Abstract
The fate of selenium in the environment is controlled, in part, by microbial selenium oxyanion reduction and Se(0) precipitation. In this study, we identified a genetic regulator that controls selenate reductase activity in the Se-reducing bacterium Enterobacter cloacae SLD1a-1. Heterologous expression of the global anaerobic regulatory gene fnr (fumarate nitrate reduction regulator) from E. cloacae in the non-Se-reducing strain Escherichia coli S17-1 activated the ability to reduce Se(VI) and precipitate insoluble Se(0) particles. Se(VI) reduction by E. coli S17-1 containing the fnr gene occurred at rates similar to those for E. cloacae, with first-order reaction constants of k = 2.07 × 10−2 h−1 and k = 3.36 × 10−2 h−1, respectively, and produced elemental selenium particles with identical morphologies and short-range atomic orders. Mutation of the fnr gene in E. cloacae SLD1a-1 resulted in derivative strains that were deficient in selenate reductase activity and unable to precipitate elemental selenium. Complementation by the wild-type fnr sequence restored the ability of mutant strains to reduce Se(VI). Our findings suggest that Se(VI) reduction and the precipitation of Se(0) by facultative anaerobes are regulated by oxygen-sensing transcription factors and occur under suboxic conditions.
The chemical speciation of selenium controls its mobility in natural waters and biological effect on animal life (5, 27, 44). While selenium is an essential micronutrient (27), the higher valence states of Se(VI) and Se(IV) are toxic at elevated concentrations and can cause severe poisoning of fish and waterfowl in contaminated environments (11, 22, 32, 40). The reduction of soluble selenate [Se(VI), SeO42−] and selenite [Se(IV), SeO32−] to the less toxic Se(0) converts selenium into an insoluble mineral form. In soils and sediments, this transformation is largely mediated by microorganisms (30, 31, 43, 47), although abiotic reduction of selenium oxyanions can also occur in the presence of the Fe(II)-Fe(III) hydroxide mineral green rust (28). Anaerobic Se-respiring bacteria can use Se(VI) and Se(IV) as terminal electron acceptors and precipitate elemental selenium granules (26, 29, 30). Aerobic and phototrophic Se-resistant bacteria can also catalyze the reduction of selenium oxyanions to form insoluble Se(0) particles (1, 15, 35, 38). However, despite the ubiquity of Se-reducing bacteria in diverse terrestrial and aquatic environments (P. Narasingarao and M. M. Haggblom, unpublished data), the mechanisms of Se(0) biomineralization are poorly understood.
Microbial reduction of selenium oxyanions generates red elemental selenium with either crystalline or amorphous structures (24, 25). Recently, Oremland et al. (29) demonstrated that the Se(0) particles formed by the Se-respiring bacteria Sulfurospirillum barnesii, Bacillus selenitireducens, and Selenihalanaerobacter shriftii are structurally unique compared to elemental selenium formed by chemical synthesis. Furthermore, the Se(0) particles precipitated by these three strains exhibit large variations in UV-visible and Raman spectral features, suggesting different species of Se-reducing bacteria produce Se(0) biominerals with different atomic structures. These structural variations were attributed to the diversity of enzymes that catalyze the reduction of selenium oxyanions (29), although the molecular determinants that mediate the reduction reactions have not been identified.
In order to elucidate the formation and biogenicity of Se(0) precipitates, analysis of the genes that control selenium reduction is required. The genetic systems that regulate Se(VI) reduction play a central role in affecting the activity of Se-reducing bacteria and their ability to precipitate Se(0). To date, only two selenate reductase genes have been characterized. The bacterial strain Escherichia coli K-12 carries a putative oxidoreductase gene required for selenate reduction (2), and the serABC operon of the anaerobe Thauera selenatis encodes a substrate-specific trimeric selenate reductase used for dissimilatory selenium respiration (19). However, selenate reductase expression in these organisms is poorly understood, and the genetic systems that regulate selenate reductase activity have not been resolved.
Enterobacter cloacae SLD1a-1 is a gram-negative facultative anaerobe isolated from selenium contaminated agricultural drainage water in San Joaquin Valley, California (24). This organism is capable of reducing both Se(VI) and Se(IV) and produces insoluble elemental selenium that accumulates as extracellular granules (25). Biochemical studies by Watts et al. (46) and Ridley et al. (34) have recently revealed that the selenate reductase in E. cloacae is a membrane-bound heterotrimeric complex that faces the periplasmic side of the cytoplasmic membrane. The selenate reductase contains molybdenum, heme, and nonheme iron as prosthetic constituents and a b-type cytochrome in the active complex. While E. cloacae can utilize alternate electron acceptors for anaerobic growth, the metabolic controls on selenate reductase activity have been debated (24, 46). Currently, the genes involved in selenate reductase expression are unknown.
In the present study, we investigated the mechanisms of Se(VI) reduction by the Se-reducing bacterium E. cloacae SLD1a-1. Direct cloning and mutagenesis experiments were used to elucidate the genetic pathway in E. cloacae that regulates selenate reductase activity. Our objectives were (i) to identify gene(s) that are required for Se(VI) reduction and Se(0) precipitation and (ii) to characterize the mineral products formed by the Se(VI) reduction process. The experimental results showed that the global anaerobic regulatory gene fnr (fumarate nitrate reduction regulator [FNR]) confers selenate reductase activity and the ability to precipitate elemental selenium.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All strains and plasmids used in the present study are listed in Table 1. All strains were grown and maintained on LB agar (Difco) unless otherwise mentioned. E. cloacae SLD1a-1 was grown at 30°C, while E. coli strains were grown at 37°C. For Se(VI) reduction experiments, cells were initially grown in sealed Pyrex bottles containing LB broth. Cell densities were monitored by determining the optical density at 600 nm, and the dissolved oxygen concentration remaining in the medium was measured by using a YSI 550A oxygen probe. Growth studies indicated that the bacterial cultures were depleted in oxygen within approximately 3 h, and suboxic conditions were present by early exponential phase. Measurements of selenate reductase activity were conducted with cells that were harvested after 16 h of growth and resuspended in minimal salt medium composed of Na2SeO4 (0.023 g/liter), K2HPO4 (0.225 g/liter), KH2PO4 (0.225 g/liter), NaCl (0.460 g/liter), (NH4)2SO4 (0.225 g/liter), MgSO4·7H2O (0.117 g/liter), NaHCO3 (0.05 g/liter), FeCl3 (0.005 g/liter), yeast extract (0.5 g/liter), Casamino Acids (0.1 g/liter), acetate (1.0 g/liter) (7), and trace element solution (1 ml/liter). Antibiotics were added as supplements to the medium at the following concentrations: ampicillin, 50 μg/ml; tetracycline, 12.5 μg/ml; gentamicin (Gm), 25 μg/ml; and kanamycin (Km), 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Enterobacter cloacae | ||
| SLD1a-1 | Wild type; selenate reductase positive | Losi and Frankenberger (24) |
| FM-1, FM-2, and FM-3 | fnr knockout mutant derivative strains of SLD1a-1 | This study |
| Escherichia coli | ||
| DH5α | General laboratory strain; selenate reductase positive | Gibco-BRL |
| HB101 | General laboratory strain; selenate reductase positive | Gibco-BRL |
| S17-1 | General laboratory strain; selenate reductase negative | Simon et al. (39) |
| Plasmids | ||
| pLAFR3 | Cosmid cloning vector; Tcr | Staskawicz et al. (42) |
| pRK415 | Broad-host-range cloning vector, Tcr | Keen et al. (14) |
| pBluescript II SK(+) | Cloning vector; Apr | Strategene |
| pGEM-T Easy | PCR cloning vector; Apr | Promega |
| pECL1e | Cosmid clone containing selenate reductase activity when expressed in S17-1 | This study |
| pECL1m | Cosmid clone containing selenate reductase activity when expressed in S17-1 | This study |
| pECL29 | 9.75-kb HindIII fragment containing selenate reductase activity cloned into pBluescript II SK (+/−) | This study |
| pECL221 | 2.25-kb HindIII-BamHI fragment of pECL29 cloned into pBluescript II SK(+/−) | This study |
| pECL222 | 3.2-kb BamHI fragment of pECL29 cloned into pBluescript II SK(+/−) | This study |
| pECL223 | 3.1-kb BamHI fragment of pECL29 cloned into pBluescript II SK(+/−) | This study |
| pECL224 | 1.4-kb BamHI-HindIII fragment cloned into pBluescript II SK(+/−) | This study |
| pECL31 | PCR product of fnr gene cloned into pGEM-T Easy | This study |
| pECL32 | FNR gene fragment from pECL31 cloned into pRK415 | This study |
Apr, ampicillin resistance; Tcr, tetracycline resistance.
Molecular methods and construction of the E. cloacae genomic library.
DNA isolations, restriction digests, ligations, and gel electrophoresis were performed by standard methods (37). Genomic library construction was performed similar to a procedure described elsewhere (18). Briefly, total genomic DNA of E. cloacae SLD1a-1 was isolated by sodium dodecyl sulfate lysis and purified on a cesium chloride gradient. Then, 60 μg of DNA was partially digested with Sau3A before size fractionation on a 10 to 40% glycerol gradient centrifuged at 194,000 × g for 33 h. DNA fragments greater than 15 kb were collected and ligated into the BamHI site in the cosmid cloning vector pLAFR3 (42) before packaging into lambda phage by using Gigapak Gold extracts (Strategene). To determine an appropriate E. coli strain host to screen the E. cloacae genomic library, three strains were tested for selenate reductase activity by plating onto LB agar supplemented with 10 mM sodium selenate. Both HB101 and DH5α demonstrated selenate reductase activity, as determined by the formation of a red colony color indicative of the formation of red elemental selenium, whereas strain S17-1 did not. Therefore, the E. cloacae genomic library was transduced into S17-1 and plated directly onto LB agar containing tetracycline and sodium selenate (10 mM). The antibiotic tetracycline was used to select for colonies containing the vector.
Nucleotide sequence analysis of the 9.75-kb fragment and PCR amplification of the fnr gene.
Nucleotide sequencing was performed by GENEWIZ, Inc. (North Brunswick, NJ). Raw sequence data was assembled and analyzed by using the DNAStar sequence analysis software (Lasergene). Database searches were conducted with identified open reading frames (ORFs) by using the BLAST algorithm (www.ncbi.nlm.nih.gov/BLAST).
Mutagenesis and PCR amplification of the E. cloacae SLD1a-1 fnr gene.
fnr gene knockout mutant strains were constructed with the sacB-based vector pJQ200SK (33). Two sets of primer pairs, FNRmutF2-FNRmutR2 and FNRmutF1-FNRmutR1, were designed to amplify approximately 500-bp products and incorporate a 580-base deletion between bases 115 and 695 of the fnr gene. Unique restriction enzyme sites, indicated by the underlined sequences, were added to the 5′ ends of FNRmutF2 (5′-GGATCCGCTCATGCTCATTCAGGGTA-3′), FNRmutR2 (5′-CTCGAGCGCGATATTCCGTGTGGT-3′), FNRmutF1 (5′-TCTAGACTACCAGCATGTTTTGATAC-3′), and FNRmutR1 (5′-GGATCCCATCGAAAACGGTGAAGCG-3′) to facilitate building of the mutant construct. Amplified PCR products were cloned into pGEM-T Easy (Promega), excised with the appropriate enzymes, and cloned into pJQ200SK. A Km resistance cassette originating from p34S-Km3 (4) was then inserted as a BamHI fragment between the cloned fragments within pJQ200SK. The mutant construct was mated into E. cloacae SLD1a-1 by using E. coli strain S17-1, and transconjugants were selected by using the appropriate antibiotics. Single colony isolates displaying Km and Gm resistance were inoculated into 3 ml of LB and grown overnight at 30°C. Overnight cultures were diluted 1:1,000 in fresh LB broth medium containing 5% sucrose. After 6 h of growth, cultures were plated onto LB agar containing 5% sucrose and Km. Colonies that grew on LB agar supplemented with Km but were unable to grow on LB agar supplemented with Km plus Gm were considered putative knockout mutants and were verified by PCR using the primer set SLD1a1FNRF (5′-GAATTCTTTATCGGCCTGGCATCAATC-3′) and SLD1a1FNRR (5′-AAGCTTGCAGCGCAAGGAGTGGTTAC-3′), positioned 32 bp downstream and 233 bp upstream of the fnr ORF, respectively. Underlined sequences for each primer indicate the added restriction enzyme sites EcoRI for SLD1a1FNRF and HindIII for SLD1a1FNRR. Putative knockout mutants were plated on LB agar containing 10 mM sodium selenate to screen for Se(VI) reductase activity. The ability of mutants to reduce Se(VI) in liquid media was also tested by using the experimental and analytical methods described below.
To clone the fnr gene for complementation of fnr mutants, the same primer set described above was used to amplify and clone the fnr gene using pECL223 as template DNA. The amplified product was cloned into pGEM-T Easy, resulting in pECL31. The fnr gene was then excised as an EcoRI-HindIII fragment from pECL31 and cloned into pRK415, resulting in pECL32. Plasmid pECL32 was mated into fnr mutant strains using E. coli S17-1, and transconjugants were selected using the appropriate antibiotics.
Chemical and mineralogical analysis.
The rate of selenate reduction was measured by monitoring changes in selenate concentrations in the minimal salt medium. At periodic intervals, aliquots of the culture were taken, filtered (0.1-μm pore size), and analyzed for dissolved selenate by using ion chromatography (Dionex). The selenate standards used for calibration were made using the same minimal salt medium as the experiment.
The elemental selenium particles precipitated by E. cloacae and the clones were analyzed by using scanning electron microscopy (SEM) to determine the shape and size of the particles formed, and X-ray absorption fine structure (XAFS) was used to determine the distances, coordination numbers, and disorder parameters for structural Se atoms. SEM analysis was performed by using a LEO 1530 VP field emission scanning electron microscope with an accelerating voltage of 2.0 kV. XAFS experiments were carried out at the National Sychrotron Light Source at Brookhaven National Laboratory using Beamline X-11B. XAFS spectra were collected by using centrifuged pellets containing both cells and selenium particles. Sodium selenate, sodium selenite, red monoclinic and gray hexagonal elemental selenium, zinc selenide, and selenocystine powders were used as reference standards. K-edge data were calibrated by defining the inflection point of the first feature in foil spectra for selenium. The foil spectra were measured between sample runs. The reduction of the XAFS data was performed by using standard procedures (21). The data analysis was performed by using the software packages WinXAS, FEFF7, and FEFF8.
Nucleotide sequence accession number.
The nucleotide sequence of the 9.76-kb HindIII fragment was deposited in GenBank under accession number DQ523830.
RESULTS
Isolation of Se-reducing clones.
A collection of approximately 1,000 clones was produced, representing roughly five genome equivalents of coverage. The precipitation of red elemental selenium as a product of Se(VI) reduction was used to screen the genomic library. Clones unable to reduce Se(VI) formed white colonies on LB agar with sodium selenate, while clones that were able to catalyze the reduction of Se(VI) to Se(0) formed bright red colonies. Screening the E. cloacae SLD1a-1 genomic library for selenate reductase activity in E. coli S17-1 resulted in the identification of two cosmid clones that caused the precipitation of red elemental selenium. Comparisons of restriction digest banding patterns indicated that the two cosmid clones shared a region of overlap. One of the cosmid clones, pECL1e, was selected for further characterization.
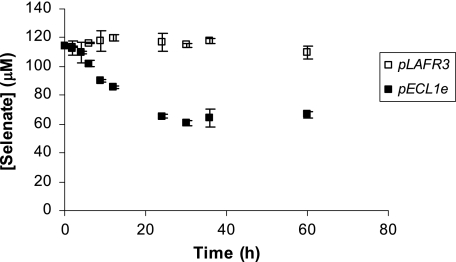
Figure 1 shows the Se(VI) reductase activity of E. coli S17-1(pLAFR3) and E. coli S17-1(pECL1e) in a minimal salt medium. The Se(VI) reduction experiments indicated that E. coli S17-1(pLAFR3) was unable to reduce Se(VI). Batch incubations showed no change in Se(VI) concentration and no change in the nutrient medium color. However, E. coli S17-1 cells containing the cosmid clone pECL1e rapidly reduced Se(VI), removing 45% of the aqueous Se(VI) from solution within 24 h. The decrease in Se(VI) was concurrent with the nutrient medium turning bright red, indicating the precipitation of Se(0).
FIG. 1.
Se(VI) reduction by E. coli(pLAFR3) (□) and E. coli(pECL1e) (▪). Points and error bars represent the averages and standard deviations of triplicate experiments.
Molecular characterization and sequence analysis of the selenate reductase activity from clone pECL1E.
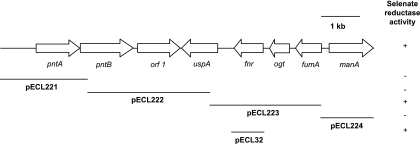
A 9.75-kb HindIII fragment was subcloned from pECL1e into pBluescript II SK(+) and designated pECL29. S17-1 cells containing pECL29 formed red colonies when plated onto LB agar supplemented with ampicillin and sodium selenate. The nucleotide sequence of the entire 9.75-kb DNA fragment was determined. Analysis of the sequence revealed the presence of eight ORFs (Fig. 2). Based on BLAST searches, the eight ORFs were assigned as the alpha (pntA) and beta (pntB) subunits of an NAD(P) transhydrogenase, a putative inner membrane protein, a universal stress protein (uspA), a fumarate nitrate reduction regulator protein (fnr), an O-6-alkylguanine-DNA/cysteine-protein methyltransferase (ogt), the fumarase A protein (fumA), and the mannose-6-phosphate isomerase protein (manA). Based on the positions of three internal BamHI sites identified by the sequence, the 9.75-kb HindIII fragment was further subcloned into four fragments, yielding pECL221 to -224. Screening each of the plasmids in S17-1 localized the selenate reductase activity to pECL223, which contained a 3.1-kb BamHI fragment that included only portions of the uspA and fumA genes, and the complete ORFs containing the fnr and ogt genes (Fig. 2). To determine whether one or both of the fnr or ogt genes were necessary for selenate reductase activity in S17-1, the fnr gene was amplified by PCR and cloned into pRK415, resulting in pECL32. S17-1 cells harboring pECL32 formed red-colored colonies when grown on LB agar supplemented with sodium selenate (Fig. 2), confirming that the fnr gene was responsible for activating selenate reductase activity in this E. coli strain.
FIG. 2.
Map of the 9.76-kb HindIII fragment cloned from E. cloacae SLD1a-1. Arrows depict gene location and transcriptional direction. Lines with plasmid designation depict specific clones tested for selenate reductase activity. + or −, positive or negative for selenate reductase activity, respectively.
Mutation of the fnr gene in E. cloacae SLD1a-1.
To verify that FNR regulated Se(VI) reductase activity in E. cloacae, a knockout mutation of the fnr gene was introduced into SLD1a-1, and three mutant strains, designated FM-1, FM-2, and FM-3, were selected for further characterization. All three mutant strains formed white colonies on the LB agar supplemented with sodium selenate, indicating that a mutation within the fnr gene in E. cloacae resulted in the inability to transform Se(VI) to Se(0).
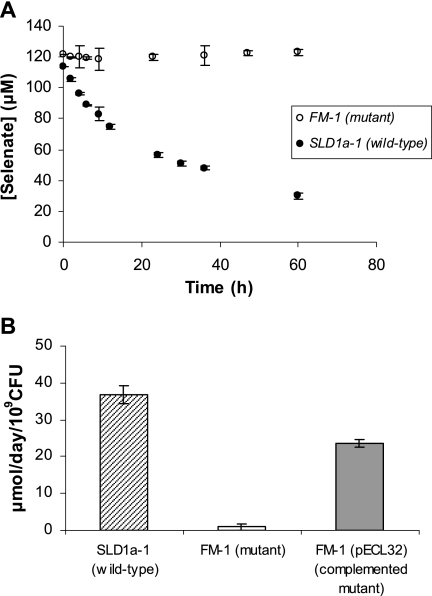
Se(VI) reductase activity of wild-type SLD1a-1 and mutant strain FM-1 in a minimal salt medium is shown in Fig. 3A. The reduction of Se(VI) by E. cloacae SLD1a-1 occurred rapidly at rates comparable to those for E. coli S17-1(pECL1e), with first-order rate constants of k = 3.36 × 10−2 h−1 and k = 2.07 × 10−2 h−1, respectively, although E. cloacae was able to reduce Se(VI) to a greater extent after the initial reduction period. The removal of aqueous Se(VI) by E. cloacae was concurrent with the nutrient medium turning bright red, indicating the precipitation of Se(0). In contrast, mutant strain FM-1 containing the knockout mutation of the fnr gene was unable to reduce Se(VI). Batch incubations showed no change in Se(VI) concentration and no change in the nutrient medium color.
FIG. 3.
(A) Se(VI) reduction by E. cloacae fnr knockout mutant strain FM-1 (○) and E. cloacae SLD1a-1 wild type (•). (B) Se(VI) reduction by the SLD1a-1 wild type, the FM-1 mutant strain, and the complemented FM-1 mutant carrying pECL32. The data and error bars represent the averages and standard deviations of triplicate experiments.
To further verify that the loss of Se(VI) reductase activity in strains FM-1, FM-2, and FM-3 was due to disruption of the fnr gene, pECL32 was introduced into the mutants. All three mutant strains containing the plasmid were rescued in their ability to precipitate Se(0). Measurements of the Se(VI) reduction rate indicated that plasmid pECL32 restored selenate reductase activity in mutant strain FM-1(pECL32), although the levels were slightly less than those expressed by the wild-type strain SLD1a-1 (Fig. 3B).
Characterization of the mineral products.
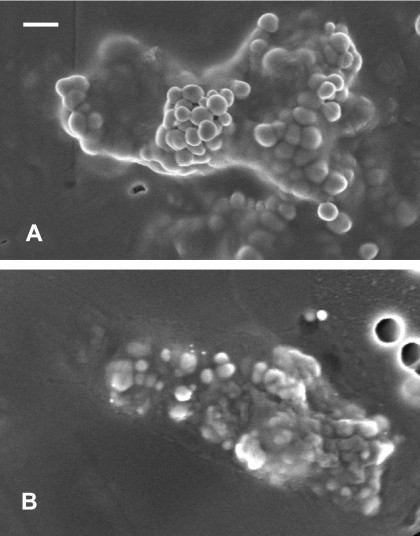
The precipitates formed by E. cloacae and E. coli S17-1(pECL1e) were examined by using SEM and X-ray absorption spectroscopy to verify the formation of selenium biominerals. Se(VI) reduction by both strains formed discrete extracelluar Se particles with identical morphologies that accumulated in the spent medium (Fig. 4). The Se particles were spherical in shape and associated with the exterior cell membrane and were also released into bulk solution. The Se particles precipitated by E. cloacae were monodispered and approximately 200 nm in diameter, whereas those formed by E. coli S17-1(pECL1e) were slightly smaller, with diameters of 100 to 150 nm.
FIG. 4.
SEM image of Se particles precipitated by E. cloacae (A) and E. coli(pECL1e) (B). Scale bar, 300 nm.
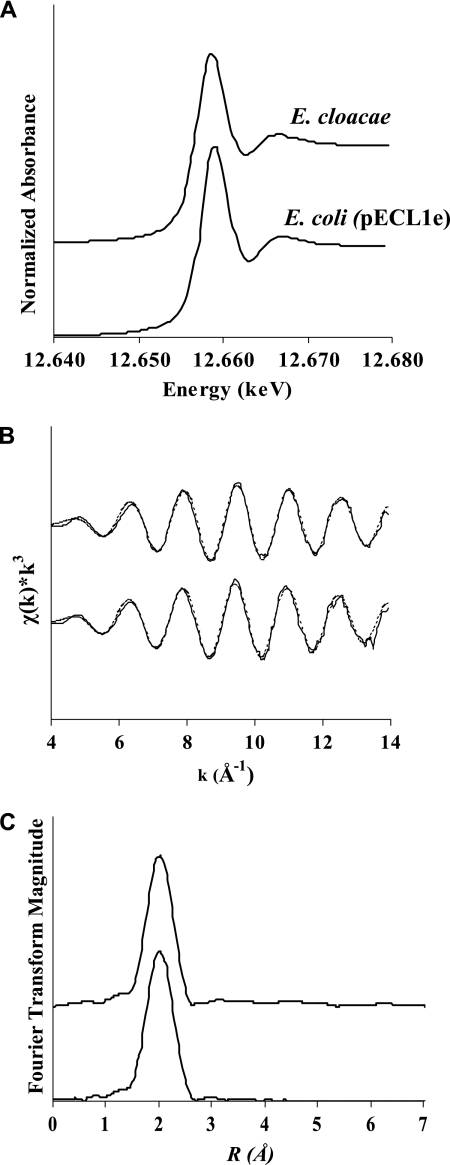
Figure 5 shows the XANES and XAFS spectra of Se particles precipitated by E. cloacae and E. coli S17-1(pECL1e). The position of the Se K-edge shifts to lower energies with decreasing oxidation states and can be used to determine the oxidation state of Se. The position of the Se K-edge for Se(VI), Se(IV), Se(0), and Se(−II) are 12.665, 12.662, 12.658, and 12.656 keV, respectively (32). The Se K-edge XANES spectra measured for Se particles formed by E. cloacae and E. coli S17-1(pECL1e) show the same peak position of 12.658 keV, indicating that the Se particles were composed of elemental selenium (Fig. 5A). The local coordination of structural Se atoms was examined by using XAFS. The k3-weighted XAFS spectra collected for Se(0) particles formed by E. cloacae and E. coli S17-1(pECL1e) are identical, indicating the structural Se atoms have identical short-range order (Fig. 5B). Fitting of the XAFS data yielded an average Se-Se coordination number of 2.8 and an average bond distance of 2.33 Å for the first shell nearest neighbors (Table 2). This local bonding structure is consistent with crystalline red elemental selenium, which contains Se8 rings with average Se-Se separation distances of 2.34 Å (8).
FIG. 5.
X-ray absorption spectra of Se particles precipitated by E. cloacae (top) and E. coli(pECL1e) (bottom). Selenium K-edge XANES spectra (A), k3-weighted χ(k) spectra (B), and the corresponding Fourier transform of the XAFS data (C) are shown.
TABLE 2.
Structural parameters from XAFS curve fitting of Se particles
| Strain | Mean ± SD
|
Debye-Waller factor (Å2) | |
|---|---|---|---|
| Se-Se bond distance (Å) | Coordination no. | ||
| E. cloacae SLD1a-1 | 2.33 ± 0.02 | 2.8 ± 0.2 | 0.0046 |
| E. coli clone E | 2.33 ± 0.02 | 2.7 ± 0.2 | 0.0051 |
DISCUSSION
Losi and Frankenberger (24) previously showed that E. cloacae SLD1a-1 catalyzes the reduction of Se(VI) only after oxygen is depleted from the nutrient medium. Similar Se(VI) reduction behavior has been observed for the gram-negative bacterium Stenotrophomonas maltophilia (6). Watts et al. (46) showed that the selenate reductase of E. cloacae is a substrate-specific molybdenum-dependent membrane-bound enzyme that reduces Se(VI) to Se(IV) but cannot reduce nitrate, nitrite, sulfate, arsenate, dimethyl sulfoxide, or trimethylamine N-oxide. Ridley et al. (34) showed that the enzyme can reduce chlorate and bromate. Although the biochemical properties of the selenate reductase are consistent with a dissimilatory respiratory enzyme, E. cloacae cannot use Se(VI) as a sole electron acceptor for anaerobic growth (46).
Expression of the E. cloacae fnr gene in E. coli S17-1 activated the ability to reduce Se(VI) and precipitate Se(0) particles. FNR is a global transcription regulator that monitors the availability of oxygen in the environment and mediates physiological changes in response to oxygen deprivation (10). Genome-wide transcription data have revealed that FNR modulates at least 103 operons (3, 13, 36). In E. coli, FNR regulates the expression of anaerobic respiratory enzymes including nitrate, fumarate, and dimethyl sulfoxide reductases (20, 41, 45). The FNR protein is located in the cytoplasm and contains a [4Fe-4S]2+ cluster ligated by four essential cysteine residues that is involved in site-specific DNA-binding activity (17). Under aerobic conditions, diffusion of oxygen into the cytoplasm results in the rapid oxidation of the [4Fe-4S]2+ cluster into [2Fe-2S]2+, which is incapable of binding onto DNA (16). Therefore, FNR regulation is active only in the absence of oxygen.
We hypothesize that the FNR transcription factor regulates selenate reductase activity as the bacterium adapts from aerobic to anaerobic growth. The selenate reductase appears to be inherent to E. coli, and its activity is observed in strains K-12 (2), HB101, and DH5α (data not shown); however, specific genes are required to induce expression. Our results indicate that oxygen-sensing transcription factors are needed to activate selenate reductase activity, which is apparently missing in E. coli S17-1.
A putative selenate reductase has been previously identified in E. coli K-12 (2). Genetic analysis based on transposon mutagenesis showed that the mutation of a novel ygfK gene encoding a putative oxidoreductase enzyme resulted in the loss of selenate reductase activity. The YgfK enzyme has an apparent molecular mass of 115 kDa with at least three distinct domains: a N-terminal NAD-binding domain, a central pyridine nucleotide-disulfide redox domain, and a C-terminal iron-sulfur binding domain. ygfK is the first of four genes in the ygfKLMN operon. Genes ygfN and ygfM encode a molybdo-protein and an FAD-containing protein, respectively. Bébien et al. (2) proposed that the selenate reductase of E. coli is a structural complex including the proteins YgfK, YgfM, and YgfN. However, the ygfKLMN operon does not appear to contain an FNR binding site in the promoter region. Furthermore, microarray studies do not show ygfK regulation by FNR (3, 13, 36). This suggests that either there are multiple selenate reductases in E. coli or the selenate reductase pathways in strain K-12 are different than those in S17-1.
E. cloacae mutants with fnr knockout mutations lost the ability to reduce Se(VI) and were unable to precipitate elemental selenium. Complementation by the wild-type fnr sequence restored all abolished phenotypes. Based on GenBank entries, our nucleotide sequence analysis is the first to identify the fnr gene in Enterobacter; however, previous studies have reported an FNR-like protein in E. cloacae associated with the anaerobic transcription of 1-aminocyclopropane-1-carboxylate deaminase and the nifLA operon (9, 12, 23). Our data indicate that the fnr gene in E. cloacae SLD1a-1 is essential for Se(VI) reduction and is linked to anaerobic electron transfer processes. Interestingly, E. cloacae is capable of reducing Se(VI) during fermentative growth on glucose (24, 25, 46), but anaerobic growth on nitrate inhibits selenate reductase activity (34).
The results of the present study suggest that the precipitation of Se(0) particles by facultative anaerobes is regulated by oxygen-sensing proteins and occurs under suboxic conditions. Due to their metabolic flexibility and ubiquity in soils and sediments, bacteria from the family Enterobacteriaceae may play a significant role in selenium transformations in oxic-anoxic transition zones. Selenate reductase genes among facultative anaerobes are potentially widespread, and their expression appears to be controlled by the global transcription regulator FNR. Genetic identification of selenate reductases and determination of their expression dynamics is required to further elucidate the geochemical impact of these organisms.
Acknowledgments
This study was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service under grant 2005-35107-16230.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Bébien, M., J. P. Chauvin, J. M. Adriano, S. Grosse, and A. Verméglio. 2001. Effect of selenite on growth and protein synthesis in the phototrophic bacterium Rhodobacter sphaeroides. Appl. Environ. Microbiol. 67:4440-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bébien, M., J. Kirsch, V. Méjean, and A. Verméglio. 2002. Involvement of a putative molybdenum enzyme in the reduction of selenate by Escherichia coli. Microbiology 148:3865-3872. [DOI] [PubMed] [Google Scholar]
- 3.Constantinidou, C., J. L. Hobman, L. Griffiths, M. D. Patel, C. W. Penn, J. A. Cole, and T. W. Overton. 2006. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K-12 adapts from aerobic to anaerobic growth. J. Biol. Chem. 281:4802-4815. [DOI] [PubMed] [Google Scholar]
- 4.Dennis, J. J., and G. J. Zylstra. 1998. Improved antibiotic-resistance cassettes through restriction site elimination using Pfu DNA polymerase PCR. BioTechniques 25:772-776. [DOI] [PubMed] [Google Scholar]
- 5.Deverel, S. J., J. L. Fio, and N. M. Dubrovsky. 1994. Distribution and mobility of selenium in groundwater in the western San Joaquin Valley of California, p. 157-184. In W. T. Frankenberger and S. Benson (ed.), Selenium in the environment. Marcel Dekker, Inc., New York, NY.
- 6.Dungan, R. S., S. R. Yates, and W. T. Frankenberger. 2003. Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ. Microbiol. 5:287-295. [DOI] [PubMed] [Google Scholar]
- 7.Focht, D. 1994. Microbiological procedures for biodegradation research, p. 407-426. In Methods of soil analysis. 2. Microbiological and biochemical properties. Soil Society of America, Madison, WI.
- 8.Foss, O., and V. Janickis. 1980. Crystal structure of the gamma-monoclinic selenium. J. Chem. Soc. Dalton Trans. 4:624-627. [Google Scholar]
- 9.Grichko, V. P., and B. R. Glick. 2000. Identification of DNA sequences that regulate the expression of the Enterobacter cloacae UW4 1-aminocyclopropane-1-carboxylic acid deaminase gene. Can. J. Microbiol. 46:1159-1165. [DOI] [PubMed] [Google Scholar]
- 10.Guest, J. R. 1995. Adaptation to life without oxygen. Philos. Trans. R. Soc. London B 350:189-202. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton, S. J. 1998. Selenium effects on endangered fish in the Colorado river basin, p. 297-313. In W. T. Frankenberger and R. A. Engberg (ed.), Environmental chemistry of selenium. Marcel Dekker, Inc., New York, NY.
- 12.Hu, B., J. B. Zhu, S. C. Shen, and G. Q. Yu. 2000. A promoter region binding protein and DNA gyrase regulate anaerobic transcription of nifLA in Enterobacter cloacae. J. Bacteriol. 182:3920-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang, Y. S., K. D. Weber, Q. Yu, P. J. Kiley, and F. R. Blattner. 2005. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 187:1135-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen, N. T., S. Tamaki, D. Y. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 15.Kessi, J., M. Ramuz, E. Wehrli, M. Spycher, and R. Bachofen. 1999. Reduction of selenite and detoxification of elemental selenium by the phototropic bacterium Rodospirillum rubrum. Appl. Environ. Microbiol. 65:4737-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoroshilova, N., C. Popescu, E. Munck, H. Beinert, and P. Kiley. 1997. Iron-sulfur cluster diassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity. Proc. Natl. Acad. Sci. USA 94:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiley, P. J., and H. Beinert. 1999. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, D. Y., R. M. Reedy, J. Bick, and P. V. Oudemans. 2002. Characterization of a chitinase gene from Stenotrophomonas maltophilia strain 34S1 and its involvement in biological control. Appl. Environ. Microbiol. 68:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krafft, T., A. Bowen, F. Theis, and J. M. Macy. 2000. Cloning and sequencing of the genes encoding the periplasmic-cytochrome B-containing selenate reductase for Thauera selenatis. DNA Sequence 10:365-377. [DOI] [PubMed] [Google Scholar]
- 20.Lambden, P. R., and J. R. Guest. 1976. Mutants of Escherichia coli unable to use fumarate as an anaerobic electron acceptor. J. Gen. Microbiol. 97:145-160. [DOI] [PubMed] [Google Scholar]
- 21.Lee, P. A., P. H. Citrin, P. Eisenberger, and B. M. Kincaid. 1981. Extended X-ray absorption fine structure: its strengths and limitations as a structural tool. Rev. Mod. Physics 53:769-806. [Google Scholar]
- 22.Lemly, A. D. 2002. Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat. Toxicol. 57:39-49. [DOI] [PubMed] [Google Scholar]
- 23.Li, J. P., and B. R. Glick. 2001. Transcriptional regulation of the Enterobacter cloacae UW4 1-aminocyclopropane-1-carboxylate (ACC) deaminase gene (acdS). Can. J. Microbiol. 47:359-367. [DOI] [PubMed] [Google Scholar]
- 24.Losi, M. E., and W. T. Frankenberger. 1997. Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl. Environ. Microbiol. 63:3079-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losi, M. E., and W. T. Frankenberger. 1998. Reduction of selenium oxyanions by Enterobacter cloacae strain SLD1a-1, p. 515-544. In W. T. Frankenberger and R. A. Engberg (ed.), Environmental chemistry of selenium. Marcel Dekker, Inc., New York, NY.
- 26.Macy, J. M., T. A. Michel, and D. G. Kirsch. 1989. Selenate reduction by a Pseudomonas species: a new mode of anaerobic respiration. FEMS Microbiol. Lett. 61:195-198. [DOI] [PubMed] [Google Scholar]
- 27.Mayland, H. F. 1994. Selenium in plant and animal nutrition, p. 29-46. In W. T. Frankenberger and S. Benson (ed.), Selenium in the environment. Marcel Dekker, Inc., New York, NY.
- 28.Myneni, S. C. B., T. K. Tokunaga, and G. E. Brown. 1997. Abiotic selenium redox transformations in the presence of Fe(II,III) oxides. Science 278:1106-1109. [Google Scholar]
- 29.Oremland, R. S., M. J. Herbel, J. S. Blum, S. Langley, T. J. Beveridge, P. M. Ajayan, T. Sutto, and A. V. Ellis. 2004. Structural and spectral features of selenium nanospheres produced by Se-respiring bacteria. Appl. Environ. Microbiol. 70:52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oremland, R. S., J. T. Hollibaugh, A. S. Maest, T. S. Presser, L. G. Miller, and C. W. Culbertson. 1989. Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl. Environ. Microbiol. 55:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oremland, R. S., N. A. Steinberg, A. S. Maest, L. G. Miller, and J. T. Hollibaugh. 1990. Measurement of in situ rates of selenate removal by dissimilatory bacterial reduction in sediments. Environ. Sci. Technol. 32:3749-3755. [Google Scholar]
- 32.O'Toole, D., and M. F. Raisbeck. 1998. Magic numbers, elusive lesions: comparative pathology and toxicology of selenosis in waterfowl and mammalian species, p. 355-396. In W. T. Frankenberger and R. A. Engberg (ed.), Environmental chemistry of selenium. Marcel Dekker, Inc., New York, NY.
- 33.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 34.Ridley, H., C. A. Watts, D. J. Richardson, and C. S. Butler. 2006. Resolution of distinct membrane-bound enzymes from Enterobacter cloacae SLD1a-1 that are responsible for selective reduction of nitrate and selenate oxyanions. Appl. Environ. Microbiol. 72:5173-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux, M., G. Sarret, I. Pignot-Paintrand, M. Fontecave, and J. Coves. 2001. Mobilization of selenite by Ralstonia metallidurans CH34. Appl. Environ. Microbiol. 67:769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmon, K., S. P. Hung, K. Mekjian, P. Baldi, G. W. Hatfield, and R. P. Gunsalus. 2003. Global gene expression profiling in Escherichia coli K-12: the effects of oxygen availability and FNR. J. Biol. Chem. 278:29837-29855. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, NY.
- 38.Sarret, G. L., L. Avoscan, M. Carriere, R. Collins, N. Geoffory, F. Carrot, J. Coves, and B. Gouget. 2005. Chemical forms of selenium in the metal resistant bacterium Ralstonia metallidurans CH34 exposed to selenite and selenate. Appl. Environ. Microbiol. 71:2331-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 40.Skorupa, J. P. 1998. Selenium poisoning of fish and wildlife in nature: lessons from twelve real-world examples, p. 315-354. In W. T. Frankenberger and R. A. Engberg (ed.), Environmental chemistry of selenium. Marcel Dekker, Inc., New York, NY.
- 41.Spiro, S. 1994. The FNR family of transcriptional regulators. Antonie Leeuwenhoek 66:23-36. [DOI] [PubMed] [Google Scholar]
- 42.Staskawicz, B., D. Dahlbeck, N. Keen, and C. Napoli. 1986. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789-5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolz, J. F., and R. S. Oremland. 1999. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 23:615-627. [DOI] [PubMed] [Google Scholar]
- 44.Tokunga, T. K., P. T. Zawislanski, P. W. Johannis, D. S. Lipton, and S. Benson. 1994. Field investigations of selenium speciation, transformation and transport in soils from Kesterson Reservoir and Lahontan Valley, p. 119-138. In W. T. Frankenberger and S. Benson (ed.), Selenium in the environment. Marcel Dekker, Inc., New York, NY.
- 45.Unden, G., S. Becker, J. Bongaerts, G. Holighaus, J. Schirawski, and S. Six. 1995. O2-sensing and O2-dependent gene regulation in facultative anaerobic bacteria. Arch. Microbiol. 164:81-90. [PubMed] [Google Scholar]
- 46.Watts, C. A., H. Ridley, K. L. Condie, J. T. Leaver, D. J. Richardson, and C. S. Butler. 2003. Selenate reduction by Enterobacter cloacae SLD1a-1 is catalyzed by a molybdenum-dependent membrane-bound nitrate reductase. FEMS Microbiol. Lett. 228:273-279. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, Y., and J. M. Moore. 1996. Selenium fractionation and speciation in a wetland system. Environ. Sci. Technol. 30:2613-2619. [Google Scholar]