Abstract
To investigate the role of superoxide dismutase (SOD) in virulence against the silkworm, Bombyx mori, mutants of Pseudomonas aeruginosa PAO1 lacking manganese-SOD (PAO1sodM), iron-SOD (PAO1sodB), or both (PAO1sodMB) were generated. The mutants were injected into the hemocoel of B. mori. The virulence decreased in the order PAO1 = PAO1sodM > PAO1sodB > PAO1sodMB. In particular, PAO1sodMB was avirulent at a dose of 105 cells or less. The sod double mutant PAO1sodMB was then complemented with either pSodM or pSodB in trans. In both the complemented strains, the virulence was partially restored. Of the two plasmids, pSodB contributed more to the virulence of P. aeruginosa against B. mori. The results of growth in B. mori hemolymph broth and microscopic analysis suggested that a longer lag phase and superoxide sensitivity correlated with decreased virulence in sod mutants. In conclusion, the SODs are required for full virulence of P. aeruginosa against B. mori and Fe-SOD is more important than Mn-SOD in the infection process.
Pseudomonas aeruginosa is ubiquitous and is found in diverse environments including soil, freshwater, and marine environments. It is also an opportunistic pathogen in three distinct groups of organisms: vertebrates, invertebrates, and plants (17, 18).
Similar to other pathogens, P. aeruginosa must overcome the oxidative stress response generated by the host for successful infection. During the process of active infection, the primary source of exogenous oxidative stress for pathogenic bacteria is attack by host phagocytic cells. Phagocytes utilize the cytotoxic effects of many of the reactive oxygen species, such as superoxide, hydrogen peroxide, and the highly toxic hydroxyl radical. These reactive oxygen species can damage the nucleic acids, proteins, and cell membranes of pathogens. On the other hand, pathogens have effective enzymatic pathways of oxidant inactivation, including those catalyzed by superoxide dismutase (SOD), catalase/peroxidase, and glutathione in combination with glutathione peroxidase and glutathione reductase (12, 13). SOD represents the first line of defense against superoxide stress by converting superoxide into hydrogen peroxide and oxygen, thereby protecting cells from the toxic effects of superoxide.
P. aeruginosa possesses both manganese-cofactored SOD (Mn-SOD) and iron-cofactored SOD (Fe-SOD), and the genes encoding these proteins (sodM, previously referred to as sodA, and sodB, respectively) have been cloned and characterized (14, 15, 16). The expression of these SODs is controlled by environmental factors. In the presence of relatively high concentrations of extracellular iron, Fe-SOD is preferentially expressed, whereas the expression of Fe-SOD decreases and Mn-SOD is produced under iron-limited conditions (5, 14). For example, P. aeruginosa PAO1 produces mainly Fe-SOD in Luria-Bertani (LB) broth. In contrast Mn-SOD is produced in low-phosphate succinate (LPS) broth (16).
Since superoxide is endogenously produced under aerobic conditions, inactivation of SOD often results in decreased viability. In fact, sodB and sodM sodB double mutants of PAO1 have been shown to grow very slowly. In addition, the double mutants demonstrated severe auxotrophy (16).
Hassett et al. (16) also reported that a mutation in sodM only slightly increased sensitivity to paraquat, a superoxide generator. However, the sodB mutant and, to a greater extent, the double mutant demonstrated a marked increase in sensitivity to paraquat (16).
The isolation of Mn-SOD-deficient P. aeruginosa from blood indicates that Fe-SOD is more important than Mn-SOD to its virulence in humans (5). However, the interaction of the pathogen with other hosts is not well understood. The silkworm, Bombyx mori, is a good insect model with which to study the interaction between insects and opportunistic bacterial pathogens since it is one of the best-characterized insect species, both phenotypically and genotypically (9, 19, 20). In this study, we constructed sodM, sodB, and double mutants in PAO1, a wild-type strain of P. aeruginosa, and then compared the virulence levels of these mutants in B. mori.
MATERIALS AND METHODS
Organisms, plasmids, and media.
The bacterial strains, plasmids, and primers used in this study are listed in Table 1. P. aeruginosa PAO1 was a kind gift of J. Kato, Hiroshima University, Hiroshima, Japan. The strain was originally donated by the Anand M. Chakrabaty laboratory, University of Illinois at Chicago.
TABLE 1.
Bacteria, plasmids, and primers used in this study
| Bacterium, plasmid, or primer | Description or sequencea | Reference or source |
|---|---|---|
| Bacteria | ||
| Pseudomonas aeruginosa | ||
| PAO1 | Wild type | Hiroshima University, Japan, via J. Kato |
| PAO1sodM | Mn-SOD mutant of PAO1; sodM::Ωaac Gmr | This study |
| PAO1sodB | Fe-SOD mutant of PAO1; sodB::ΩTcTcr | This study |
| PAO1sodMB | SOD double mutant of PAO1; sodM::Ωaac sodB::ΩTc Gmr Tcr | This study |
| Escherichia coli | ||
| DH5α | F−recA1 endA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 Δ(lacZYA-argF)U169 λ− (φ80dlacZΔM15) | 25 |
| S17-1 | thi pro hsdR recA; chromosomal RP4; Tra−; Tpr Sm/Spr | 29 |
| Plasmids | ||
| pGEM-T Easy | PCR cloning vector; AmprlacZ f1 ori/ori | Promega |
| pBBR1MCS2 | Broad-host-range vector; Kmr | 22 |
| pK18mobsacB | Allelic-exchange suicide vector; KmrsacB oriT (RP4) lacZ | 27 |
| pHP45Ωaac | Source of gentamicin resistance cassette; Ampr Gmr | 3 |
| pHP45ΩTc | Source of tetracycline resistance cassette; Ampr Tcr | 11 |
| pGSodM | 3.0-kb sodM fragment in pGEM-T Easy | This study |
| pGSodB | 1.1-kb sodB fragment in pGEM-T Easy | This study |
| pSodM | 3.0-kb sodM fragment in pBBR1MCS2 | This study |
| pSodB | 1.1-kb sodB fragment in pBBR1MCS2 | This study |
| pGSodM5′ | 2.5-kb fragment of 5′ sodM in pGEM-T Easy | This study |
| pGSodM3′ | 0.5-kb fragment of 3′ sodM in pGEM-T-Easy | This study |
| pGSodB5′ | 0.5-kb fragment of 5′ sodB in pGEM-T Easy | This study |
| pGSodB3′ | 0.7-kb fragment of 3′ sodB in pGEM-T Easy | This study |
| pGSodMHind | 3.0-kb sodM fragment containing HindIII site in pGEM-T Easy | This study |
| pGSodBHind | 1.1-kb sodB fragment containing HindIII site in pGEM-T Easy | This study |
| pGSodMΩaac | 2.9-kb mutated sodM fragment in pGEM-T Easy | This study |
| pGSodBΩTc | 3.3-kb mutated sodB fragment in pGEM-T Easy | This study |
| pKSodM | 2.9-kb mutated sodM fragment in pK18mobsacB | This study |
| pKSodB | 3.3-kb mutated sodB fragment in pK18mobsacB | This study |
| Primers | ||
| sodMf(−2350) | TTCAATTGTCCGCCTCTAT | This study |
| sodMr626 | CATCACGGGTAACACTAGG | This study |
| sodMf133HindIII | GCGAAGCTTGGCACGCCCTATGC | This study |
| sodMr124HindIII | CTTAAGCTTCGGCGTTCAGGTTGT | This study |
| sodBf(−346) | CGCGGTTTCTGTCTTC | This study |
| sodBr760 | GGCCTTCCTTGTTCGT | This study |
| sodBf110HindIII | CCTAAGCTTTGAACCTGAACAACCTGA | This study |
| sodBr109HindIII | TCAAAGCTTCCACGTAGGTGTTGTGGT | This study |
Abbreviations for phenotype: Ampr, ampicillin resistance; Gmr, gentamicin resistance; Spr, spectinomycin resistance; Smr, streptomycin resistance; Tpr, trimethoprim resistance.
Cultures of Escherichia coli and P. aeruginosa were routinely grown in LB medium at 37°C and 30°C, respectively. For the superoxide dismutase assay and mortality study, P. aeruginosa strains were grown in LB and LPS (7) broth. Antibiotics were used at the following concentrations (E. coli and P. aeruginosa): 50 μg/ml ampicillin, 5 μg/ml streptomycin, 50 μg/ml trimethoprim, 15 and 50 μg/ml gentamicin (Gm), 15 and 50 μg/ml tetracycline (Tc), and 30 and 200 μg/ml kanamycin (Km). In all the experiments, PAO1sodM, PAO1sodB, and PAO1sodMB were cultured in the medium containing Gm, Tc, and both Gm and Tc, respectively. The strains of P. aeruginosa carrying pBBR1MCS2 and derivatives were cultured in the media supplemented with Km. Eggs of silkworm (B. mori) hybrids of Kinshu × Showa were obtained from Ueda Sanshu (Ueda, Nagano, Japan). Larvae were reared on an artificial diet (Nihon Nosan Kogyo, Yokohama, Japan) at 25°C. In all the studies, B. mori worms at the fourth larval instar were used.
Construction of superoxide mutants.
The 3.0-kb sodM fragment was amplified by PCR using KOD Plus polymerase (TOYOBO Co., Ltd., Osaka, Japan) and the primers sodMf(−2350) and sodMr626 (95°C for 2 min, 35 cycles of 15 s at 94°C and 30 s at 55°C, and 3 min at 68°C). The A-overhanging sodM fragment was cloned into pGEM-T Easy to obtain pGSodM. In addition to the above primers, we used specific primers (sodMf133HindIII and sodMr124HindIII) to create suitable restriction sites in sodM. The 2.5-kb 5′ region and 0.5-kb 3′ region of sodM were amplified and cloned into pGEM-T Easy to create pGSodM5′ and pGSodM3′, respectively. To construct pGSodMHind, a small EcoRI-HindIII fragment was eliminated from pGSodM5′ and subsequently ligated with the 0.5-kb EcoRI-HindIII fragment of pGSodM3′. Then, the Ωaac cassette from pHP45Ωaac was inserted into the artificial HindIII site of pGSodMHind, designated pGSodMΩaac. To construct pKSodM, a 2.9-kb BamHI-EcoRI fragment of pGSodMΩaac harboring a disrupted sodM gene was ligated into pK18mobsacB. To create pSodM, a 3.0-kb EcoRI fragment of pGSodM was cloned into a broad-host-range plasmid, pBBR1MCS2. pKsodM and pSodM were used for the disruption and complementation of sodM, respectively. For the disruption and complementation of sodB, pKsodB and pSodB were constructed using a similar procedure.
The plasmid pKsodM or pKsodB was mobilized from the E. coli strain S17-1 (29) into P. aeruginosa PAO1, followed by selection on LB containing 10% sucrose and appropriate antibiotics to obtain sodM- or sodB-disrupted mutants. Similarly, to create a sodM sodB double mutant, the gentamicin-resistant sodM mutant was mated with E. coli S17-1 containing pKsodB. The sodM, sodB, and sodM sodB double mutant strains were designated PAO1sodM, PAO1sodB, and PAO1sodMB, respectively.
Similarly plasmids pSodM and pSodB were transferred into the sod mutants by biparental mating for complementation. Plasmids were extracted from the complemented strains by the alkaline lysis procedure and analyzed.
B. mori mortality studies.
Virulence of the sod mutants toward B. mori was estimated by the method reported by Chieda et al. (6). Briefly, the inocula were cultured in LB and LPS broth with appropriate antibiotics, since P. aeruginosa mainly produced Fe-SOD and Mn-SOD in LB and LPS, respectively (5, 16). The cells were harvested at mid-logarithmic phase by centrifugation at 20,630 × g for 1 min at 4°C. The pellets were resuspended with sterilized water, and the concentration was adjusted according to the optical density at 660 nm (OD660). The bacterial concentration was adjusted based on the standard curve showing the correlation between OD660 and viable cell number determined on LB using PAO1. Prior to inoculation, the bacterial suspension was stained with 10 μg/ml propidium iodide (PI; Dojindo Laboratories, Kumamoto, Japan) to determine the viability of the bacteria. Ten microliters of the bacterial suspension was injected with a syringe into fourth-instar B. mori larvae. The mortality of the inoculated larvae was monitored. Five larvae were injected per dilution, and three replicates per trial were performed.
Bacterial growth in the hemolymph of B. mori.
The hemolymph was collected from the fourth instar of B. mori. To inactivate the host's defense reactions, comprised of a phenol oxidase cascade and phagocytosis, the collected hemolymph was immediately treated at 60°C for 10 min. After centrifugation at 20,630 × g for 15 min at 25°C, the supernatant was filter sterilized (0.45 μm) and the filtrate was designated B. mori hemolymph (BMH) broth. The strains were grown overnight in LB broth with appropriate antibiotics at 30°C. BMH broth (3 ml) was inoculated with 3 μl of the LB culture and incubated at 25°C until the OD660 reached 0.1. At this point, a 30-μl aliquot of preculture was inoculated into 3 ml of fresh BMH broth with appropriate antibiotics and shaken at 70 rpm under aerobic conditions at 25°C to mimic the hemolymph of B. mori with the exception of the host's defense reactions. The OD660 was automatically recorded every 10 min with an Advantec TVS062CA biophotorecorder (Advantec Toyo Co. Ltd., Tokyo, Japan).
Microscopic analysis.
The hemolymph of the B. mori worms inoculated with 105 cells of various strains of P. aeruginosa was collected every 6 h after inoculation. Inoculation was carried out as described above. The hemolymph smears on the slide were stained with Giemsa solution, mounted with Permount (Fisher Scientific, Fair Lawn, NJ), and observed under a light microscope.
Growth of wild-type strain in B. mori.
PAO1(pBBR1MCS2) was cultured in LB containing Km. B. mori worms were inoculated with 105 cells of the strain as described above. To monitor the bacterial growth, the hemolymph was collected and diluted and then spread on LB plates supplemented with Km.
RESULTS
Inactivation of sodM and sodB of P. aeruginosa.
The sodM, sodB, and sodM sodB double mutants of P. aeruginosa PAO1 were created. The insertion of Ω cassettes in the mutants was confirmed by Southern hybridization (data not shown). In the complemented strains, expected plasmids were detected (data not shown).
Mortality assay.
In order to study the possible impact of SOD on the virulence of P. aeruginosa PAO1 against B. mori, various doses of SOD mutants were inoculated into the larvae. Inocula were cultured in LB and LPS broth and collected during the logarithmic growth phase. The results of the PI staining indicated that almost all bacteria were viable at this stage in all strains (data not shown). The inocula were adjusted at 108, 107, 106, and 105 CFU/ml based on OD660. Data from three inoculation experiments were analyzed (Fig. 1).
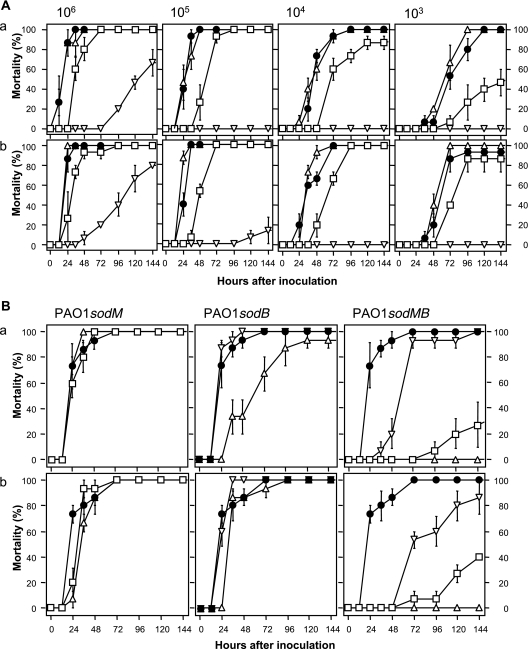
FIG. 1.
Mortality assay of the sod mutants (A) and the complemented strains (B) of P. aeruginosa PAO1 in B. mori. Each strain was precultured in LB (a) or LPS (b). P. aeruginosa PAO1 (circles), PAO1sodM (triangles), PAO1sodB (squares), and PAO1sodMB (inverted triangles) were injected into the fourth instar of B. mori larvae at various doses (A). Similarly, the complemented strain was inoculated (B). PAO1(pBBR1MCS2), the sod mutants carrying pBBR1MCS2, the sod mutants carrying pSodM, and the sod mutant carrying pSodB are represented as circles, triangles, squares, and inverted triangles, respectively. In the inoculation test using the complemented strains, the dose was 105 cells. Five larvae were injected per dilution. Each data point is an average of three replicates, and the error bar indicates the standard error of the mean.
Inoculation with PAO1 and PAO1sodM at 106, 105, 104, and 103 cells resulted in mortalities of 100, 100, 90, and 50%, respectively, within 72 h, and almost all larvae died within 144 h with no significant differences between these strains (Fig. 1A). On the other hand, PAO1sodB displayed a decreased rate of mortality in comparison with the above two strains. The mutant showed a marked decline in virulence when the inoculation dose was low. In contrast, almost all larvae inoculated with PAO1sodMB at 105 cells or less survived until 144 h. The virulence decreased in the order PAO1 = PAO1sodM > PAO1sodB > PAO1sodMB. Although the type of medium did not influence this order, PAO1sodB was more virulent when it was precultured in LPS.
The virulence of complemented strains was also investigated (Fig. 1B). In this assay, PAO1 harboring pBBR1MCS2 was used as a positive control. In the case of sodM, no significant difference was observed among PAO1(pBBR1MCS2), PAO1sodM(pBBR1MCS2), and PAO1sodM(pSodM). The virulence of PAO1sodB was completely restored when the sodB gene was reintroduced in the mutant by the plasmid pSodB. The sod double mutant, PAO1sodMB, was complemented with either pSodM or pSodB. In both of the complemented strains, the virulence was partially restored. Of the two plasmids, pSodB contributed more to the virulence of P. aeruginosa against B. mori.
Bacterial growth in the extracted hemolymph of B. mori.
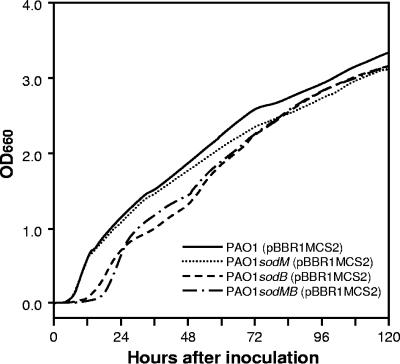
Growth of P. aeruginosa strains in BMH broth is shown in Fig. 2. All strains could propagate in the broth, and the OD660 reached 3.0 at around 120 h after inoculation. The growth rates during the logarithmic phase were similar among the strains. For example, the required times for the OD660 to increase from 0.1 to 2.0 were 46.7, 51.2, 52.8, and 47.3 h for PAO1, PAO1sodM, PAO1sodB, and PAO1sodMB, respectively. However, longer lag phase times were observed in PAO1sodB and PAO1sodMB than in PAO1 and PAO1sodM. The OD660 of the cultures of PAO1and PAO1sodM reached 0.1 within 6.3 h after inoculation. However, the PAO1sodB and PAO1sodMB strains required 13.2 and 17.3 h, respectively, to reach this OD value.
FIG. 2.
Growth of P. aeruginosa strains in BMH broth. The strains were grown overnight in LB broth at 30°C. Three microliters of the LB culture was subcultured into 3 ml of BMH broth and incubated at 25°C until an OD660 of 0.1 was reached. Then, a 30-μl aliquot of preculture was inoculated into 3 ml of fresh BMH broth and shaken at 70 rpm under aerobic conditions at 25°C. The OD660 was automatically recorded with an Advantec TVS062CA biophotorecorder (Advantec Toyo Co. Ltd., Tokyo, Japan). Three experiments were repeated to ensure reproducibility and gave equivalent results. Each plot represents the average of triplicates.
Microscopic analysis.
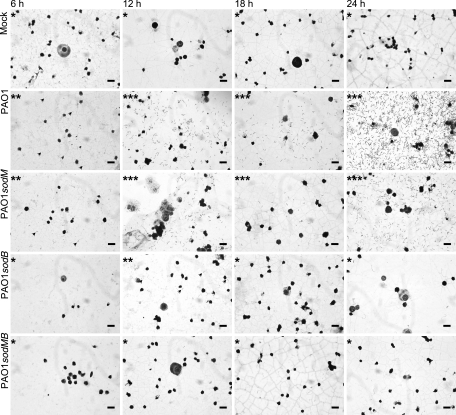
Bacterial propagation in the hemocoel of the B. mori worms infected with P. aeruginosa strains at a dose of 105 cells was investigated using light microscopy (Fig. 3). A few cells of bacteria were observed 6 h after inoculation with PAO1. Although an increase in the bacterial population was achieved by 12 h, a significant decrease occurred 18 h after inoculation. The cell number then increased markedly at 24 h after inoculation. A similar change of cell density was observed in the case of PAO1sodM. In contrast, PAO1sodB was detected at low density only at 12 h after inoculation. At other time points (6, 18, and 24 h), no bacterial cells were observed. Cells of PAO1sodMB were not detected at any observation times.
FIG. 3.
Light microscopic observation of the hemolymph from B. mori inoculated with P. aeruginosa strains. From each strain 105 cells were injected into the fourth-instar larvae. The hemolymph was collected every 6 h after inoculation. The smear of collected hemolymph was stained with Giemsa solution. A single asterisk means that bacterial cells were not observed. A double asterisk means that the bacterial density was low. A triple asterisk means that the bacterial density was high. On the plate, some of the bacterial cells are shown with arrowheads. Bars, 20 μm.
Bacterial growth in B. mori.
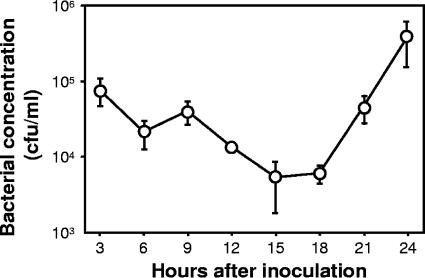
Growth of PAO1(pBBR1MCS2) in the hemocoel of B. mori larvae was monitored (Fig. 4). The bacterial concentrations were 7.8 × 104, 2.1 × 104, and 4.0 × 104 CFU/ml after 3, 6, and 9 h after inoculation, respectively. The concentration gradually decreased until 15 h and then increased. At the 24th hour after inoculation, the population reached approximately 3.9 × 105 CFU/ml.
FIG. 4.
Growth of P. aeruginosa PAO1(pBBR1MCS2) in B. mori. Cells (105) were injected into the fourth-instar larvae. A hemolymph sample of a single larva was collected every 3 h after inoculation. Three samples were independently obtained from different larvae. The number of viable cells in the hemolymph was estimated by a plate count procedure. Each plotted point represents the average of triplicates. Error bars indicates standard errors.
DISCUSSION
P. aeruginosa opportunistically infects not only vertebrate animals but also invertebrate animals. The contribution of SOD to bacterial virulence has previously been discussed for many animal pathogens, including Vibrio shiloi (1), Haemophilus ducreyi (4), Aeromonas salmonicida subsp. salmonicida (8), Mycobacterium tuberculosis (10), Staphylococcus aureus (21), Streptococcus agalactiae (23), Shigella spp. (24), Salmonella enterica serovar Choleraesuis (26), and Helicobacter pylori (28). P. aeruginosa PAO1 possesses two SODs, Mn-SOD and Fe-SOD (15). To understand the role of these SODs in the infection process of invertebrates, we constructed sod mutants and inoculated these mutants into the silkworm, B. mori. Previously it was shown that P. aeruginosa PAO1 produced mainly Fe-SOD in LB broth, whereas it produced Mn-SOD in LPS broth (16). This result meant that PAO1sodB that had been precultured in LB did not possess SOD in the cell. Similarly, no SOD was produced by PAO1sodM when the mutant was cultured in LPS. To avoid the effect of the medium on the virulence of the sod mutants, we separately prepared the inocula cultured in LB and LPS broth and then inoculated B. mori in this study.
In comparison with PAO1, PAO1sodM was of approximately equal virulence, but the virulence of PAO1sodB declined. The double mutant did not have a mortality effect on B. mori at doses of ≤105 cells. To confirm whether the virulence in the double mutant could be restored by complementation with sodM and sodB, B. mori worms were inoculated with PAO1sodMB harboring pBBR1MCS2, pSodM, or pSodB. The virulence of the complemented strain of PAO1sodMB was in the following order: PAO1sodMB(pSodB) > PAO1sodMB(pSodM) > PAO1sodMB(pBBR1MCS2). The difference in culture conditions (LB or LPS) had no effect on the order of virulence against B. mori (Fig. 1). These results indicate that both Mn-SOD and Fe-SOD are functional but that Fe-SOD is more important than Mn-SOD in the virulence of P. aeruginosa against B. mori.
Hassett et al. reported that Fe-SOD is the key enzyme in aerobic metabolism and against oxidative stress (16). In their studies, the sodB mutant grew much more slowly than the sodM mutant or the wild-type strain. The double mutant demonstrated an even lower rate of aerobic growth than the sodB mutant. The double mutant was especially incapable of growth in glucose minimal medium, suggesting auxotrophy. This auxotrophy in PAOsodB and PAO1sodMB constructed in the present study was confirmed, and the cells cultured in the media used in this study also had the same characteristics (data not shown). To ascertain the effect of growth speed and auxotrophy on virulence, the growth in BMH broth under aerobic conditions at 25°C, which mimics in vivo conditions, was monitored. The growth speeds were similar during the logarithmic phase among all the strains in this broth. However, the length of the lag phase was prolonged in PAO1sodB and PAO1sodMB. This phenotypic change could be one reason for the decreased virulence seen in these strains. However, the reduced virulence could not be completely explained by only the length of the lag phase, because all strains were able to grow under the environmental conditions. The initial concentration in BMH broth was approximately 106 CFU/ml. This concentration was equivalent to the bacterial cell density in hemolymph inoculated with 105 cells, since the total volume of hemolymph was 100 to 200 μl in fourth-larval-instar silkworms. At this concentration, PAO1sodMB was avirulent (Fig. 1).
Microscopic analysis was then performed to clarify the behavior of the bacterial cells injected into hemolymph. A few of the cells of PAO1sodB could be detected at 12 h after inoculation, while no cells of the double mutant PAO1sodMB were observed. In the sod mutants of PAO1, sensitivity to paraquat, a superoxide generator, was increased in the order PAO1 < sodM mutant < sodB mutant < sodM sodB double mutant (16). In the interaction between host and microorganism, superoxide is generated by macrophages and other phagocytic cells. This first defense mechanism is conserved in insects (2). SODs have been reported to enhance bacterial survival by inhibition of the macrophage oxidative burst. Thus, the increase of sensitivity to oxidative stress would be another factor contributing to the reduced virulence of certain strains. More detailed analysis is needed to clarify the interaction between the sod mutants and the phagocytic cells in B. mori.
The bacterial population decreased 15 to 18 h after inoculation (Fig. 4). The growth curve of the viable bacterial cells in the hemocoel of B. mori infected with PAO1(pBBR1MCS2) also supported the decline of the bacterial cells in our microscopic observations. This population decline may have been due to the antibacterial substances produced by the host cells. B. mori produces antibacterial substances, including cecropin, attacin, lebocin, and moricin. These compounds are mainly released from the fat body and from hemocytes after bacterial infection (30). Since the population of PAO1sodB was low at 12 h after inoculation, almost all of the mutant cells would have been killed by this subsequent defense response induced by the host cell. The sensitivity to these antibacterial substances must be investigated in the sod mutants.
Taken together, the results of this study suggest that SODs are required for the full virulence of P. aeruginosa against B. mori and that Fe-SOD is more important than Mn-SOD in the infection process.
Acknowledgments
This work was supported in part by a grant-in-aid (15208007) from the Japan Society for the Promotion of Science. Y. Chieda is a Research Fellow supported by a scholarship from the Japan Society for the Promotion of Science for Young Scientists.
We thank Mathias Keller for supplying us with pK18mobsacB and E. coli strain S17-1. We also thank Junichi Kato, Joachim Frey, and Marie-Héléne Blondelet-Rouault for providing strains PAO1, pHP45ΩTc, and pHP45Ωaac, respectively. Thanks are also given to Michael E. Kovach for the donation of pBBR1MCS2.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Banin, E., D. Vassilakos, E. Orr, R. J. Martinez, and E. Rosenberg. 2003. Superoxide dismutase is a virulence factor produced by the coral bleaching pathogen Vibrio shiloi. Curr. Microbiol. 46:418-422. [DOI] [PubMed] [Google Scholar]
- 2.Bergin, D., E. P. Reeves, J. Renwick, F. B. Wientjes, and K. Kavanagh. 2005. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect. Immun. 73:4161-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blondelet-Rouault, M. H., J. Weiser, A. Lebrihi, P. Branny, and J. L. Pernodet. 1997. Antibiotic resistance gene cassettes derived from the Ω interposon for use in E. coli and Streptomyces. Gene 190:315-317. [DOI] [PubMed] [Google Scholar]
- 4.Bong, C. T., K. R. Fortney, B. P. Katz, A. F. Hood, L. R. San Mateo, T. H. Kawula, and S. M. Spinola. 2002. A superoxide dismutase C mutant of Haemophilus ducreyi is virulent in human volunteers. Infect. Immun. 70:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britigan, B. E., R. A. Miller, D. J. Hassett, M. A. Pfaller, M. L. McCormick, and G. T. Rasmussen. 2001. Antioxidant enzyme expression in clinical isolates of Pseudomonas aeruginosa: identification of an atypical form of manganese superoxide dismutase. Infect. Immun. 69:7396-7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chieda, Y., K. Iiyama, C. Yasunaga-Aoki, J. M. Lee, T. Kusakabe, and S. Shimizu. 2005. Pathogenicity of gacA mutant of Pseudomonas aeruginosa PAO1 in the silkworm, Bombyx mori. FEMS Microbiol. Lett. 244:181-186. [DOI] [PubMed] [Google Scholar]
- 7.Cox, C. D. 1986. Role of pyocyanin in the acquisition of iron from transferrin. Infect. Immun. 52:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacanay, A., S. C. Johnson, R. Bjornsdottir, R. O. Ebanks, N. W. Ross, M. Reith, R. K. Singh, J. Hiu, and L. L. Brown. 2003. Molecular characterization and quantitative analysis of superoxide dismutases in virulent and avirulent strains of Aeromonas salmonicida subsp. salmonicida. J. Bacteriol. 185:4336-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doira, H. 1980. Silkworms as a model for human diseases. Jikken Dobutsu 29:369-372. (In Japanese.) [PubMed] [Google Scholar]
- 10.Edwards, K. M., M. H. Cynamon, R. K. Voladri, C. C. Hager, M. S. DeStefano, K. T. Tham, D. L. Lakey, M. R. Bochan, and D. S. Kernodle. 2001. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 164:2213-2219. [DOI] [PubMed] [Google Scholar]
- 11.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 12.Haas, A., and W. Goebel. 1992. Microbial strategies to prevent oxygen-dependent killing by phagocytes. Free Radic. Res. Commun. 16:137-157. [DOI] [PubMed] [Google Scholar]
- 13.Hassett, D. J., and M. S. Cohen. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 3:2574-2582. [DOI] [PubMed] [Google Scholar]
- 14.Hassett, D. J., L. Charniga, K. Bean, D. E. Ohman, and M. S. Cohen. 1992. Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect. Immun. 60:328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassett, D. J., W. A. Woodruff, D. J. Wozniak, M. L. Vasil, M. S. Cohen, and D. E. Ohman. 1993. Cloning and characterization of the Pseudomonas aeruginosa sodA and sodB genes encoding manganese- and iron-cofactored superoxide dismutase: demonstration of increased manganese superoxide dismutase activity in alginate-producing bacteria. J. Bacteriol. 175:7658-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassett, D. J., H. P. Schweizer, and D. E. Ohman. 1995. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J. Bacteriol. 177:6330-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarrell, K. F., and A. M. Kropinski. 1982. The virulence of protease and cell surface mutants of Pseudomonas aeruginosa for the larvae of Galleria mellonella. J. Invertebr. Pathol. 39:395-400. [DOI] [PubMed] [Google Scholar]
- 19.Kaito, C., N. Akimitsu, H. Watanabe, and K. Sekimizu. 2002. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32:183-190. [DOI] [PubMed] [Google Scholar]
- 20.Kaito, C., K. Kurokawa, Y. Matsumoto, Y. Terao, S. Kawabata, S. Hamada, and K. Sekimizu. 2005. Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol. Microbiol. 56:934-944. [DOI] [PubMed] [Google Scholar]
- 21.Karavolos, M. H., M. J. Horsburgh, E. Ingham, and S. J. Foster. 2003. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 149:2749-2758. [DOI] [PubMed] [Google Scholar]
- 22.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 23.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 69:5098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash, R., H. Devaraj, and S. N. Devaraj. 2004. Identification of an atypical form of 30 kDa SOD—a possible virulence factor in clinical isolates of Shigella spp. Mol. Cell. Biochem. 267:91-98. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 26.Sansone, A., P. R. Watson, T. S. Wallis, P. R. Langford, and J. S. Kroll. 2002. The role of two periplasmic copper- and zinc-cofactored superoxide dismutases in the virulence of Salmonella choleraesuis. Microbiology 148:719-726. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 28.Seyler, R. W., Jr., J. W. Olson, and R. J. Maier. 2001. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect. Immun. 69:4034-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 30.Yamakawa, M., and H. Tanaka. 1999. Immune proteins and their gene expression in the silkworm, Bombyx mori. Dev. Comp. Immunol. 23:281-289. [DOI] [PubMed] [Google Scholar]