng, J. S., W. C. Tsai, and C. C. Chou. 2002. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int. J. Food Microbiol. 77:11-18. [DOI] [PubMed] [Google Scholar]
- 29.Rasko, D. A., M. R. Altherr, C. S. Han, and J. Ravel. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303-329. [DOI] [PubMed] [Google Scholar]
- 30.Rasko, D. A., J. Ravel, O. A. Okstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A. B. Kolsto, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 32.Ronner, U., U. Husmark, and A. Henriksson. 1990. Adhesion of bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550-556. [DOI] [PubMed] [Google Scholar]
- 33.Rupp, C. J., C. A. Fux, and P. Stoodley. 2005. Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl. Environ. Microbiol. 71:2175-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu, J. H., and L. R. Beuchat. 2005. Biofilm formation and sporulation by Bacillus cereus on a stainless steel surface and subsequent resistance of vegetative cells and spores to chlorine, chlorine dioxide, and a peroxyacetic acid-based sanitizer. J. Food Prot. 68:2614-2622. [DOI] [PubMed] [Google Scholar]
- 35.Sauer, K., M. C. Cullen, A. H. Rickard, L. A. Zeef, D. G. Davies, and P. Gilbert. 2004. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 186:7312-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma, A., and S. K. Anand. 2002. Biofilms evaluation as an essential component of HACCP for food/dairy processing industry—a case. Food Control 13:469-477. [Google Scholar]
- 37.Spiers, A. J., J. Bohannon, S. M. Gehrig, and P. B. Rainey. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50:15-27. [DOI] [PubMed] [Google Scholar]
- 38.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 39.Thormann, K. M., S. Duttler, R. M. Saville, M. Hyodo, S. Shukla, Y. Hayakawa, and A. M. Spormann. 2006. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J. Bacteriol. 188:2681-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thormann, K. M., R. M. Saville, S. Shukla, and A. M. Spormann. 2005. Induction of rapid detachment in Shewanella oneidensis MR-1 biofilms. J. Bacteriol. 187:1014-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veening, J. W., O. P. Kuipers, S. Brul, K. J. Hellingwerf, and R. Kort. 2006. Effects of phosphorelay perturbations on architecture, sporulation, and spore resistance in biofilms of Bacillus subtilis. J. Bacteriol. 188:3099-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb, J. S., M. Givskov, and S. Kjelleberg. 2003. Bacterial biofilms: prokaryotic adventures in multicellularity. Curr. Opin. Microbiol. 6:578-585. [DOI] [PubMed] [Google Scholar]
- 43.Wiencek, K. M., N. A. Klapes, and P. M. Foegeding. 1990. Hydrophobicity of Bacillus and Clostridium spores. Appl. Environ. Microbiol. 56:2600-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong, A. C. 1998. Biofilms in food processing environments. J. Dairy Sci. 81:2765-2770. [DOI] [PubMed] [Google Scholar]