Abstract
Following a pressure treatment of a clonal Staphylococcus aureus culture with 400 MPa for 30 min, piezotolerant variants were isolated. Among 21 randomly selected survivors, 9 were piezotolerant and all formed small colonies on several agar media. The majority of the isolates showed increased thermotolerance, impaired growth, and reduced antibiotic resistance compared to the wild type. However, several nonpiezotolerant isolates also demonstrated impaired growth and the small-colony phenotype. In agglutination tests for the detection of protein A and fibrinogen, the piezotolerant variants showed weaker agglutination reactions than the wild type and the other isolates. All variants also showed defective production of the typical S. aureus golden color, a characteristic which has previously been linked with virulence. They were also less able to invade intestinal epithelial cells than the wild type. These S. aureus variants showed phenotypic similarities to previously isolated Listeria monocytogenes piezotolerant mutants that contained mutations in ctsR. Because of these similarities, possible alterations in the ctsR hypermutable regions of the S. aureus variants were investigated through amplified fragment length polymorphism analysis. No mutations were identified, and subsequently we sequenced the ctsR and hrcA genes of three representative variants, finding no mutations. This work demonstrates that S. aureus probably possesses a strategy resulting in an abundance of multiple-stress-resistant variants within clonal populations. This strategy, however, seems to involve genes and regulatory mechanisms different from those previously reported for L. monocytogenes. We are in the process of identifying these mechanisms.
Staphylococcus aureus is a gram-positive, facultatively anaerobic, cluster-forming, nonmotile coccus that is implicated in conditions such as toxic shock syndrome and nosocomial infections, of which it is the leading cause (1, 2a, 28, 33). Approximately 20% of healthy individuals almost always carry one strain, while a large proportion of the population (∼60%) harbors S. aureus intermittently. A minority of people (∼20%) almost never carry S. aureus (20). This bacterium can easily infect a host through an open wound and cause a possibly fatal opportunistic infection. Another problem related to S. aureus is the production of toxins in foods linked with enterotoxicosis in humans (21). This disease is usually the result of improper food-handling techniques. In addition, lately S. aureus has been found to be able to invade and colonize the intestinal tract, which may act as a reservoir and may protect the bacterial cells from antibiotic treatments (13).
Efficient inactivation of the bacterium in foods is usually achieved by conventional heat inactivation procedures such as pasteurization. During the last decade, increased consumer demand for minimally processed products has led to the development of new methods of food preservation. High-hydrostatic-pressure (HHP) treatment is one such nonthermal preservation process, in which pressures between 200 and 700 MPa are used to inactivate vegetative cells of microorganisms including pathogens like S. aureus. However, inactivation of bacterial spores requires higher pressures of around 1,000 MPa (11, 12).
As HHP has been used commercially only in the last decade, there is still much work to be done in identifying the mechanisms through which microorganisms respond to this stress. Although tailing in inactivation curves suggests the existence of piezotolerant strains of several microorganisms, only a few of them have been isolated. Hauben et al. reported the isolation of piezotolerant mutants of Escherichia coli after numerous repeated selective HHP cycles (11), while Fujii et al. reported the isolation of a piezotolerant strain of Saccharomyces cerevisiae (10). Most of the mechanisms involved in piezotolerance are not pressure specific and seem to play a role in resistance to other stresses, like heat. For E. coli, it has been demonstrated that proteins like Lon, ClpX, and DnaK play a role in piezotolerance (2). Genes corresponding to the above-mentioned proteins are also present in gram-positive bacteria like S. aureus, and they are under the control of regulatory proteins like HrcA and CtsR. In previous work, Karatzas and Bennik were able to isolate piezotolerant mutants of Listeria monocytogenes and identify the mechanism related to their phenotype. These mutants demonstrated reduced virulence, impaired growth, small colony size, loss of motility, and multiple-stress resistance compared to the wild type (18). For the majority of mutants, a hypermutable short sequence repeat region in the ctsR gene of L. monocytogenes was responsible for their increased piezotolerance (17, 19). Interestingly, this region has been selected by evolution as part of a strategy for the development of an abundance of stress-resistant mutants within a few hours of growth of a pure culture, which would ensure the survival of the population under unfavorable conditions (17). It is possible that this mechanism of strategically located hypermutable regions for the generation of population variability is widespread among several species of bacteria. It has been reported that short sequence repeats are overrepresented in stress genes, resulting in mutants with high stress resistance (29). Short sequence repeats are known to play a very important role in fitness, survival, and pathogenicity in Haemophilus influenzae and Neisseria meningitidis, as contingency loci are located in genes encoding evasins, lipopolysaccharide biosynthesis proteins, adhesins, iron acquisition proteins, and restriction modification systems (24, 31, 32).
In this study, we investigated the existence of stress-resistant subpopulations within a clonal S. aureus culture. With the use of high-hydrostatic-pressure technology, we were able to isolate multiple-stress-resistant variants. These mutants, like the L. monocytogenes isolates, demonstrated impaired growth, small colony size, and increased thermotolerance and piezotolerance compared to the wild type. In addition, they demonstrated a reduced ability to produce the golden color typical of wild-type S. aureus as well as reduced invasiveness. Small-colony variants of S. aureus have been implicated previously in increased antibiotic resistance (27). Interestingly, the variants we isolated showed reduced antibiotic tolerance compared to the wild type. As the ctsR gene of S. aureus contains a short sequence repeat region in the locus corresponding to that in L. monocytogenes, we looked for possible mutations by using amplified fragment length polymorphism (AFLP) technology and sequencing of the ctsR and hrcA genes of three piezotolerant variants. However, no mutations were found, revealing that S. aureus has an alternative strategy for creating an abundance of piezotolerant and stress-tolerant variants.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A methicillin-susceptible strain of S. aureus isolated from ham (National Agricultural Research Foundation, Lycovrissi, Greece) and L. monocytogenes Scott A (Department of Food Science, Wageningen Agricultural University, The Netherlands) were used in this study. The methicillin-susceptible S. aureus strain was resistant to ampicillin, amoxicillin, penicillin, nalidixic acid, and sulfonamides. Stock cultures were kept at −80°C in 15% (vol/vol) glycerol or microbank tubes (Pro-Lab Diagnostics, Neston, Wirral, United Kingdom), transferred into sterile brain heart infusion (BHI) broth (Oxoid, Hampshire, England) with or without 5% Tween 80 (ICI Surfactants, Wilmington, DE), and incubated twice at 37°C overnight (0.3% [vol/vol] inoculum). Cultures were grown with shaking (160 rpm), and Tween 80 was added only to those that would be subjected to HHP or heat treatment to alleviate the clumping of bacterial cells.
High-hydrostatic-pressure treatment.
Cultures were placed in sterile plastic stomacher bags (Seward, London, United Kingdom) that were sealed while avoiding an excess of air bubbles. Pouches were submerged in glycol (Resato, Roden, The Netherlands), which was the fluid medium through which the pressure was transferred, and subjected to 400 MPa for 30 min or 500 MPa for 15 min in a high-pressure unit (Resato, Roden, The Netherlands) at 20°C. The viability of S. aureus cells was determined before and after the pressure treatment. Decimal dilutions of samples in saline solution (Oxoid, Hampshire, England) were prepared, followed by plating in triplicate onto BHI agar (1% [wt/vol] agar). Plates were incubated at 37°C for 5 days.
Selection of piezotolerant isolates.
The objective was the isolation of piezotolerant mutants appearing within a short period of time among a population of wild-type S. aureus cells. We first purified the stock culture on BHI agar plates and inoculated an individual colony into BHI broth. The culture was subcultured daily at 37°C to stationary phase for 5 successive days by using a 0.3% (vol/vol) inoculum of stationary-phase culture from the previous day (∼1010 CFU ml−1). The stationary-phase culture from the fifth day was subjected to pressure treatment with 400 MPa for 30 min. Treated cell suspensions were plated and numbers of CFU were determined as described above. Importantly, the culture derived from the isolate showed a level of inactivation by pressurization identical to that of the initial −80°C wild-type stock culture. Following the HHP treatment, 21 surviving isolates were randomly selected and stored at −80°C in 15% (vol/vol) glycerol.
Agglutination test.
An agglutination test was performed using the Staphylo Monotec kit (Fluka, Buchs, Switzerland) to confirm the identity of the isolates as S. aureus. The test reagent consists of monodispersed particles coated with fibrinogen and immunoglobulin G, which bind to the cell-associated coagulase and cell wall protein A, respectively. The times needed for agglutination for each isolate and the wild type were recorded.
Thermotolerance of isolates.
BHI broth (100 ml) was inoculated (0.1%, vol/vol) with overnight cultures of the isolates incubated with shaking (160 rpm) at 37°C. Cultures were grown until mid-exponential phase (optical density at 600 nm [OD600], 0.4 to 0.6), and samples were placed in 7-ml plastic tubes (Sterilin, Staffordshire, United Kingdom) and incubated in a water bath at 58°C for 20 min. Samples were taken before and after treatment, decimal dilutions in saline solution were prepared using saline tablets (Oxoid, Hampshire, England), and viability was determined.
Stability of phenotypes.
All 21 individual isolates were assessed for the stability of the phenotypes. Isolates were subcultured for 10 consecutive days using 0.3% (vol/vol) inocula in fresh BHI medium. On day 10, cultures were inoculated using a 0.3% (vol/vol) inoculum in 100 ml of BHI broth, incubated at 37°C with shaking (160 rpm), and tested for their growth characteristics, colony morphologies, and piezotolerance as described above.
Colony morphology.
Stationary-phase cultures of the wild type and all isolates were grown as described above and passaged on Baird Parker agar, BHI agar, Columbia blood agar (COLBA), and plate count agar plates (Oxoid, Hampshire, England). Plates were incubated at 37°C for 24 h, and colonies were examined for their size, color, and hemolysis characteristics on COLBA plates.
Analysis of growth kinetics.
Growth characteristics of all isolates were assessed at 37°C with shaking (160 rpm) or at 20°C under static conditions. Five microliters from the culture of each survivor was inoculated into 200 μl of fresh BHI. Samples were placed onto a Sero-Well microtiter plate (Sterilin, Staffordshire, United Kingdom), and bacterial growth was assessed by measuring the OD600s of the samples with a Bio-Rad model 680 microplate reader (Bio-Rad, Hercules, CA). Growth curves were constructed in triplicate. Doubling times were calculated based on values obtained from the exponential phase of growth.
Antibiotic disk diffusion test.
The antibiotic disk diffusion test was conducted according to the recommendations of the British Society for Antimicrobial Chemotherapy (3). Antibiotic disks were provided by Oxoid (Hampshire, England).
Determination of MICs.
The MICs of a range of antibiotics and disinfectants were determined using the broth doubling dilution method according to the recommendations of the British Society for Antimicrobial Chemotherapy (4). Kanamycin, gentamicin, and nalidixic acid were purchased from Sigma-Aldrich (Poole, United Kingdom), while all disinfectants were obtained from Antec International Ltd. (Sudbury, Suffolk, United Kingdom), except for triclosan, which was obtained from Ciba Geigy (United Kingdom). Virkon-S is a blend of an inorganic peroxygen compound, inorganic salts, organic acid, anionic detergent, fragrance, and dye. Farm Fluid-S is a blend of tar acids, organic acids, and surfactants, and Antec quaternary active sterilizer (AQAS) contains quaternary ammonium biocide, nonionic surfactant, and excipients.
Gentamicin protection assay.
The gentamicin protection assay was performed for the wild-type strain and all isolates as described previously by Elsinghorst (9). In brief, Caco-2 human colon adenocarcinoma cells (European Collection of Cell Cultures number 86010202) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 2 mM glutamine, 1% nonessential amino acids, and 10% fetal bovine serum. Penicillin or streptomycin (Invitrogen) was used at a concentration of 100 U ml−1 in DMEM until 3 days before cells were used for infection studies. The culture medium was changed every 2 to 3 days. Just before experiments, Caco-2 cells were washed three times with sterile phosphate-buffered saline (PBS), and subsequently, 2 ml of antibiotic-free DMEM was added to each well. The OD600s of stationary-phase cultures of the S. aureus isolates and the wild type were measured, and all cultures were adjusted to similar OD600s. We previously confirmed that there was a good correlation between OD600 measurements and cell numbers for all strains, as assessed by comparing CFU and OD values. Fifty microliters of the adjusted bacterial suspensions was added to the wells, resulting in ∼106 CFU per well and yielding an estimated ratio of bacteria to cells (multiplicity of infection) of ∼2.5:1. Cells were incubated for 1 h at 37°C and subsequently washed twice with PBS and suspended in PBS containing 100 μg ml−1 gentamicin. After 2 h at 37°C, cells from cultures exposed to gentamicin were rinsed twice with sterile PBS and lysed with 2 ml of 1% Triton X-100 (vol/vol) in PBS. Following a brief incubation for 5 min at 37°C, cell lysates were serially diluted and plated onto COLBA to quantify the number of intracellular bacteria. As several isolates demonstrated different susceptibilities to gentamicin, we also performed the assay with each one of the isolates by suspending the corresponding bacterial cells in fresh DMEM without Caco-2 cells.
Detection of mutations in the short sequence repeat region of ctsR by AFLP analysis.
In this study, we present a simple method for the detection of L. monocytogenes piezotolerant mutants containing mutations in the short sequence repeat region of the ctsR gene. We also applied this method to investigate whether the S. aureus piezotolerant variants contained similar mutations in the hypermutable regions of their ctsR genes.
Primers LmctsrglyF and LmctsrglyR (Table 1) were used for the PCR amplification of the ctsR short sequence repeat region of L. monocytogenes, resulting in 56- and 53-bp fragments from the wild type and the AK01 strain, respectively. The technique would also detect all previously described mutations in the short sequence repeat region (17). Chromosomal DNA was isolated by the method described by Pospiech and Neumann (26) and used as a template in PCRs. PCR products were loaded on a 5% agarose gel (Oxoid, Hampshire, England) containing 500 μg ml−1 ethidium bromide (Sigma-Aldrich, Poole, United Kingdom), followed by electrophoresis at 105 mV.
TABLE 1.
Primers used in this study
| Primer name | Sequence |
|---|---|
| LmctsrglyF | 5′-ATA TCG TTG AAA GTA AAC GT-3′ |
| LmctsrglyR | 5′-TTT CAC TTT AAT AAT CCT AAT ATA TAG-3′ |
| SactsrglyF | 5′-TAT GAA ATC GAA AGT AAA CG-3′ |
| SactsrglyR | 5′-ATT TTA GTG ATT CGG ATG TA-3′ |
| ctsrF | 5′-AAA TGG AAG GGC TAA CAT CA-3′ |
| ctsrR | 5′-TTG ATG CCA TGT TTC GTA GC-3′ |
| hrcaF | 5′-AAT AAC ATT CTC GTC AGA CG-3′ |
| hrcaR | 5′-GAC CAA TCT ATT GAA AGT GTC-3′ |
This technique was employed to detect similar mutations in the ctsR short sequence repeat region of S. aureus. Primers used in this case were SactsrglyF and SactsrglyR (Table 1), designed to produce a 53-bp fragment from the wild-type strain. Primers were designed to result in PCR fragments with lengths similar to those of the successfully tested L. monocytogenes fragments, as longer fragments may compromise results. All primers were provided by MWG Biotech AG, Ebersberg, Germany. All gel images were analyzed with Quantity One software (Bio-Rad, Hemel Hempstead, United Kingdom).
Sequencing of ctsR and hrcA genes.
We previously demonstrated that a single codon deletion in a repeat region of ctsR resulted in increased piezotolerance, tolerance to other stresses, and a loss of motility in L. monocytogenes (19). In this study, we investigated the involvement of mutations in ctsR in piezotolerance by analyzing the sequence of ctsR and the region upstream of ctsR (∼100 bp) in three representative, stable, piezotolerant S. aureus mutants, namely, isolates 1, 8, and 21. PCR amplification of the ctsR gene was performed using standard methods (30). Chromosomal DNA from all stable piezotolerant isolates was isolated by the method described by Pospiech and Neumann (26) and used as a template in PCRs. To serve as controls, DNA was isolated from the wild-type strain. Primers ctsrF and ctsrR were used for PCR amplification and DNA sequence analysis of the ctsR gene, while hrcaF and hrcaR were used for the hrcA gene. All primers were provided by MWG Biotech AG (Ebersberg, Germany), and amplification and sequence analysis were performed in duplicate. All primer sequences are presented in Table 1.
Statistical analysis.
The t test for two samples, assuming equal variances, was used to determine statistical differences between the means of log reductions in CFU of each isolate and of the wild type. P values of less than 0.05 were considered to indicate statistical significance.
RESULTS
Piezotolerance of isolates.
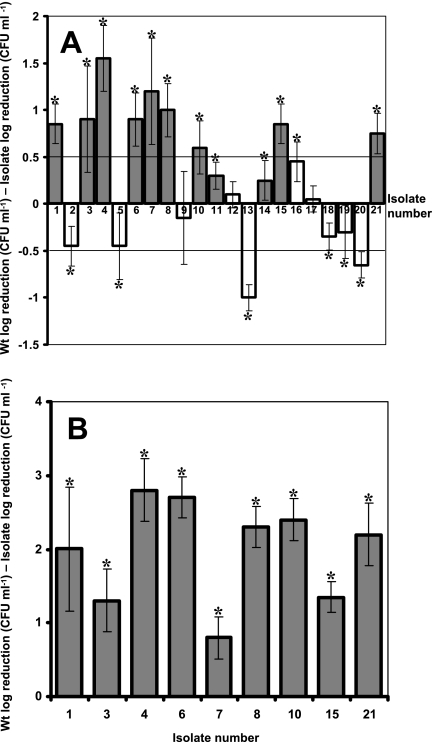
To distinguish between piezotolerant and wild-type isolates, we arbitrarily classified as piezotolerant any isolate that demonstrated a reduction in CFU of less than 0.5 log compared to the wild type when challenged at 400 MPa for 30 min. Among 21 isolates, 9 mutants, those numbered 1, 3, 4, 6, 7, 8, 10, 15, and 21, fulfilled that criterion and were regarded as piezotolerant (Fig. 1A). Three isolates, 11, 14, and 16, were also more tolerant than the wild type, but differences were less pronounced with these isolates than with the other strains (Fig. 1A). The survival of all piezotolerant variants was also tested at 450 MPa for 15 min (Fig. 1B). All tolerant variants showed increased piezotolerance, with reductions in CFU ml−1 being 0.8 to 2.8 log lower than that of the wild type at this higher pressure. Interestingly, the most piezotolerant isolates at the lower pressure were not always the most tolerant at the higher pressure, as clearly demonstrated in the cases of isolates 3 and 7. In addition, other isolates, 13 and 20, were regarded as piezosensitive, demonstrating a reduction in CFU ml−1 of more than 0.5 log compared to the wild type. Isolates 2, 5, 18, and 19 were also more sensitive than the wild type, although differences were not as great as with other strains. Isolates 9, 12, and 17 showed a level of piezotolerance identical to that of the wild type.
FIG. 1.
Piezotolerance of isolates obtained from a wild-type culture of S. aureus after exposure to 400 MPa for 30 min at 20°C. The piezotolerance of all isolates was assessed following exposure to 400 MPa for 30 min (A), and that of only the piezotolerant variants was assessed following exposure to 450 MPa for 15 min (B). The level of piezotolerance is presented as the log difference in reduction in CFU between each isolate and the wild type (Wt). Positive values denote higher resistance than that of the wild type, while negative values denote higher sensitivity. Dark bars represent small-colony variants, while light bars represent isolates with normal colony morphologies. Trendlines at 0.5 and −0.5 represent the threshold above or below which the isolates were determined to be piezotolerant or piezosensitive, respectively. Reductions in numbers of viable CFU were determined in triplicate for each isolate, and error bars represent the standard deviations of the log differences between reductions in CFU of the wild type and the isolates. Asterisks denote significant differences between the log reductions in CFU of the isolates and the wild type (P < 0.05).
Agglutination test.
All isolates were identified as S. aureus by an agglutination test. However, all piezotolerant variants, except isolate 1, showed weak agglutination reactions that required at least 4 s longer to give a positive result than that of the wild type (data not shown). Also, the coagulation was not very strong, producing small clumps. In addition, isolate 16 required 12 s longer for agglutination than the wild type, although it did not demonstrate a small-colony-variant phenotype and was not highly piezotolerant. The weakest agglutination reactions occurred with isolates 3, 10, 15, and 21, which required at least 15 s longer than the wild type.
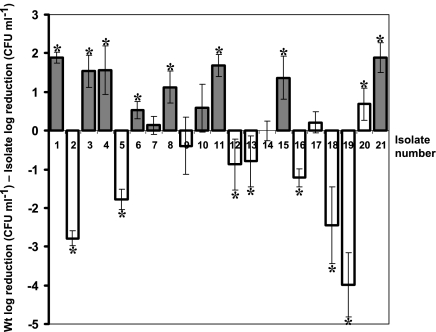
Thermotolerance of isolates.
In general, all piezotolerant variants apart from isolate 7 were also resistant to heat compared to the wild type, although the magnitude of this resistance varied (Fig. 2). Piezotolerant isolates 6 and 10 showed differences in reduction of only 0.52 and 0.58 log compared to the wild type, respectively. In addition, isolates 11 and 20, although not piezotolerant, appeared to be more thermotolerant than the wild type and had 1.68 and 0.68 log less reduction than the wild type, respectively, at 58°C after 20 min. Isolates 2, 5, 18, and 19 were thermosensitive, having a difference of more than 1 log from the wild type, with this difference even reaching 4 logs for isolate 19.
FIG. 2.
Thermotolerance of isolates expressed as the log difference in reductions in CFU between each isolate and the wild type (Wt). Positive values denote higher resistance than that of the wild type, while negative values denote higher sensitivity. Dark bars represent small-colony variants, while light bars represent isolates with normal colony morphologies. Experiments were performed on cells in the exponential phase which were exposed to 58°C for 30 min. Reductions in numbers of viable CFU were determined in triplicate for each isolate, and error bars represent standard deviations of the log differences between reductions in CFU of the wild type and the isolates. Asterisks denote significant differences between the log reductions in CFU of the isolates and the wild type (P < 0.05).
Stability of phenotypes.
All variants showed stable phenotypes following sequential growth in BHI for 10 days. Phenotypes were assessed by measuring colony size and piezotolerance.
Colony morphology.
All piezotolerant variants showed colony sizes decreased by two to threefold on all different solid growth media used. Isolates 11 and 14 were also small-colony variants without being piezotolerant (Fig. 1A). All other isolates showed colony morphologies similar to that of the wild type. When grown on plate count agar, all piezotolerant isolates showed defective production of the typical S. aureus golden color compared to all other strains. On COLBA, all piezotolerant isolates formed colonies with very well-defined shapes and a vivid white color, in contrast to the less well- defined and more transparent pale white colonies of the other isolates and the wild type. However, all isolates showed the same level of hemolysis as the wild type.
Analysis of growth kinetics.
At 37°C with shaking, all isolates grew similarly (data not shown). However, differences were observed when growth occurred under static conditions at 20°C (Table 2). All piezotolerant variants with the exception of isolate 4 showed increased doubling times, and several also had reduced OD600s in stationary phase, denoting impaired growth. Most nonpiezotolerant isolates showed growth patterns similar to that of the wild type. A few isolates demonstrated increased doubling times or reduced OD600s at stationary phase but never both characteristics together. Piezosensitive variant 13 had a reduced doubling time, and it and isolate 4 were the only strains that reached significantly higher OD600s at stationary phase than the wild type.
TABLE 2.
Doubling times of isolates and OD600s at stationary phasea
| Strain | Doubling time (h) | OD600 at stationary phase |
|---|---|---|
| Piezotolerant strains | ||
| 1 | 0.98 | 1.11 |
| 3 | 1.19 | 1.33 |
| 4 | 0.75 | 1.48* |
| 6 | 1.03 | 1.33 |
| 7 | 1.12 | 1.34 |
| 8 | 1.61 | 0.93 |
| 10 | 1.03 | 1.46 |
| 15 | 0.99 | 1.16 |
| 21 | 1.42 | 0.949 |
| Wild-type-like strains | ||
| Wild type | 0.77 | 1.37 |
| 2 | 0.95 | 1.32 |
| 5 | 0.71 | 1.24 |
| 9 | 0.86 | 1.45 |
| 11 | 0.71 | 1.17 |
| 12 | 1.05 | 1.33 |
| 14 | 1.05 | 1.43 |
| 16 | 0.93 | 1.39 |
| 17 | 0.88 | 1.44 |
| 18 | 1.10 | 1.32 |
| 19 | 0.97 | 1.33 |
| Piezosensitive strains | ||
| 13 | 1.07 | 1.6* |
| 20 | 0.84 | 1.35 |
Bold numbers represent a difference of more than ±0.2 for doubling times and more than ±0.1 for OD600s at stationary phase compared to the values for the wild type. Asterisks denote values significantly higher than those of the wild type that exceed the positive thresholds mentioned in the text.
Antibiotic disk diffusion test.
The antibiotic susceptibilities of several isolates were different from those of the wild type. In general, the piezotolerant variants were more susceptible than the wild type to the antibiotics tested (Table 3). Specifically, isolates 1, 3, 4, 6, 7, 9, 10, 11, 14, 15, and 19 showed intermediate resistance to amikacin, isolates 8 and 21 were sensitive to amikacin, and the remaining isolates were as resistant as the wild type. Isolates 1, 3, 4, 6, 7, 8, 10, 11, 14, 15, 20, and 21 were sensitive to kanamycin, while the remainder showed intermediate resistance similar to that of the wild type. Isolate 5 was resistant to oxacillin, while all other isolates were sensitive. The isolates sensitive to neomycin were 3, 6, 10, 14, 19, and 21, while the rest of the isolates and the wild type were resistant. All piezotolerant variants, including 11 and 14, were susceptible to gentamicin, and all other isolates were resistant (Table 3).
TABLE 3.
Diameters of zones of growth inhibition in disk diffusion tests of isolates with several antibioticsa
| Isolate | Diam (mm) of zone of growth inhibition on disk with:
|
||||
|---|---|---|---|---|---|
| Amikacin (30) | Kanamycin (30) | Oxacillin (1) | Neomycin (10) | Gentamicin (10) | |
| Wild type | 15 (R) | 15-16 (I) | 16 (R) | 13-14 (R) | 17 (R) |
| 1 | 18 (I) | 18 | 21 | 16 (R) | 21 |
| 2 | 15-16 (R) | 16 (I) | 18 | 14 (R) | 17 (R) |
| 3 | 18 (I) | 20 | 22-23 | 17 | 20 |
| 4 | 18 (I) | 20 | 18 | 16 (R) | 20 |
| 5 | 15 (R) | 17 (I) | 16 (R) | 15 (R) | 17 (R) |
| 6 | 18 (I) | 19 | 19 | 17-19 | 21 |
| 7 | 17 (I) | 19 | 18 | 16 (R) | 20 |
| 8 | 20 | 20-22 | 24 | 16 (R) | 21 |
| 9 | 16 (I) | 17 (I) | 22-25 | 14 (R) | 17 (R) |
| 10 | 18 (I) | 19 | 20 | 17-18 | 20 |
| 11 | 17 (I) | 16 | 24 | 15 (R) | 22 |
| 12 | 15 (R) | 16 (I) | 19 | 15-16 (R) | 17 (R) |
| 13 | 15 (R) | 16 (I) | 19 | 13-15 (R) | 17 (R) |
| 14 | 18 (I) | 19 | 19 | 17 | 21 |
| 15 | 16-17 (I) | 18 | 21 | 16 (R) | 20 |
| 16 | 15-16 (R) | 16-18 (I) | 19 | 14 (R) | 17 (R) |
| 17 | 15 (R) | 16 (I) | 18 | 14 (R) | 18 (R) |
| 18 | 15 (R) | 17 (I) | 22-26 | 13-14 (R) | 17 (R) |
| 19 | 16 (I) | 17 (I) | 23-25 | 17 | 17 (R) |
| 20 | 15 (R) | 18-19 | 18 | 13 (R) | 17 (R) |
| 21 | 20 | 20 | 21 | 18 | 21 |
R, resistant; I, intermediate. Numbers in parentheses after the names of the antibiotics are concentrations (μg ml−1) on the disk.
Determination of MICs.
We tested whether the differences found by the disk diffusion test were reflecting differences in the MICs. Isolates 6, 8, and 21 and the wild type were examined to determine the MICs of several antibiotics and disinfectants for these strains. Indeed, the 4- to 5-mm difference in the diameters of growth inhibition zones detected by the disk diffusion method for kanamycin corresponded to a lower MIC of this drug (12.5 to 25 μg ml−1) than the 50 to 100 μg ml−1 for the wild type (Table 4). Similarly, the differences between these isolates and the wild type found with the disk diffusion method for gentamicin and nalidixic acid reflected differences in the MICs. No major differences in the MICs of the disinfectants AQAS and Virkon-S for the wild type and the three isolates were detected. However, there were significant differences in the MICs of Farm Fluid-S and triclosan (Table 4).
TABLE 4.
MICs of several antibiotics and disinfectants for the wild type and isolates 6 and 21a
| Strain | MIC of:
|
||||||
|---|---|---|---|---|---|---|---|
| KAN | GENT | NAL | AQAS | FFS | VS | TRIC | |
| Wild type | 50-100 | 50 | 400 | 0.0019-0.0039 | 0.0156 | 0.0625 | 0.05-0.025 |
| 6 | 12.5-25 | 1.5-3.1 | 50-100 | 0.0019-0.001 | 0.0078-0.0019 | 0.0625 | 0.0001-0.0002 |
| 8 | 12.5-25 | 1.5-3.1 | 50-100 | 0.0019-0.001 | 0.0019-0.001 | 0.0625 | 0.0031 |
| 21 | 25 | 1.5-6.2 | 50-100 | 0.001-0.004 | 0.0156-0.0078 | 0.0625 | 0.0031 |
MICs of kanamycin (KAN), gentamicin (GENT), nalidixic acid (NAL), and triclosan (TRIC) are presented as micrograms per milliliter, while those of AQAS, Farm Fluid-S (FFS), and Virkon-S (VS) are presented as percentages.
Gentamicin protection assay.
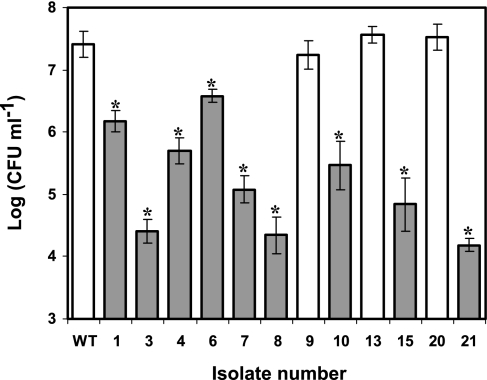
All piezotolerant variants demonstrated a reduced ability to invade Caco-2 human colon adenocarcinoma epithelial cells compared to the wild type and the rest of the isolates (Fig. 3). The level of invasiveness of the wild type was significantly higher (between 10- and 3,000-fold) than that of the piezotolerant variants. In control experiments, none of the strains, including the wild type, were able to survive the gentamicin assay when no intestinal epithelial cells were present in the wells. This finding demonstrates that the results presented were not affected by the different susceptibilities of the various strains to the antibiotic, and the results thus clearly represent the invasiveness of each strain.
FIG. 3.
Results of the gentamicin protection assay for determination of the invasiveness of the wild-type strain and isolates 1, 3, 4, 6, 7, 8, 9, 10, 13, 15, 20, and 21 in Caco-2 intestinal epithelial cells for 2 h at 37°C. Dark bars represent small-colony variants, while light bars represent isolates with normal colony morphologies. Asterisks denote significant differences between the log CFU ml−1 values of the isolates and the wild type (P < 0.05).
Detection of mutations in the short sequence repeat region of ctsR by AFLP analysis.
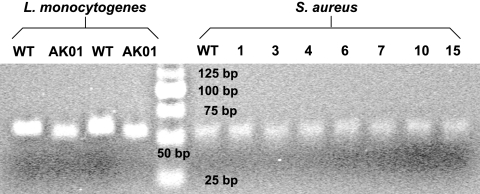
PCR-based AFLP analysis was employed for the detection of possible mutations in the region of the ctsR gene coding for the glycine-rich region of CtsR. The PCR-amplified locus of ctsR was 50 bp in size, and the most common mutation involves 3-bp deletions. The PCR-based AFLP technique is able to successfully identify such small differences in L. monocytogenes and can distinguish between Scott A (wild type) and strain AK01, which has a 3-bp deletion (17), as presented in Fig. 4. The above-mentioned observations were also confirmed following analysis with Quantity One software. Using this test, we confirmed that none of the S. aureus isolates contained a 3-bp deletion in the hypermutable region of the ctsR gene (Fig. 4).
FIG. 4.
Use of AFLP technology for the detection of possible 3-bp deletions in the hypermutable region of the ctsR gene of S. aureus. The AFLP technique is able to detect the typical 3-bp deletion contained in the hypermutable region of ctsR of L. monocytogenes AK01. No difference between the wild type (WT) and piezotolerant variants was detected. Results shown are representative of results from at least six experiments performed.
Sequencing of ctsR and hrcA genes.
In S. aureus, as in several other gram-positive microorganisms, like L. monocytogenes, ctsR is the first gene of the ctsR-clpC operon. The sequence of the 461-bp-long ctsR gene was obtained together with ∼100 bp upstream and ∼220 bp downstream. hrcA is the first gene of the dnaK operon (977 bp), and in addition to the sequence of this gene we obtained sequences extending ∼180 bp upstream and ∼130 bp downstream. Sequencing of ctsR and hrcA genes of isolates 1, 8, and 21 revealed no mutations relative to the wild-type sequences.
DISCUSSION
The existence of piezotolerant variants in pure cultures of S. aureus was investigated. Following HHP treatment of a stationary-phase S. aureus culture, a number of piezotolerant variants were isolated. These variants appeared in the population within 5 days of growth on liquid and solid media post-pressure treatment. Among 21 randomly selected isolates, 9 (42%) were piezotolerant. In general, they demonstrated impaired growth at 20°C without shaking, a small-colony phenotype, defects in carotenoid production linked with the typical golden color, possible defects in coagulase and protein A production, reduced invasiveness, and increased susceptibility to several antibiotics compared to the wild type. To our knowledge, this is the first report describing a piezotolerant strain of S. aureus and the existence of a piezotolerant subfraction in a pure culture of this organism.
The characterization of piezotolerant food-borne pathogens has been restricted to a limited number of incidental isolates of several microorganisms, like E. coli (11), Saccharomyces cerevisiae (10), and L. monocytogenes (18). However, in most cases the isolation of these strains is achieved following many subsequent selective HHP cycles (11) or treatment with mutagenic substances (16). As we have shown previously with L. monocytogenes, piezotolerant mutants can be abundant within clonal bacterial populations following a few hours of growth (17). In this study, piezotolerant variants of S. aureus were isolated by an HHP treatment of a clonal population of the organism following 5 days of growth on liquid and solid media.
The differences in piezotolerance levels between the variants and the wild type were significant, reaching in the case of isolate 4 even 2.8 log at 450 MPa for 20 min. Most of the piezotolerant variants isolated in this study were also thermotolerant. Several workers have shown that piezotolerance is driven not by unique stress resistance mechanisms but by those involved in resistance to other stresses, like heat (15, 16). In work done with L. monocytogenes, the upregulation of stress genes such as those for Clp-ATPases or cold shock proteins resulted in increased piezotolerance, revealing the similar mechanisms involved in these processes (19, 35). In addition, following an HHP treatment with 55 MPa, a total of 55 proteins were upregulated and the majority were stress proteins (34). In addition, it seems that increased piezotolerance coincides with small-colony morphology and impaired growth (18). As described previously, piezotolerance is linked to tolerance to other stresses. Variants of E. coli (25) and Salmonella (our unpublished results) that are resistant to acid show small-colony morphologies and impaired growth.
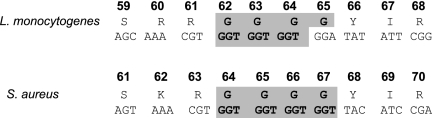
In previous work, Karatzas et al. showed that L. monocytogenes possesses a hypermutable region in the ctsR gene which, through mutations, renders CtsR inactive, resulting in the upregulation of class III stress genes (clpC and clpP, etc.) and increased stress resistance in a number of cells within a population (17, 19). As part of a strategy for the development of an abundance of stress-resistant subpopulations, these mutants appear within hours in a pure L. monocytogenes culture (17). Various studies stress the significance of short sequence repeat regions, but in only a few instances has a role for short sequence repeats in specific cellular processes been demonstrated experimentally (6, 31). The S. aureus piezotolerant variants in the present study showed similarities to the piezotolerant variants of L. monocytogenes isolated previously, namely, impaired growth, small colony size, increased thermotolerance, reduced invasiveness, and high abundance within the population, leading to easy isolation through a single HHP isolation step. In addition, the ctsR gene of S. aureus contains four GGT repeats in the hypermutable locus encoding the glycine-rich region of CtsR, while the corresponding gene in L. monocytogenes contains only three (Fig. 5). These repeats may increase the hypermutagenic nature of the region and probably allow the isolation of a high number of ctsR mutants. By adapting an AFLP-based method developed for L. monocytogenes, we proved that the hypermutable regions of all isolates of S. aureus contained no triplet deletions as observed in the L. monocytogenes mutants. In addition, we sequenced the ctsR and hrcA genes of three randomly selected piezotolerant variants (1, 8, and 21), finding no mutations in these genes. It is possible that ctsR mutants appear in a population of S. aureus but may not be as resistant as or are more resistant than the variants we isolated. This may be the result of a lower impact of these mutations on the functionality of CtsR as a repressor or differences between the CtsR regulons of L. monocytogenes and S. aureus. However, in S. aureus, CtsR possesses a higher position in the regulatory network of stress proteins than its counterpart in L. monocytogenes, coregulating with HrcA the hrcA-dnaK and groESL operons, and a possible mutation would have a greater impact (7).
FIG. 5.
Protein sequences and corresponding DNA sequences of the glycine-rich regions of the ctsR gene products from L. monocytogenes and S. aureus. The S. aureus gene contains four repeats instead of the three in the L. monocytogenes gene.
In various studies, small-colony variants of S. aureus and other bacterial species have been found to correlate with increased antibiotic resistance (27). However, in this study the small-colony variants showed higher susceptibility to several antibiotics than the wild type. This finding clearly demonstrates that there are small-colony variants in S. aureus populations that are not antibiotic resistant and they might be confused with those described in the other reports. To our knowledge, it has not been reported before that increased resistance to stresses like heat and high hydrostatic pressure coincides with reduced antibiotic resistance. It could be speculated that the impaired growth of the piezotolerant variants is responsible for their increased sensitivity to antibiotics. However, antibiotic-resistant small-colony variants also had impaired growth, without compromising their resistance (27). It is possible that the overexpression of stress proteins may affect the expression or the proper function of efflux pumps, resulting in this phenotype. Another explanation could be that mutations in the piezotolerant variants lead to target alteration, resulting in changes in antibiotic susceptibility. However, this possibility is unlikely, as changes in susceptibility to two different classes of antibiotics, namely, aminoglycosides (amikacin, kanamycin, neomycin, and gentamicin) and β-lactams (oxacillin), which do not share a common bacterial target, were observed. Challenging with disinfectants did not reveal major differences in the MICs for the piezotolerant variants and the wild type, probably due to the multiplicity of cellular targets. However, in the case of triclosan, the MICs for the piezotolerant variants were lower than that for the wild type. This result could be explained by the possible downregulation of efflux pumps in the piezotolerant variants (23).
Since these variants are more thermotolerant and piezotolerant, they may survive better in food and eventually colonize the intestinal tract. This is one of the first reports to investigate the ability of S. aureus to invade and colonize intestinal epithelial cells, since only recently has this ability been discovered (13). In addition, we wanted to assess the invasiveness of these naturally occurring variants as an indication of their virulence. However, the piezotolerant variants of S. aureus showed reduced invasiveness with Caco-2 epithelial cells compared to the wild type. A possible mechanism may be the overexpression of stress proteins, which previously has been implicated in reduced-virulence properties of the L. monocytogenes piezotolerant mutants (19). An interesting finding is that the piezotolerant variants of S. aureus showed reduced production of the typical S. aureus golden color compared to the wild type. This characteristic has been shown previously to play an important role in virulence, as an S. aureus mutant with disrupted carotenoid biosynthesis had impaired neutrophil survival and was less pathogenic in a mouse subcutaneous abscess model than the wild type (22). In addition, the heterologous expression of the S. aureus carotenoid in the nonpigmented Streptococcus pyogenes conferred enhanced neutrophil survival and increased virulence in animals. The mechanism causing the piezotolerant phenotype is possibly involved in the downregulation of the surface structure proteins coagulase and protein A, as suggested by weak agglutination reactions with a specific antibody detecting these two proteins. Protein A has been shown to play an important role in virulence by inhibiting phagocytosis (14). It has been a long-held belief that coagulase plays a role in virulence by protecting cells from phagocytic and immune defenses through clumping. However, experimental data have not supported this hypothesis (5).
High-hydrostatic-pressure treatment has been linked with the isolation of piezotolerant and multiple-stress-resistant mutants, which probably are less virulent according to our findings (19). However, the property of facilitating the isolation of these variants does not seem to be related to an inability of the HHP method to kill bacteria effectively. There are high pressures that can kill all life forms, but technological advances are needed to deliver such pressures in a cost-effective manner. The ability of the HHP method to allow the isolation of stress-resistant mutants is related to the isostatic nature of the HHP stress (12). Every cell is challenged with the same pressure, and each cell's survival depends on its ability to resist this stress, giving a survival advantage to piezotolerant cells. With stresses like heat, where the intensity of the stress is not the same through the mass of the sample, survival depends also on other factors, like the position of the cell in the sample and the emergence of protective clumps. These factors may result in the increased survival of wild-type cells, making it harder to isolate thermotolerant mutants. In this study, we were unsuccessful in isolating stress-resistant and thermotolerant mutants through heat treatment, probably due to the reasons described above (data not shown).
The discovery and characterization of piezotolerant strains that appear naturally in populations of microorganisms are essential for the optimization of combined processing and the successful application of new techniques, like high pressure in food systems. Importantly, these strains also demonstrate a multiple-stress-resistant phenotype, and their existence affects the application of classical food preservation techniques like heat treatment. Such insights may explain phenomena like tailing in inactivation curves and help us resolve these problems by designing processes that would specifically target these strains. It is important to understand that when we apply a food process, the real targets are not the typical wild-type strains but resistant strains that are often present in populations of the microorganism, as their existence may be part of a strategy for survival based on genetic phenomena, as has been described previously for L. monocytogenes and in this work for S. aureus (17).
This study demonstrates that similar to L. monocytogenes, S. aureus possesses a strategy, possibly linked with genetic alteration, that results in the generation of piezotolerant and multiple-stress-resistant mutants within a short period of time. These variants demonstrated impaired growth, a small-colony phenotype, defects in carotenoid production linked with the typical golden color, reduced invasiveness, possible defects in coagulase and protein A production, and increased susceptibility to several antibiotics compared to the wild type. However, although S. aureus contains a region similar to the L. monocytogenes hypermutable short sequence repeat region in its ctsR gene, these variants contained no 3-bp deletions and three randomly selected variants contained no mutations in their ctsR and hrcA genes. We recently obtained evidence that the deletion of a region including genes involved in regulatory processes may be responsible for the phenotypes described in this work. In addition, we are conducting whole-genome microarrays to determine the changes in gene expression caused by this specific mutation. These data will be reported in subsequent publications.
Acknowledgments
Angelos Zervos was financially supported by the State Scholarships Foundation of Greece (IKY).
We thank Tristan Cogan and Amanda Dodson for their significant contributions to the invasion assays.
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Adams, M. R., and M. O. Moss. 2000. Food microbiology, 2nd ed., p. 253-259. Royal Society of Chemistry, Cambridge, United Kingdom.
- 2.Aertsen, A., K. Vanoirbeek, P. De Spiegeleer, J. Sermon, K. Hauben, A. Farewell, T. Nyström, and C. W. Michiels. 2004. Heat shock protein-mediated resistance to high hydrostatic pressure in Escherichia coli. Appl. Environ. Microbiol. 70:2660-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Aires de Sousa, M. A., C. Bartzavali, I. Spiliopoulou, I. Santos Sanches, M. I. Crisóstomo, and H. de Lencastre. 2003. Two international methicillin-resistant Staphylococcus aureus clones endemic in a University Hospital in Patras, Greece. J. Clin. Microbiol. 41:2027-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J. M. 2005. BSAC standardized disc susceptibility testing method (version 4). J. Antimicrob. Chemother. 56:60-76. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, J. M. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. 1):5-16. [DOI] [PubMed] [Google Scholar]
- 5.Baddour, L. M., M. M. Tayidi, E. Walker, D. McDevitt, and T. J. Foster. 1994. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. J. Med. Microbiol. 41:259-263. [DOI] [PubMed] [Google Scholar]
- 6.Bayliss, C. D., D. Field, and E. R. Moxon. 2001. The simple sequence contingency loci of Haemophilus influenzae and Neisseria meningitides. J. Clin. Investig. 107:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastanet, A., J. Fert, and T. Msadek. 2003. Comparative genomics reveal novel heat shock regulatory mechanisms in Staphylococcus aureus and other Gram-positive bacteria. Mol. Microbiol. 47:1061-1073. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Elsinghorst, E. A. 1994. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 10.Fujii, S., H. Iwahashi, K. Obuchi, T. Fujii, and Y. Komatsu. 1996. Characterization of a barotolerant mutant of the yeast Saccharomyces cerevisiae: importance of trehalose content and membrane fluidity. FEMS Microbiol. Lett. 141:97-101. [DOI] [PubMed] [Google Scholar]
- 11.Hauben, K. J. A., D. H. Bartlett, C. C. F. Soontjens, K. Cornelis, E. Y. Wuytack, and C. W. Michiels. 1997. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl. Environ. Microbiol. 63:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heremans, K. 1982. High pressure effects on proteins and other biomolecules. Annu. Rev. Biophys. Bioeng. 11:1-21. [DOI] [PubMed] [Google Scholar]
- 13.Hess, D. J., M. J. Henry-Stanley, E. A. Erickson, and C. L. Wells. 2003. Intracellular survival of Staphylococcus aureus within cultured enterocytes. J. Surg. Res. 114:42-49. [DOI] [PubMed] [Google Scholar]
- 14.Higgins, J., A. Loughman, K. P. van Kessel, J. A. van Strijp, and T. J. Foster. 2006. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol. Lett. 258:290-296. [DOI] [PubMed] [Google Scholar]
- 15.Hörmann, S., C. Scheyhing, J. Behr, M. Pavlovic, M. Ehrmann, and R. F. Vogel. 2006. Comparative proteome approach to characterize the high-pressure stress response of Lactobacillus sanfranciscensis DSM 20451T. Proteomics 6:1878-1885. [DOI] [PubMed] [Google Scholar]
- 16.Iwahashi, H., S. Fujii, K. Obuchi, S. C. Kaul, A. Sato, and Y. Komatsu. 1993. Hydrostatic pressure is like high temperature and oxidative stress in the damage it causes to yeast. FEMS Microbiol. Lett. 108:53-58. [DOI] [PubMed] [Google Scholar]
- 17.Karatzas, K. A. G., V. P. Valdramidis, and M. H. J. Wells-Bennik. 2005. Contingency locus in ctsR of Listeria monocytogenes ScottA: a strategy for occurrence of abundant piezotolerant isolates within clonal populations. Appl. Environ. Microbiol. 71:8390-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karatzas, K. A. G., and M. H. J. Bennik. 2002. Characterization of a Listeria monocytogenes Scott A isolate with high tolerance towards high hydrostatic pressure. Appl. Environ. Microbiol. 68:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karatzas, K. A. G., J. A. Wouters, G. M. C. Gahan, C. Hill, T. Abee, and M. H. J. Bennik. 2003. The CtsR regulator of Listeria monocytogenes contains a variant glycine repeat region that affects piezotolerance, stress resistance, motility and virulence. Mol. Microbiol. 49:1227-1238. [DOI] [PubMed] [Google Scholar]
- 20.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Loir, Y., F. Baron, and M. Gautier. 2003. Staphylococcus aureus and food poisoning. Gen. Mol. Res. 2:63-76. [PubMed] [Google Scholar]
- 22.Liu, G. Y., A. Essex, J. T. Buchanan, V. Datta, H. M. Hoffman, J. F. Bastian, J. Fierer, and V. Nizet. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 202:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol. Lett. 166:305-309. [DOI] [PubMed] [Google Scholar]
- 24.Moxon, E. R., and D. S. Thaler. 1997. The tinkerer's evolving tool-box. Nature 387:659-662.9192885 [Google Scholar]
- 25.Nadal, G. M., L. A. Lewis, R. Saby, P. T. Umekubo, and I. N. Hirshfield. 2006. Escherichia coli small colony variants: a newly proposed acid resistance mechanism, abstr. K-018. Abstr. 106th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 26.Pospiech, A., and B. Neumann. 1995. Isolation of genomic DNA from Gram positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 27.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295-305. [DOI] [PubMed] [Google Scholar]
- 28.Quinn, P. J., M. E. Carter, B. Markey, and G. R. Carter. 2002. Clinical veterinary microbiology, p. 118-126. Mosby International Limited, London, United Kingdom.
- 29.Rocha, E. P. C., I. Matic, and F. Taddei. 2002. Over-representation of repeats in stress response genes: a strategy to increase versatility under stressful conditions? Nucleic Acids Res. 30:1886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.van Belkum, A., S. Scherer, L. van Alphen, and H. Verburg. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62:275-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Woude, M. W., and A. J. Bäumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaudaux, P. E., P. Francois, F. D. Lew, and F. A. Waldvogel. 2000. Host factors predisposing to and influencing therapy of foreign body infections, p. 1-27. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices. ASM Press, Washington, DC.
- 34.Welch, T. J., A. Farewell, F. C. Neidhardt, and D. H. Bartlett. 1993. Stress response of Escherichia coli to elevated hydrostatic pressure. J. Bacteriol. 175:7170-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wemekamp-Kamphuis, H. H., A. K. Karatzas, J. A. Wouters, and T. Abee. 2002. Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure. Appl. Environ. Microbiol. 68:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]