Abstract
Angiostrongylus cantonensis is a common cause of human eosinophilic meningitis. Recent outbreaks of this infection have shown that there is a need to determine the distribution of this nematode in the environment in order to control transmission. A. cantonensis is generally identified morphologically in the molluscan intermediate host by microscopic examination, which can be labor-intensive. The aim of this study was to develop a PCR-based method to detect A. cantonensis directly from molluscan tissue. A total of 34 Parmarion cf. martensi (Simroth) semislugs, 25 of which were naturally infected with A. cantonensis, were used to develop this assay. Tissue pieces (approximately 25 mg) were digested with pepsin-HCl to recover third-stage larvae for morphological identification or were used for DNA extraction. PCR primers were designed to amplify 1,134 bp from the Angiostrongylus 18S rRNA gene, and the amplicons produced were sequenced for identification at the species level. Both microscopy and the PCR-DNA sequencing analysis indicated that the same 25 semislugs were positive for A. cantonensis, showing that the two methods were equally sensitive and specific for this application. However, morphological detection requires access to living mollusks, whereas molecular analysis can also be performed with frozen tissue. The PCR-DNA sequencing method was further evaluated using tissue from Veronicella cubensis (Pfeiffer) slugs and mucus secretions from infected P. martensi. To our knowledge, this is the first use of a PCR-based method to confirm the presence of A. cantonensis in mollusks collected in the environment.
Eosinophilic meningitis caused by Angiostrongylus (Parastrongylus) cantonensis, the rat lungworm, is reemerging as a zoonotic disease in humans. Rats and mollusks are the definitive and intermediate hosts of A. cantonensis, respectively (1). Adult A. cantonensis worms live in the pulmonary arteries of rats, where females produce first-stage larvae that eventually are passed in the rat feces. The first-stage larvae then penetrate or are ingested by a molluscan intermediate host. After two molts, they become third-stage larvae, which are infective for mammals. Rats become infected when they ingest mollusks carrying third-stage larvae. The larvae migrate to the central nervous system, where they develop into young adults before returning to the pulmonary arteries to become sexually mature adult worms. Various species of fish, frogs, crustaceans, and flatworms can act as paratenic or transport hosts after they ingest infected mollusks.
Humans become infected by eating raw or undercooked infected mollusks, as well as food items contaminated with infective third-stage larvae, infected mollusks, or infected paratenic hosts. Whether produce contaminated with mollusk secretions can serve as a vehicle for angiostrongyliasis remains to be confirmed (7, 15, 25). Following ingestion, the third-stage larvae migrate to the brain and spinal cord tissues. They may molt twice to become young adults. In humans they infrequently migrate to the lungs. Instead, they remain in the central nervous system, causing tissue damage and subsequent inflammation. The disease is mostly self-limited, with full recovery within a month, but severe cases can result in persistent neurological problems or even death (1). Up to 1992 at least 2,500 cases had been reported worldwide, but this is most likely a vast underestimate of the true incidence, in part due to diagnostic challenges (10, 17). In 2000, a food-borne outbreak affected 12 American tourists visiting Jamaica (27). In Hawaii, human infections have been detected since 1962 and are still sporadically associated with consumption of contaminated food items (9, 18, 20). This illustrates the fact that human A. cantonensis infections continue to have public health relevance.
A. cantonensis is a known cause of human disease in Southeast Asia (4, 24) and has spread to new territories, probably via human cargo ships that unintentionally carried infected rats and/or snails (17). This parasite is endemic in most of the Pacific Basin, including the Hawaiian Islands, parts of Africa, Cuba, Puerto Rico, the Bahamas, and the Dominican Republic. Recently, A. cantonensis was detected in rats and mollusks in new geographic locations, including Jamaica and Haiti (19, 26). A. cantonensis was detected in rats in New Orleans in 1988 (7), and the first human case there was detected in 1995 (23). Recent reports of infections in captive animals suggest that the parasite may have spread to Mississippi and Florida (11, 13). It is likely that expansion of the range of A. cantonensis will continue as long as there are rats and mollusks that can maintain its life cycle, leading to an increasing risk for human disease. The introduction of exotic mollusks that are associated with human habitats into regions where the organism is endemic, including Parmarion cf. martensi (Simroth) in Hawaii, also increases the exposure of humans to third-stage larvae. Monitoring the territorial spread of this nematode and proper identification of its local rat and mollusk hosts are crucial for preventing and controlling human disease. In this study we developed a PCR-based method for detection and identification of A. cantonensis in mollusks. The molecular approach described here was also applied to mucus samples from naturally infected mollusks in order to assess use of the procedure for detection of secreted larvae.
MATERIALS AND METHODS
Slugs and nematodes.
Mollusks were collected in the field at various locations in Hawaii and sent to the CDC. These mollusks included 69 P. martensi semislugs (semislugs are snails with a shell that is too small for the snail to retract into) and 12 Veronicella cubensis (Pfeiffer) slugs. Sixty-eight of the P. martensi semislugs were sent alive to the CDC to facilitate isolation and morphological identification of Angiostrongylus larvae. All 12 V. cubensis slugs and one P. martensi semislug were received frozen. Free-living rhabditoid nematodes (n = 14) belonging to an unknown species were collected from the containers in which the P. martensi semislugs were kept. One Dipetalonema filarial nematode was isolated from the abdominal cavity of an owl monkey (Aotus nancymaae) captured in Peru. The partial 18S rRNA genes of these non-Angiostrongylus nematodes were PCR amplified and sequenced using generic nematode primers (GenBank accession numbers DQ531722 and DQ531723).
Morphological identification of A. cantonensis.
Tissue pieces (6 to 53 mg) were cut from the posterior end of the mollusk's foot and placed in 1 ml of 0.01% pepsin-0.7% HCl in individual wells of a 24-well culture dish (Corning Inc., Corning, NY) for digestion of the tissue (14). The larvae that were released were identified as A. cantonensis on the basis of morphological features (2). To collect mucus secretions, slugs were kept individually in polystyrene petri dishes (60 by 15 mm; Becton Dickinson & Co, Franklin Lakes, NJ) overnight, with occasional prodding to stimulate secretion. The mucus samples were treated with pepsin-HCl to facilitate collection of larvae for identification by microscopy.
For the experiment with mucus samples spiked with A. cantonensis, larvae were isolated from infected slug tissue as described above and washed with Livsey spring water. The spiking procedure was performed using visualization with a dissecting microscope. Larvae were counted, and defined numbers of larvae (1 to 37 larvae) were transferred to approximately 0.3-g aliquots of mucus from noninfected slugs using a glass pipette that had been modified using a flame to decrease the diameter of the tip. The spiked mucus was then subjected to DNA extraction and PCR analysis.
DNA extraction.
DNA was extracted from purified nematode larvae and mucus samples by digestion with 0.1 μg proteinase K/ml in a buffer consisting of 50 mM Tris-HCl (pH 8.5), 1% laureth-12, and 1 mM EDTA overnight at 56°C, followed by incubation at 95°C for 10 min to inactivate the proteinase K. The mucus secretions required an additional purification step with QIAquick columns (QIAGEN Inc., Valencia, CA) to remove PCR inhibitors. DNA in intact fresh or frozen mollusk tissue was extracted using the FastDNA method (Q-Biogene, Carlsbad, CA) with the modifications described previously (12). Disruption of samples in an FP120 cell disruptor was performed at speed 5.5 for 30 s. PCR inhibitors were removed by further purification with a QIAquick PCR purification kit (QIAGEN Inc., Valencia, CA) used according to the manufacturer's instructions. Purified DNA was stored at 4°C until it was used in PCRs.
PCR amplification.
Primers AngioF1 and AngioR1 (Table 1) were designed to amplify a 1,134-bp fragment of the 18S rRNA gene of Angiostrongylus spp. based on BLAST searches and multiple alignments of 18S rRNA sequences from various nematodes and mollusks. Primer sequences were revised using the Primer Express software (Applied Biosystems, Foster City, CA) to check for potential secondary structures and primer-dimers. The 50-μl PCR mixtures contained 0.4 μM of each primer and AmpliTaq Gold PCR Master Mix (Applied Biosystems, Foster City, CA). Amplification was carried out with a GeneAmp 9700 PCR thermal cycler (Applied Biosystems, Foster City, CA) using the following cycling parameters: 95°C for 5 min, 45 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 1 min, and 72°C for 10 min. The products were detected on 1.5% agarose gels stained with ethidium bromide. Positive PCR products were purified and sequenced as described below, using AngioF1, AngioR1, NEM2, NEM3, and NEM3R (Table 1) as sequencing primers.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′-3′) | Positiona | Reference |
|---|---|---|---|
| NEMFG1 | TCTCCGATTGATTCTGTCGGCGATTATATG | 5′ of sequence | 5 |
| AngioF1 | ATCATAAACCTTTTTTCGAGTATCCAG | 456-482 | This study |
| NEM2F | GCGGTTAAAAAGCTCGTAGTTGG | 549-571 | 5 |
| NEM2R | CCAACTACGAGCTTTTTAACCGC | 571-549 | 5 |
| NEM3F | GCGGCTTAATTTGACTCAACAC | 1098-1119 | 5 |
| NEM3R | GTGTTGAGTCAAATTAAGCCG | 1119-1098 | 5 |
| AngioR1 | TCTCGAGACAGCTCAGTCCCGG | 1590-1569 | This study |
| NEM4R | CTCGAAACGGCTCAGTCCCGG | 1589-1569 | 5 |
| CRYPTOR | GCTTGATCCTTCTGCAGGTTCACCTAC | 3′ of sequence | 5 |
Based on the A. cantonensis 18S rRNA gene GenBank entry (accession number AY295804).
DNA sequencing analysis.
PCR products were purified with a StrataPrep PCR purification kit (Stratagene, La Jolla, CA) used according to the manufacturer's instructions. Cycle sequencing reactions were performed using BigDye V3.1 chemistry (Applied Biosystems, Foster City, CA) and a selection of the primers listed in Table 1 depending on the application. The cycle sequencing reaction mixtures were purified using MultiScreen-HV plates (Millipore, Billerica, MA) and were analyzed with an ABI Prism 3100 sequence analyzer using the data collection software v 2.0 and DNA sequence analysis software V. 5.1 (Applied Biosystems, Foster City, CA). Sequences were assembled, edited, and aligned by using DNA Star SeqMan (DNASTAR Inc., Madison, WI).
RESULTS
PCR-based identification of A. cantonensis in slugs.
Microscopic evaluation of pepsin-digested tissue revealed third-stage A. cantonensis larvae in 25 of 34 P. martensi semislugs. This morphological identification was confirmed by DNA sequencing analysis of the 18S rRNA gene after PCR amplification using DNA extracted from the larvae and generic primers NEMFG1 and NEM4R (Table 1). The use of morphology for detection of A. cantonensis in mollusks required careful visual evaluation of features that are diagnostic for this species by highly trained professionals (2). Moreover, the PCR primers available at the start of this project also amplified DNA originating from the slugs themselves, so the morphological findings could be confirmed by molecular analysis only after we ensured that the isolated larvae were cleared of all molluscan DNA. To make detection of infected mollusks easier, new primers that allowed amplification of Angiostrongylus spp. DNA without interaction with slug DNA were developed.
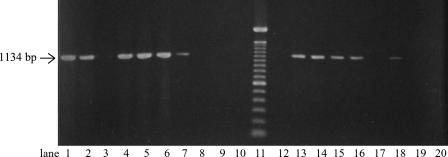
PCR primers were designed to amplify a 1,134-bp DNA fragment of the Angiostrongylus spp. 18S rRNA gene. The primers were initially tested with DNA from the isolated larvae, as well as with DNA extracted directly from tissue pieces from the 34 semislugs mentioned above, and identical PCR results were obtained for the two types of starting material (Fig. 1). No amplification was observed from the nine semislugs that were negative for larvae as determined by microscopy. To ensure that negative PCR results were not caused by PCR inhibitors in the DNA samples, generic primers NEM2 and NEM3R were utilized to amplify a 600-bp fragment from the molluscan 18S rRNA in each sample analyzed. These results allowed us to omit larval isolation and use this method with DNA extracted directly from the mollusks.
FIG. 1.
Gel containing selected samples from the Angiostrongylus spp. PCR. The arrow indicates the size (1,134 bp) of the expected PCR products. Lane 11 contained a 100-bp ladder DNA size marker. Lanes 1 though 3 show results obtained with fresh P. martensi tissue (lane 1, larvae isolated from an infected individual; lane 2, tissue from the same individual; lane 3, tissue from noninfected P. martensi). Lanes 4 through 10 show the effect on storage of Angiostrongylus-infected tissue from P. martensi (lane 4, 1 month of storage at −18°C; lane 5, 1 month of storage at −18°C followed by storage for 24 h at 5°C; lane 6, 1 month of storage at −18°C followed by storage for 24 h at room temperature; lane 7, 2 weeks of storage in 70% ethanol; lane 8, 4 weeks of storage in 70% ethanol; lane 9, 2 weeks of storage in 95% ethanol; lane 10, 4 weeks of storage in 95% ethanol). Lanes 12 through 16 show results for mucus secretions spiked with known numbers of A. cantonensis larvae (lane 12, no larvae; lane 13, one larva; lane 14, two larvae; lane 15, 10 larvae; lane 16, 37 larvae). Lane 17 and 18 show results for mucus excreted from two A. cantonensis-infected P. martensi individuals. Lanes 19 and 20 show results for non-Angiostrongylus nematodes (lane 19, free-living nematode belonging to an unknown species; lane 20, Dipetalonema sp.).
PCR detection of A. cantonensis in mollusk tissue stored under different conditions.
Freezing the tissue before PCR analysis did not compromise PCR amplification, as 11 of 13 slugs that were sent frozen to CDC were positive as determined by the Angiostrongylus spp. PCR. The effect of freezing and storage of mollusks prior to PCR was analyzed further by storing tissue pieces from infected P. martensi at −18°C for 1 month. After thawing, these pieces were either immediately processed or kept at 5°C or room temperature for 24 h before DNA extraction. DNA from all tissue pieces could be amplified in the Angiostrongylus spp. PCR (Fig. 1). In contrast, keeping the mollusk tissue in 70% ethanol for 4 weeks or in 95% ethanol for 2 weeks or more resulted in no DNA amplification (Fig. 1).
PCR detection of A. cantonensis in mollusk secretions.
Mucus secretions from 46 P. martensi semislugs were tested for the presence of A. cantonensis. Since PCR analysis was incompatible with the pepsin treatment necessary for morphological identification, it was not possible to examine the same samples by both methods. Twelve mucus samples from naturally infected slugs were analyzed by microscopy, while 34 mucus samples, 13 of which were from slugs infected with A. cantonensis, were utilized for DNA extraction and PCR. Microscopy analysis revealed one and four motile larvae in two of the samples. One of the samples analyzed by PCR was positive for A. cantonensis. The possibility of false-negative results due to PCR inhibitors was again excluded by successfully amplifying slug DNA using generic primers NEM2 and NEM3R. To ensure that the methodology was appropriate, secretions from noninfected slugs were experimentally spiked with known numbers of larvae and then subjected to DNA extraction and PCR. This spiking experiment revealed that DNA from A. cantonensis could indeed be amplified from mucus, and as little as one larva in a sample was enough for a positive PCR (Fig. 1). Thus, assuming that the sensitivity of both microscopy and PCR was 100%, only 3 of 25 (12%) naturally infected slugs shed A. cantonensis larvae in their mucus.
Evaluation of primer specificity.
We used the PCR method described above with two other nematode species available for PCR analysis, one Dipetalonema nematode and 14 individuals of an unidentified free-living nematode species. The Angiostrongylus-specific 1,134-bp product was never amplified from DNA extracted from these nematodes (Fig. 1).
All amplicons produced by the Angiostrongylus spp. PCR were sequenced for identification at the species level. All sequences obtained from the mollusks and mucus secretions analyzed in this study (as described above) were identical to the 18S rRNA sequence from A. cantonensis deposited in the GenBank database (accession number AY295804). However, cross-reactivity of the primers was detected when the PCR was applied to a larger set of snails and slugs that were collected for a geographical survey study of the presence of A. cantonensis in the molluscan fauna of Hawaii (16). Of 49 mollusks with a positive result in the PCR, 3 were found to be false positives for A. cantonensis by sequence analysis. The sequences of the three PCR products were identical to each other but differed from the A. cantonensis sequence by 1.1%. BLAST searches could not identify the origin of the amplified sequence, but the highest similarity scores were obtained for the nematode Troglostrongylus wilsoni (GenBank accession number AY295820). Thus, using the PCR alone for detection of A. cantonensis may produce false-positive results due to cross-reactivity with other nematode species.
DISCUSSION
In this study, P. martensi semislugs and V. cubensis slugs, collected from the field in Hawaii, were analyzed for the presence of A. cantonensis. Whenever possible, tissue from each mollusk was examined both by microscopy and by a new PCR assay followed by sequence analysis. The two methods agreed for all 34 samples analyzed. Thus, the PCR followed by sequence analysis as described here was as specific and sensitive as morphological detection; the difference was that the PCR method could be used with frozen mollusk tissue. In our experiments, the mollusk tissue could be stored frozen for at least 1 month and then kept refrigerated or at room temperature for 24 h after thawing. This allows easy storage and transport of specimens from the collection site to a laboratory that can perform the analysis.
The PCR method was successfully applied to mucus secretions from P. martensi. Spiking experiments revealed that the PCR could detect a single A. cantonensis larva in approximately 0.3 g of secretions. Despite this, the majority of naturally infected P. martensi semislugs examined in this study did not have detectable levels of larvae in their mucus. Since the notion that mollusks infected with A. cantonensis can shed larvae in their mucus trails was first suggested, other workers have found no or very few A. cantonensis larvae secreted from infected mollusks (3, 7, 8). Similar results have been obtained for excretion of other Angiostrongylus species (6, 15, 25, 28).
As determined by experimental spiking of larvae into mucus, the PCR could detect one larva per sample analyzed. The corresponding sensitivity of the PCR in mollusk tissue is more difficult to determine since equivalent spiking experiments are not feasible. The tissue from naturally infected mollusks examined by microscopy was estimated to contain between 1 and 19 larvae per mg of tissue depending on the individual, and all mollusks identified as positive by microscopy were determined to be positive by the Angiostrongylus spp. PCR. Thus, assuming that the larvae were evenly distributed in the foot of each mollusk, the PCR was able to detect at least one larva per mg of tissue.
The PCR method presented here may also be useful for detecting Angiostrongylus costaricensis, a parasitic nematode that causes gastrointestinal disease (22). A. costaricensis has a life cycle similar to that of A. cantonensis, including the fact that mollusks are intermediate hosts that transmit the disease to humans (21). The PCR primers described in this study are based on regions where these two parasites have almost identical sequences (GenBank accession number DQ116748), making it possible to use this PCR to examine mollusks for the presence of A. costaricensis, as well as A. cantonensis. The two species can be differentiated by sequence analysis. In addition to amplifying the intended Angiostrongylus species sequence, the PCR primers were found to interact with an unidentified nematode species. This finding emphasizes the importance of sequence analysis of the PCR amplicons. It also highlights the need for further evaluation of the specificity of the PCR primers for DNA from more nematode species, especially those that are associated with mollusks.
Acknowledgments
We express our gratitude to Rachel Kaneta (currently at Linfield College, McMinnville, OR) for help with collecting the mollusks in Hawaii. We are also grateful to Mark Eberhard (Division of Parasitic Diseases, NCID, CDC) for invaluable assistance with morphological identification of the A. cantonensis larvae.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Alto, W. 2001. Human infections with Angiostrongylus cantonensis. Pac. Health Dialog 8:176-182. [PubMed] [Google Scholar]
- 2.Ash, L. R. 1970. Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J. Parasitol. 56:249-253. [PubMed] [Google Scholar]
- 3.Ash, L. R. 1976. Observations on the role of mollusks and planarians in the transmission of Angiostrongylus cantonensis infection to man in New Caledonia. Rev. Biol. Trop. 24:163-174. [Google Scholar]
- 4.Beaver, P. C., and L. Rosen. 1964. Memorandum on the first report of angiostrongylus in man, by Nomura and Lin, 1945. Am. J. Trop. Med. Hyg. 13:589-590. [DOI] [PubMed] [Google Scholar]
- 5.Bimi, L., A. R. Freeman, M. L. Eberhard, E. Ruiz-Tiben, and N. J. Pieniazek. 2005. Differentiating Dracunculus medinensis from D. insignis, by the sequence analysis of the 18S rRNA gene. Ann. Trop. Med. Parasitol. 99:511-517. [DOI] [PubMed] [Google Scholar]
- 6.Bonetti, V. C., and C. Graeff-Teixeira. 1998. Angiostrongylus costaricensis and the intermediate hosts: observations on elimination of L3 in the mucus and inoculation of L1 through the tegument of mollucs. Rev. Soc. Bras. Med. Trop. 31:289-294. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, B. G., and M. D. Little. 1988. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am. J. Trop. Med. Hyg. 38:568-573. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X. G., H. Li, and Z. R. Lun. 2005. Angiostrongyliasis, mainland China. Emerg. Infect. Dis. 11:1645-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, H. 2005. A letter regarding a case of eosinophilic meningitis. Hawaii Med. J. 64:60. [PubMed] [Google Scholar]
- 10.Chye, S. M., S. R. Lin, Y. L. Chen, L. Y. Chung, and C. M. Yen. 2004. Immuno-PCR for detection of antigen to Angiostrongylus cantonensis circulating fifth-stage worms. Clin. Chem. 50:51-57. [DOI] [PubMed] [Google Scholar]
- 11.Costa, L. R. R., J. J. McClure, T. G. Snider III, and T. B. Stewart. 2000. Verminous meningoencephalomyelitis by Angiostrongylus (= Parastrongylus) cantonensis in an American miniature horse. Equine Vet. Educ. 12:2-6. [Google Scholar]
- 12.da Silva, A. J., F. J. Bornay-Llinares, I. N. Moura, S. B. Slemenda, J. L. Tuttle, and N. J. Pieniazek. 1999. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 4:57-64. [DOI] [PubMed] [Google Scholar]
- 13.Duffy, M. S., C. L. Miller, J. M. Kinsella, and A. de Lahunta. 2004. Parastrongylus cantonensis in a nonhuman primate, Florida. Emerg. Infect. Dis. 10:2207-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graeff-Teixeira, C., and P. Morera. 1995. Método de digestão de moluscos em ácido clorídrico para isolamento de larvas de metastrongilídeos. Biociencias 3:85-89. [Google Scholar]
- 15.Heyneman, D., and B. L. Lim. 1967. Angiostrongylus cantonensis: proof of direct transmission with its epidemiological implications. Science 158:1057-1058. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth, R. G., R. Kaneta, S. J. J., H. S. Bishop, Y. Qvarnstrom, A. J. da Silva, and D. Robinson. Distribution of Parmarion cf. martensi Simroth 1893, a new semi-slug pest on Hawaii Island, and its potential as a vector for human angiostrongyliasis. Pac. Sci., in press.
- 17.Kliks, M. M., and N. E. Palumbo. 1992. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc. Sci. Med. 34:199-212. [DOI] [PubMed] [Google Scholar]
- 18.Kuberski, T., R. D. Bart, J. M. Briley, and L. Rosen. 1979. Recovery of Angiostrongylus cantonensis from cerebrospinal fluid of a child with eosinophilic meningitis. J. Clin. Microbiol. 9:629-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindo, J. F., C. Waugh, J. Hall, C. Cunningham-Myrie, D. Ashley, M. L. Eberhard, J. J. Sullivan, H. S. Bishop, D. G. Robinson, T. Holtz, and R. D. Robinson. 2002. Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg. Infect. Dis. 8:324-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh, C. M. 1998. Eosinophilic meningitis/angiostrongyliasis from eating aquaculture-raised snails: a case report. Hawaii Med. J. 57:652-654. [PubMed] [Google Scholar]
- 21.Morera, P. 1973. Life history and redescription of Angiostrongylus costaricensis Morera and Cespedes, 1971. Am. J. Trop. Med. Hyg. 22:613-621. [DOI] [PubMed] [Google Scholar]
- 22.Morera, P., and R. Cespedes. 1970. Angiostrongylus costaricensis n. sp. (Nematoda: Metastrongyloidea), a new lungworm occurring in man in Costa Rica. Rev. Biol. Trop. 18:173-185. [PubMed] [Google Scholar]
- 23.New, D., M. D. Little, and J. Cross. 1995. Angiostrongylus cantonensis infection from eating raw snails. N. Engl. J. Med. 332:1105-1106. [DOI] [PubMed] [Google Scholar]
- 24.Nomura, S., and P. H. Lin. 1945. First case report of human infection with Haemostrongylus ratt Yokogawa. Taiwan No Ikai 3:589-592. [Google Scholar]
- 25.Prociv, P., D. M. Spratt, and M. S. Carlisle. 2000. Neuro-angiostrongyliasis: unresolved issues. Int. J. Parasitol. 30:1295-1303. [DOI] [PubMed] [Google Scholar]
- 26.Raccurt, C. P., J. Blaise, and M. C. Durette-Desset. 2003. Presence of Angiostrongylus cantonensis in Haiti. Trop. Med. Int. Health 8:423-426. [DOI] [PubMed] [Google Scholar]
- 27.Slom, T. J., M. M. Cortese, S. I. Gerber, R. C. Jones, T. H. Holtz, A. S. Lopez, C. H. Zambrano, R. L. Sufit, Y. Sakolvaree, W. Chaicumpa, B. L. Herwaldt, and S. Johnson. 2002. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N. Engl. J. Med. 346:668-675. [DOI] [PubMed] [Google Scholar]
- 28.Ubelaker, J. E., G. R. Bullick, and J. Caruso. 1980. Emergence of third-stage larvae of Angiostrongylus costaricensis Morera and Cespedes 1971 from Biomphalaria glabrata (Say). J. Parasitol. 66:856-857. [PubMed] [Google Scholar]