Abstract
The utilization of the fuel oxygenate methyl tert-butyl ether (MTBE) and related compounds by microorganisms was investigated in a mainly theoretical study based on the YATP concept. Experiments were conducted to derive realistic maintenance coefficients and Ks values needed to calculate substrate fluxes available for biomass production. Aerobic substrate conversion and biomass synthesis were calculated for different putative pathways. The results suggest that MTBE is an effective heterotrophic substrate that can sustain growth yields of up to 0.87 g g−1, which contradicts previous calculation results (N. Fortin et al., Environ. Microbiol. 3:407-416, 2001). Sufficient energy equivalents were generated in several of the potential assimilatory routes to incorporate carbon into biomass without the necessity to dissimilate additional substrate, efficient energy transduction provided. However, when a growth-related kinetic model was included, the limits of productive degradation became obvious. Depending on the maintenance coefficient ms and its associated biomass decay term b, growth-associated carbon conversion became strongly dependent on substrate fluxes. Due to slow degradation kinetics, the calculations predicted relatively high threshold concentrations, Smin, below which growth would not further be supported. Smin strongly depended on the maximum growth rate μmax, and b and was directly correlated with the half maximum rate-associated substrate concentration Ks, meaning that any effect impacting this parameter would also change Smin. The primary metabolic step, catalyzing the cleavage of the ether bond in MTBE, is likely to control the substrate flux in various strains. In addition, deficits in oxygen as an external factor and in reduction equivalents as a cellular variable in this reaction should further increase Ks and Smin for MTBE.
Methyl tert-butyl ether (MTBE) and the related compounds ethyl tert-butyl ether (ETBE) and tert-amyl methyl ether, are widely used as oxygenating compounds in gasoline. Extensive use of these compounds for over 20 years has led to pollution mainly caused by unnoticed leakages and accidental spills. High water solubility facilitates the spread of these pollutants, which now severely threaten water resources (4, 29, 47, 51) by their unpleasant odor and taste and suspected carcinogenicity. Consequently, a main concern is the environmental fate of the ether oxygenates and the development of measures against pollution. In this respect microbial degradation has been considered (11, 14, 48). However, MTBE and structurally related compounds prove recalcitrant to microbial attack. This is thought to be mainly caused by the ether bond in these compounds and the presence of a tertiary carbon atom. The search for degradative microorganisms was without success for a long time (25) or resulted at best in the enrichment of degrading consortia (6, 15, 28, 44). However, provision of alkanes as a primary source of carbon and energy led to the enrichment of strains that are able to attack MTBE and related compounds cometabolically (52). Extensive search has also resulted in the enrichment of axenic cultures capable of degrading MTBE and using it as sole source of carbon and energy for growth. A first hint came from Mo et al. (34), who described bacterial isolates with MTBE-degrading activity belonging to the genera Methylobacterium, Rhodococcus, and Arthrobacter. Recently, the spectrum of strains has been extended to Rubrivivax gelatinosus PM1 (18), now Methylibium petroleiphilum PM1 (36), Hydrogenophaga flava ENV735 (21), and Mycobacterium austroafricanum IFP2012 (16, 17). We have recently isolated strain L108 from a polluted site in Germany, which is able to grow on MTBE and ETBE (42) and, like M. petroleiphilum PM1, belongs to the Ideonella-Leptothrix-Rubrivivax branch of the β-proteobacteria.
Despite the success in isolating single strains capable of degrading MTBE, their abundance seems to be rather low even at polluted sites. This can be inferred from the very long half-lives of MTBE and its main degradation intermediate tert-butyl alcohol (TBA) in in situ investigations and microcosm studies (5, 7, 46, 65). Moreover, degradation was often absent in samples that were derived from contaminated sites (27). Rittmann (41) explained this deficit by the fact that two factors must coincide to favor (productive) MTBE degradation: a high oxygen concentration, which is rather uncommon in organically contaminated aquifers, and the presence of microorganisms with specific oxygenase activity (45). An alternative explanation assumes energy spillage during MTBE conversion (15), the mechanism of which, however, was not specified.
The present investigation considers the fate of MTBE and related compounds from the viewpoint of their qualities as heterotrophic substrates. The question was with which efficiency carbon from these substrates could be incorporated into biomass. The stoichiometric balances and the corresponding yield coefficients were calculated by taking into account the stoichiometries of various proven and putative pathways. Theoretical yields were related to growth kinetics, i.e., by taking into account nonproductive substrate consumption for maintenance, a rate term common and specific to living cells, resulting in threshold substrate concentrations that must be exceeded to enable growth. The various stoichiometric and kinetic parameters provide the framework for the productive degradation of MTBE and related compounds and define the potentials and the restrictions of the elimination of these pollutants by autocatalytically controlled microbial processes. It can be concluded that in situ MTBE concentrations are often below the threshold enabling productive degradation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The MTBE-degrading strain L108 and its mutant L10 (TBA+, MTBE−) (42) were grown on mineral salts solution containing (in mg/liter): NH4Cl, 760; KH2PO4, 340; K2HPO4, 485; CaCl2·6H2O, 27; MgSO4·7H2O, 71.2; and 1 ml/liter of trace element solution (TES). TES was composed of (in g/liter): FeSO4·7H2O, 4.98; CuSO4·5H2O, 0.785; CoCl2, 5; MnSO4·4H2O, 0.81; ZnSO4·7H2O, 0.44; Na2MoO4·2H2O, 0.25. The medium was supplemented with a vitamin mixture comprising (in μg/liter): biotin, 20; folic acid, 20; pyridoxine-HCl, 100; thiamine-HCl, 50; riboflavin, 50; nicotinic acid, 50; dl-Ca-pantothenate, 50; p-amino-benzoic acid, 50; liponic acid, 50, and cyanocobalamin, 50. Batch cultivation on MTBE and TBA was as previously described (42). Continuous cultivation was performed with strain L10 in a laboratory fermentor (Biostat D; B. Braun Biotech, Melsungen, Germany) at a working volume of 0.8 l at 30°C and pH 6.5. The fermentor was gassed with a mixture of 90% air and 10% carbon dioxide at a rate of 50 liters/h; the suspension in the fermentor was stirred at 600 rpm. Acetate at a concentration of 3 g/liter (supplied as the sodium salt) served as a substrate. The mineral salts solution medium did not contain CoCl2 when acetate was the growth substrate.
Kinetic constants.
Batch cultivation on MTBE aimed at deriving kinetic constants was performed in 50-ml bottles that were sealed gas-tight with butyl rubber stoppers. These contained a 20-ml suspension of strain L108 at a biomass concentration of 0.05 g/liter. MTBE was added to the individual flasks in amounts yielding concentrations from 0.015 to 0.25 mM. The bottles were incubated for 2 h at 22°C to equilibrate MTBE in the liquid phase, and thereafter samples of 0.5 ml were taken over a period of 10 h, added to 5-ml gas chromatography vials that contained 1.5 ml of 0.1 N NaOH, and immediately sealed. The degradation of MTBE was monitored in duplicates, and the rates were determined by linear regression analysis; the confidence intervals of the combined sets of data were in general >0.9. Rates refer to the initial MTBE concentration obtained after equilibration between liquid and gas phases.
Analytics.
Biomass concentration was monitored by measurements of the optical densities of cultures, and the mass of samples was dried to weight constancy at 105°C. Acetate was determined by high-pressure liquid chromatography on a Nucleogel ION 300 OA column (Macherey-Nagel, Düren, Germany) at 70°C with 0.01 N H2SO4 as the mobile phase (0.6 ml/min). Detection was performed with a photo diode array detector at 210 nm. MTBE, tert-butyl formate (TBF), and TBA were quantified by headspace gas chromatography (42).
Calculations.
The calculation of growth yields was performed according to the YATP concept (1, 54), assuming synthesis of 10.5 g of bacterial dry mass/mol of ATP (2, 3, 54). Biomass synthesis was assumed to start from the central carbon precursor 3-phosphoglycerate (PGA [62]). Hence, the assimilatory pathways forming this metabolite were formulated. C4H8O2N1 was taken as the elemental composition of biomass (3).
Theory.
The metabolic routes and stoichiometry considered here are discussed below.
Cleavage of the ether bond and formation of TBA.
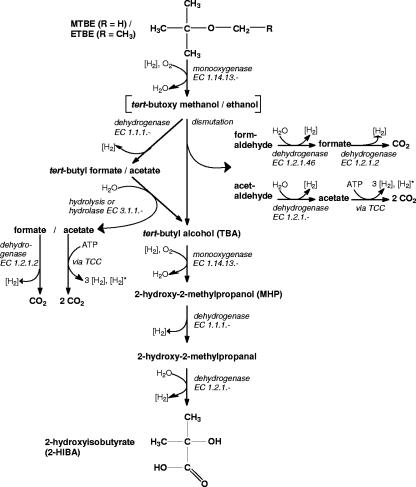
MTBE degradation is initiated by the monooxygenase-catalyzed cleavage of the ether bond (Fig. 1) (8, 14, 26, 50, 52). The reaction is believed to yield tert-butoxy methanol, an unstable and/or reactive intermediate (13, 14, 48, 52) that may undergo dismutation to TBA and formaldehyde (19). Alternatively, a dehydrogenase reaction may form TBF (19, 23, 43). This is supported by the occasional detection of TBF in the culture broth of MTBE-degrading bacteria (26, 50) such as strain L108 (43). Abiotic reactions or an esterase will then cleave TBF into TBA and formate (14). TBA is a key intermediate observed in various investigations (10, 13, 43, 52). Formaldehyde and formate are likely to be oxidized by common dehydrogenases. The balance equations of the two routes leading to TBA are identical (equation 1a [see below]). We also consider the degradation of ETBE, which is a substrate of strain L108 (42) and Rhodococcus ruber IFP 2001 (8), with TBA and acetaldehyde or acetate being the corresponding products of ether bond cleavage (Fig. 1). Acetaldehyde and acetate might be metabolized via acetyl-coenzyme A (CoA). For the sake of simplicity we assumed their complete oxidation (equation 1b), although they may serve as a carbon source, as in the case with R. ruber IFP 2001 (8). The balances, including a [H2]-consuming monooxygenase step and the [H2]-delivering oxidations of formaldehyde or acetaldehyde, respectively, to carbon dioxide read as follows:
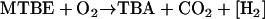 |
(1a) |
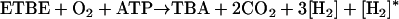 |
(1b) |
[H2] and [H2]* refer to NAD(P)H + H+ and FADH2, respectively.
FIG. 1.
Potential conversion routes of MTBE or ETBE to 2-HIBA. TCC, tricarboxylic acid cycle.
Formation of 2-HIBA.
2-Hydroxyisobutyrate (2-HIBA) is a likely central metabolite in the degradation pathway of MTBE as it is frequently detected in cultures growing on MTBE accompanied by the presumed intermediate 2- methyl-2-hydroxypropanol (17, 52). Hydroxylation of a methyl group of TBA followed by two consecutive dehydrogenase steps yields 2-HIBA (Fig. 1). Recently, the corresponding enzymes were detected in M. austroafricanum IFP 2012 (32). The sum equation including the monooxygenase reaction to TBA and two consecutive dehydrogenase steps yielding 2-HIBA reads:
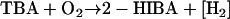 |
(2) |
and the complete sequences:
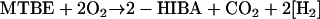 |
(3a) |
 |
(3b) |
Connection with the central metabolism.
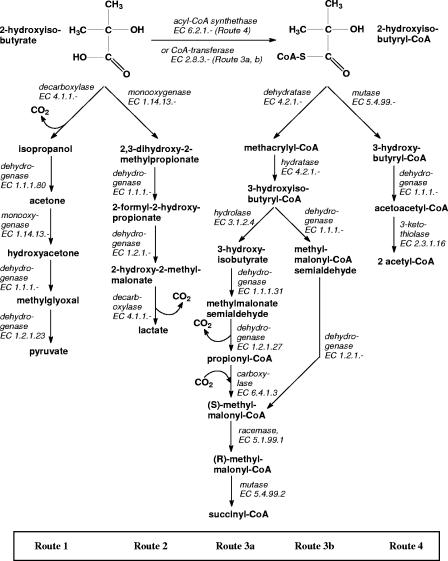
Information about the assimilation of 2-HIBA is scarce. However, observed metabolites (11, 22, 33, 42) suggest the routes depicted in Fig. 2.
FIG. 2.
Putative pathways connecting 2-HIBA to the general metabolism. TCC, tricarboxylic acid cycle.
(i) Direct conversion of 2-HIBA.
Conversion of 2-HIBA without activation by CoA was proposed (48, 52). Initiation by a decarboxylase would yield pyruvate via acetone (Fig. 2, route 1), whereas a monooxygenase reaction would lead to lactate (10, 52) (route 2). The former route was favored by several authors (10, 11, 13, 17, 22, 52). Indeed, acetone was found as a metabolite in MTBE degradation in M. austroafricanum IFP 2012, and high and inducible acetone monooxygenase activity could be demonstrated in MTBE-grown cultures supporting this route (16). In addition, hydroxyacetone was detected during degradation of MTBE by M. vaccae JOB5 (22). In contrast, strain L108 showed weak growth on acetone at best, whereas it utilized lactate quickly. Both balance equations are equivalent when lactate is assumed to oxidize to pyruvate:
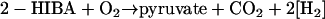 |
(4a) |
For comparison of the various pathways and calculation of biomass synthesis from a common basis, we formally transformed the key metabolites into the conventional (3, 62) intermediate for biomass synthesis PGA. Pyruvate conversion to PGA was assumed to involve pyruvate carboxylase and phosphoenolpyruvate carboxykinase reactions, thus extending equation 4a to:
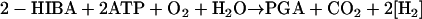 |
(4b) |
(ii) Metabolism via 2-HIBA-CoA.
Alternative routes including activation by thioester formation were formulated, which are motivated by the observed dependency of MTBE (TBA, 2-HIBA) degradation by strain CIP I-2052 (13, 39), M. austroafricanum IFP 2012 (17), and strain L108 (42) on Co2+. Since the addition of vitamin B12 stimulated MTBE degradation by strain L108 (42), we assumed that a cobalamin-dependent mutase reaction was involved in the MTBE degradation and we could show that strain L108 and the derivative strain L10 express a mutase that directly converts 2-HIBA-CoA into 3-hydroxybutyryl-CoA (42). The respective balance equation of the mutase pathway of 2-HIBA-CoA degradation (Fig. 2, route 4), including the formation of PGA, reads:
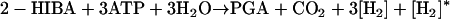 |
(5) |
The formation of 2-HIBA-CoA was assumed to be an ATP-dependent process (acyl-CoA synthetase, EC 6.2.1.-) resulting in AMP and pyrophosphate and thus requiring the formal addition of two energy equivalents (ATP) included in the above balance equation.
We also considered a dehydratase pathway initiated by the conversion of 2-HIBA-CoA to methacrylyl-CoA (52) (Fig. 2). This enzymatic step is speculative at present, but strains L108 and L10 grew on the proposed intermediates methacrylate and methylmalonate (42). 3-Hydroxyisobutyryl-CoA as a central intermediate in this pathway may be oxidized by two consecutive dehydrogenase reactions, yielding methylmalonyl-CoA (the direct oxidation variant of 2-HIBA-CoA degradation; route 3b). Alternatively, the thioester may be assimilated via the free acid, 3-hydroxyisobutyrate, resulting in propionyl-CoA through methylmalonate semialdehyde dehydrogenase (EC 1.2.1.27) which, after carboxylation by propionyl-CoA carboxylase (EC 6.4.1.3), will give methylmalonyl-CoA (the indirect oxidation variant of 2-HIBA-CoA degradation; route 3a). The simultaneous presence and function of both routes was shown in Streptomyces cinnamonensis (31). The methylmalonyl-CoA mutase (EC 5.4.99.2) will finally convert methylmalonyl-CoA into succinyl-CoA (Fig. 2, routes 3a and 3b). In this case we considered CoA activation of 2-HIBA by transesterase (CoA transferase, EC 2.8.3.-) activity with succinyl-CoA as the energy-neutral CoA donor which contributed positively to the energy balance (equation 5 versus equations 6a and 6b).
Route 3b results in:
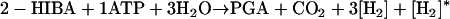 |
(6a) |
whereas route 3a requires an additional ATP (propionyl-CoA carboxylation, EC 6.4.1.3):
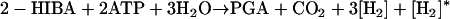 |
(6b) |
The overall balances of PGA synthesis from MTBE (ETBE) by taking into account the various routes are summarized in equations 7 to 10 (Table 1) .
TABLE 1.
Mass and energy balances for synthesis of PGA from MTBE and ETBEa
| Route | MTBE or ETBE | Substrate | Variantb
|
Equation no. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moles consumed
|
|
Moles gained
|
|||||||||
| ATP | O2 | H2O | → | PGA | CO2 | [H2] | [H2]* | ||||
| 1 or 2 | MTBE | 1 | 2 | 3 | 1 | 1 | 2 | 4 | 0 | 7a (3a + 4b) | |
| ETBE | 1 | 3 | 3 | 1 | 1 | 3 | 6 | 1 | 7b (3b + 4b) | ||
| 4 | MTBE | 1 | 3 | 2 | 3 | 1 | 2 | 5 | 1 | 8a (3a + 5) | |
| ETBE | 1 | 4 | 2 | 3 | 1 | 3 | 7 | 2 | 8b (3b + 5) | ||
| 3b | MTBE | 1 | 1 | 2 | 3 | 1 | 2 | 5 | 1 | 9 (3a + 6a) | |
| 3a | MTBE | 1 | 2 | 2 | 3 | 1 | 2 | 5 | 1 | 10 (3a + 6b) | |
Biomass synthesis.
The balance equation (2, 3) for biomass synthesis from PGA reads:
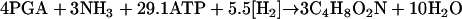 |
(11) |
Combination of equation 11 with any of the equations 7 through 10 (Table 1) gives the overall balance of MTBE/ETBE assimilation. The demand and gain in energy (ATP and reduction equivalents) of each combination will define the requirements of substrate dissimilation to satisfy the energy demand of biomass synthesis. The reduction equivalents [H2] and [H2]* were converted into ATP quantities with an efficiency according to [H2] → (P/O)ATP and [H2]* → (P/O − 1)ATP, respectively. The energy balances were calculated with (P/O)-coefficients of 1, 2, or 3, respectively (for details, see references 2 and 3). The P/O quotient reflects the efficiency of energy transduction from reduction equivalents into ATP at the passage of electrons via the respiratory chain. It is defined as the energy-rich phosphate bonds formed (P) per oxygen (O) reduced to water.
Implication of growth kinetics.
Yield calculations are based on the stoichiometry of the assumed metabolic routes, taking into account the energy demands for biomass synthesis. However, they do not consider the amount of energy required for cell maintenance, i.e., turnover of cell constituents and homeostasis that is commonly lumped in the maintenance coefficient ms. This parameter is correlated to the yield coefficient:
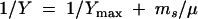 |
(12) |
where Y is the experimental yield coefficient that is derived at growth rate μ and Ymax is the maximum (experimental) yield coefficient. Equation 12 allows deriving ms from experimental data sets by linear regression. The specific maintenance rate may be correlated to the specific growth rate by an extended Monod equation which includes a rate term b (h−1) for biomass loss due to maintenance costs.
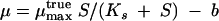 |
(13) |
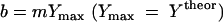 |
(14) |
At S ≫ Ks, the rate parameters approach:
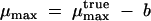 |
(15) |
i.e., the true maximum rate cannot be reached because there is always biomass decay. A consequence of the foregoing is the existence of a limiting substrate concentration, Smin, at which μ becomes zero:
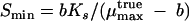 |
(16) |
The maintenance coefficient m, usually derived from growth experiments as the substrate-based coefficient ms may be transformed into an energy equivalent-based maintenance coefficient me that is based on the energy equivalents that were gained after complete substrate oxidation to CO2. me has the advantage that substrates can be compared directly. Moreover, me can be calculated for substrates for which ms is hardly accessible for practical reasons, such as MTBE.
This maintenance rate has a major impact of the growth rate on biomass formation and on Smin. The maximum specific growth rate on MTBE was derived from the specific substrate consumption rates qs with μmax = qs max × Yexp. Relevant figures of specific substrate consumption were taken from experiments with pure cultures, as summarized elsewhere (14), and by assuming experimental yield coefficients of 0.2 to 0.5 g g−1 (13, 18, 53, 58). The protein content of biomass was assumed to be 55%.
RESULTS
Balance equations and maximum theoretical yield coefficients.
The mass and energy balances of the synthesis of PGA from MTBE (ETBE) via the various routes are summarized in Table 1. It is clear that different quantities of reduction equivalents arise during assimilation and become available for biomass formation. Routes involving CoA activation of 2-HIBA were the most energy efficient but varied, e.g., due to the requirement of two energy equivalents for 2-HIBA-CoA synthesis in the mutase pathway (route 4). Succinyl-CoA as the final intermediate was assumed to function as the CoA-donor for 2-HIBA activation by a transferase mechanism, thus reducing the expenditure of ATP to one in the direct oxidation route (route 3b) and two in the indirect route (route 3a) (Fig. 2).
The energy and reduction equivalents involved in PGA synthesis (equations 4 to 6) and biomass synthesis from PGA (equation 11) are summed up and summarized in Table 2. In accordance with the foregoing, most efficient biomass synthesis should result from pathways including 2-HIBA-CoA activation due to its higher yield of reduction equivalents that is caused by the fact that it contains one less inefficient monooxgenase step.
TABLE 2.
Balance of energy equivalents in the synthesis of biomass from MTBE
| Route | Varianta
|
Equationb | ||||
|---|---|---|---|---|---|---|
| Moles required
|
Moles produced
|
|||||
| MTBE | ATP | Biomass | [H2] | [H2]* | ||
| 1 or 2 | 4 | 37.1 | 3 | 10.5 | 0 | 7a |
| 4 | 4 | 41.1 | 3 | 14.5 | 4 | 8a |
| 3b | 4 | 33.1 | 3 | 14.5 | 4 | 9 |
| 3a | 4 | 37.1 | 3 | 14.5 | 4 | 10 |
Based on the pathways indicated in Fig. 2.
Calculations were based on the equations indicated in Table 1. [H2] and [H2]* stand for the reduction equivalents NAD(P)H + H+ and FADH2, respectively, which are converted into ATP according to energy transduction efficiencies of P/O and P/O − 1, respectively (for further details, see the text).
The derived yield coefficients and corresponding carbon conversion efficiencies (CCE) are given in Table 3. CCE expresses the fraction of carbon of a heterotrophic substrate that is incorporated into biomass; this value can be deduced from the yield coefficient by referring to the carbon content of biomass (of defined composition), i.e., 47% in the present case (equation 11). The yield coefficients were derived by taking into account the amount of substrate assimilated (Table 1 and equation 11) and the amount of substrate dissimilated in order to satisfy the energy requirements for biomass synthesis (energy balances for dissimilation were derived from connecting the central intermediates shown in the various routes of MTBE metabolism as outlined in Fig. 2 to common sequences for oxidizing these metabolites to CO2, i.e., finally to the tricarboxylic acid cycle [data not shown]). According to the figures in Table 3, MTBE is an effective heterotrophic substrate, regardless of the pathway used for 2-HIBA degradation. The CCE were similar to those obtained at P/O = 2 with acetate (0.477), glucose (0.614), or methanol (0.671) (1, 2). Balanced growth, i.e., carbon conversion liberating enough energy equivalents during substrate assimilation to enable biomass synthesis without additional substrate dissimilation for energy generation, was obtained for several MTBE and ETBE assimilation routes at P/O = 2. Conditions of energy excess, i.e., the liberation of energy equivalents exceeding the requirement for carbon incorporation into biomass (2, 3), were obtained for P/O = 3 for most assimilation routes. The maximum theoretical yield from MTBE was Ymaxtheor = 0.869 g g−1. This corresponds to a maximum carbon conversion of 0.6 C-mol of biomass (C-mol of substrate)−1 reflecting the oxidation of two carbon atoms of MTBE and elimination as CO2 during the formation of PGA. With ETBE the CCE was lower (0.5) since the acetate liberated from this compound was assumed to be further oxidized to carbon dioxide (Fig. 1). This loss in carbon was accompanied by the generation of reduction equivalents with the result that the total amount of energy equivalents formed at PGA synthesis was sufficient or even in excess of that required for synthesizing biomass from this general carbon precursor, efficient energy transduction provided (Table 3).
TABLE 3.
Yield coefficients (Y) during conversion of MTBE and ETBE
| Route | MTBE or ETBE | Yield coefficient (Y [g g−1])a
|
Equationb | ||
|---|---|---|---|---|---|
| P/O = 1 | P/O = 2 | P/O = 3 | |||
| 1 or 2 | MTBE | 0.500 (0.345) | 0.711 (0.49) | 0.826 (0.569) | 7a |
| ETBE | 0.479 (0.319) | Balanced | Excess | 7b | |
| 4 | MTBE | 0.500 (0.345) | 0.789 (0.544) | Excess | 8a |
| ETBE | 0.450 (0.300) | Balanced | Excess | 8b | |
| 3b | MTBE | 0.717 (0.494) | Balanced | Excess | 9 |
| 3a | MTBE | 0.534 (0.368) | 0.827 (0.570) | Excess | 10 |
Carbon conversion efficiencies (CCE; in C-mol/C-mol) are indicated in parentheses. Balanced, no dissimilation of substrate required in addition to assimilation to synthesize biomass; excess, more energy generated during assimilation than required for biomass synthesis. The maximum biomass yield (at balanced or excess cases) was as follows: YMTBE = 0.869 (CCE = 0.6); YETBE = 0.750 (CCE = 0.5).
Calculations were based on the equations in Table 1.
Maintenance coefficients and effective biomass yields.
Maintenance coefficients required for the calculation of rate-dependent biomass yields were derived from chemostat cultivation of strain L10 on acetate at dilution rates of 0.03 to 0.12 h−1. This substrate was used since chemostat growth on MTBE is difficult to establish and TBA as a possible alternative is susceptible to losses by volatilization. Linear regression analysis of the experimental data (equation 12) gave an ms of 0.7 mmol of acetate g−1 h−1. This term was transformed into the energy-based maintenance coefficient me, with acetate delivering [3(P/O) + (P/O − 1) −1]ATP per mol after its oxidation to CO2 via common routes. MTBE would thus deliver [9(P/O) + 2(P/O − 1) + 1]ATP, [9(P/O) + 2(P/O − 1)]ATP, and [8(P/O) + (P/O − 1) + 1]ATP per mol via the dehydratase pathway (equation 9), the mutase pathway (equation 8a), and the acetone pathway (equation 7a) (Table 1), respectively, assuming that the final metabolites in Fig. 2 are connected to the general metabolism via common sequences. For MTBE and P/O = 2, ms was 0.20, 0.21, and 0.23 mmol g−1 h−1, respectively, for the three pathways.
The reported maximum growth rates on MTBE range between 0.01 and 0.06 h−1 (14), and preliminary investigation with our strain L108 gave a μmax with MTBE of 0.035 h−1. The Ks values for MTBE were 0.51 to 0.58 mM for M. petroleiphilum PM1 (18), 0.95 to 1.48 mM for M. vaccae JOB5 (23, 50), and 0.53 mM for strain L108 during batch growth; for modeling we used a Ks of 1 mM. Ymax was assumed to be 0.0765 g mmol−1 MTBE. This value corresponds to balanced carbon conversion according to our calculation with P/O = 2 (Table 2). With μmax = 0.035 h−1, Ks = 1 mM, ms = 0.2 mmol g−1 h−1, and b = 0.0153 h−1 Smin (equation 16) corresponds to 0.77 mM MTBE. The results with strain PM1 indicate that Smin for other organisms might be lower than calculated here, since it was observed that biomass (protein) still increased during the degradation of 0.28 mM MTBE (18). However, reevaluation of these data may be required since substrate decline and protein increase were not fairly coupled and data in figures were inconsistent with those in the text (18). Reduction of the assumed Ks would result in proportionally reduced Smin. A Ks of 17 μM was indeed reported for a microbial consortium (15).
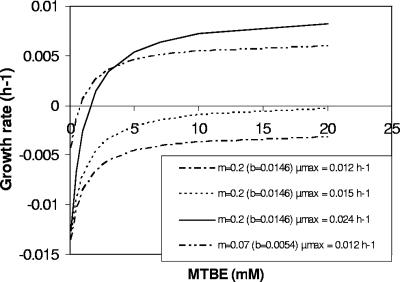
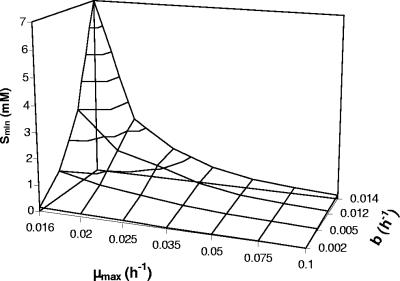
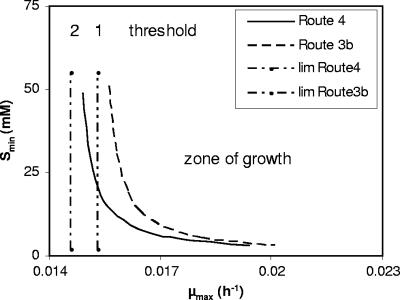
When the assumed μmax is reduced, Smin increases since more substrate is required for μ to become positive (equation 16), as shown in Fig. 3. It is evident that minor changes in μmax can result in drastically increased threshold concentrations for productive degradation. Decreasing the maintenance requirement b, in contrast, leads to decreased Smin. The fact that Smin is reduced when μmax is increased may seem counterintuitive at a first glance. It results from the steeper slope (μmax × Ks) of the first-order part of the Monod curve at a higher μmax that intercepts with the abscissa at a lower concentration. The interplay of μmax and b as a determinant of Smin is shown in Fig. 4. Reduced maintenance needs extend the concentration range for productive degradation. For example, at μ = 0.016 h−1 a sevenfold decrease in b resulted in a 50-fold decrease in Smin (Fig. 4). Smin is so sensitive to changes of the maximum growth rate that it may be influenced by the catabolic pathway despite having only slightly different energy efficiencies. This is illustrated for the mutase pathway (route 4) and the dehydratase/direct oxidation variant pathway (route 3b) (Fig. 5). The threshold concentration for productive degradation was lower for the less-energy-efficient mutase pathway than for the dehydratase route. The acetone pathway (route 1) produced figures similar to those produced by the mutase pathway. There is a limiting concentration at which growth with the dehydratase pathway would halt (threshold 1), whereas the mutase pathway would further enable growth (threshold 2). At a μmax of 0.016 h−1 the Smin for the dehydratase pathway was about twice as high as for the mutase pathway (Fig. 5), and at a μmax of 0.015 h−1 growth was restricted to the mutase pathway. It seems curious but is significant that, since b is dependent on the biomass yield (equation 14), less-efficient biomass synthesis may be an advantage at a low maximum growth rate since it minimizes b.
FIG. 3.
Kinetic characteristics of MTBE utilization at low μmax. The calculations were performed by using equation 13 with Ks = 1 mM and Ymax = 0.0694 g mmol−1 for the mutase pathway (Fig. 2, route 4) and ms = 0.21 mmol g−1 h−1 (b = 0.0146 h−1) and ms = 0.07 mmol g−1 h−1 (b = 0.0054 h−1), respectively, as indicated.
FIG. 4.
Impact of the maximum growth rate (μmax) and the rate of negative growth (b) on Smin. The calculations were performed by using equation 13 with Ks = 1 mM and various values for μmax and b as indicated.
FIG. 5.
Impact of the MTBE degradation pathway on Smin. The calculations (equation 16) were performed by taking into account MTBE degradation via the mutase pathway (Fig. 2, route 4) (b = 0.0146 h−1) and the dehydratase/direct oxidation variant pathway (Fig. 2, route 3b) (b = 0.0153 h−1), respectively. The terms lim-Route4 and lim-Route3b indicate threshold rates at which productive degradation of MTBE would not further be possible by using the respective pathways (Smin = ∞).
DISCUSSION
Calculations based on the YATP concept showed that MTBE and ETBE can be effective substrates for heterotrophic bacteria. Growth yields of up to 0.6 and 0.5 C-mol/C-mol are possible with MTBE and ETBE, respectively. The realization of this potential in growth-associated processes, however, seems to be restricted by the slow kinetics of the microbial conversion of MTBE. Calculations show that actual growth yields may approach zero because the biochemically determined rates of energy generation are in the order of the cells' maintenance demands. Our conclusion differs from those of an earlier attempt to explain low biomass formation from MTBE. Fortin et al. (15) used Payne's concept of available electrons (37) to explain the large discrepancy between expected and obtained yield coefficients. They introduced a dissipation term representing energy not utilized for biomass synthesis. This is formally right but does not give a mechanistic explanation. We overcame this shortcoming by coupling stoichiometric and kinetic terms, thereby showing that the energy is likely to fulfill the specific maintenance needs of the MTBE-degrading population. YATP calculations have the added advantage to consider the actual metabolism, thus allowing it to be tested in detail once MTBE degradation pathways are elucidated unequivocally. YATP calculations may also be used to identify individual reaction mechanisms by comparing theoretical and observed contribution to the energy generation. For the time being our calculations rely on incomplete information about MTBE metabolism.
The results in Fig. 3 and 4 showed that the threshold concentration for growth is strongly influenced by the maintenance demands. It is commonly agreed that the maintenance coefficient itself can depend on the growth rate. There is less agreement in which direction the rate influences m. Pirt (38) introduced a growth-rate-dependent term for increased maintenance at slower growth. Recent experiments with Nitrobacter growing at maximum rates of 0.02 h−1, i.e., similar to those observed with MTBE, supported this model (57). Other examples with Nitrobacter indicated a difference in maintenance requirements for cultures grown in a chemostat as opposed to reactors with biomass retention (55). In the latter, decreasing growth was found to be accompanied by a gradual decrease in the maintenance coefficient (9, 12, 30, 35, 56, 63). An assumed reduction of m to 1/3 in our calculations decreased Smin significantly (Fig. 3). With m = 0.07 mmol g−1 h−1 (b = 0.0054 h−1), Smin was reduced to 0.82 mM for μmax = 0.012 h−1 and to 0.29 mM for μmax = 0.024 h−1. Both are concentrations of environmental concern, since the threshold concentration for the taste and smell of MTBE in drinking water is as low as 0.2 μM. It should be noted that in a field situation the apparent threshold concentration may be affected by other factors, among them deficits in oxygen and NAD(P)H as the substrates of the initial cleavage reaction. All of these deficits would increase the apparent Ks with respect to MTBE and Smin. It should be noted that the supply of oxygen stimulated MTBE degradation in field studies (45) and was associated with an increase in the titer of degradative microorganisms (49).
It was not our goal to identify the biochemical reasons for slow rates of microbial MTBE conversion. However, the occasional occurrence of various metabolites in cultivation supernatants, e.g., TBF (19, 23, 43), 2-HIBA (17, 42, 52), and in particular TBA (17, 21, 52) indicates that several enzymatic steps may contribute to the limited degradation rates. Structural features of the mother compounds, such as the ether bond and the tertiary carbon atom, may certainly contribute to the low overall rates but do not fully explain them. For example, the tertiary carbon atom is retained up to 2-HIBA (Fig. 1). Despite the overall positive energy balance of MTBE, energetic reasons may hamper the first degradation phase since TBA, like MTBE itself, is attacked by an energy-inefficient monooxygenase. It may be speculated that the poor energy balance of the initial degradation may have been a serious obstacle for the evolution of MTBE pathways, since a relatively long degradation sequence had to evolve and gather in one organism to provide its host a selective advantage in MTBE-contaminated environments. Some strains seem to have overcome this difficulty by decoupling growth and biomass maintenance from MTBE degradation by using other fuel components, e.g., n-alkanes, as additional carbon sources (50, 52). A similar strategy of mixed substrate utilization has been observed in organisms that grow on substrates of low bioavailability (64).
Some investigations suggested a higher yield on MTBE than calculated here. This was the case, e.g., when MTBE-contaminated water was treated in a fluidized bed reactor where extensive biofilm formation occurred (58). It is common knowledge that biofilm formation is accompanied by extensive synthesis of extracellular polymeric substances, which may explain the observed differences since extracellular polymeric substances, (i) do not require net energy during synthesis from MTBE and, (ii) being nonliving, i.e., inert biomasses, do not require energy for maintenance.
It appears that, due to the limited efficiency of the existing degradation pathways for MTBE and related compounds, relatively high environmental concentrations are required to bring about the productive degradation of these compounds. This observation provokes questions about the mechanisms that drive the evolution of catabolic pathways for emerging contaminants. It is not daring to state that a new chemical will not exert any selective pressure on microbial communities as long as it is biochemically or physiologically inert. A minimum degree of catabolic transformation for detoxification or energy or carbon delivery is required for an emerging contaminant to drive evolution toward its faster or more substantial degradation. The example of MTBE suggests that the utilization as a sole source of energy and carbon can be regarded as the endpoint of an evolutionary adaptation to a new contaminant occurring in several steps. The first attempts to isolate MTBE-degrading microorganisms were only successful when an additional growth-supporting carbon source was provided. n- Alkanes susceptible to attack by unspecific monooxygenases turned out to be particularly effective since these enzymes also fortuitously oxidized MTBE (50, 52). Strains isolated in this manner proved capable of degrading MTBE and related oxygenates; however, this process was not self-sustained in terms of a utilization of the oxygenates as a sole source of carbon and energy for growth. It is believed that the degradation of xenobiotic compounds in an initial phase after their release is mediated by enzymes that were already existent in the preindustrial phase (24). However, even the cometabolic conversion of a compound may provide a selective advantage when the indigent substrate is toxic or its conversion adds to the energy or carbon balance of the degrader. Any evolutionary improvement of the conversion will enhance these positive effects and may help the degrader to approach conversion rates that deliver energy and/or carbon at rates sufficient for growth. Evolution may substitute the fortuitous character of these reactions by increasing the specificity of the catalysts. This appears to have happened since the first cases of MTBE contamination were reported less than 30 years ago.
The evolution of MTBE degradation is far from being understood. Evidently, hydroxylations of the methyl groups of MTBE, TBA, and acetone are key steps. The responsible alkane monooxygenases of both the heme (cytochrome P450) and non-heme (alkB) types show wide diversity (59-61) caused by the fact that n-alkanes are widespread natural chemicals. It is interesting that sequence motifs of the cytochrome P450 type monooxygenase EthABCD involved in MTBE oxidation in R. ruber (8) were also found in our gram-negative isolate L108 (unpublished results), suggesting horizontal gene transfer. The identification of a newly described mutase involved in the direct conversion of 2-HIBA to 3-hydroxybutyric acid (42) is also noteworthy in that it exhibited similarities but also distinct differences to the isobutyryl-CoA mutase large subunit of S. cinnamonensis. An exchange of Phe for Ile (corresponding to position 80 in the S. cinnamonensis sequence) in strains PM1 and L108, was remarkable (42) since this position influences the substrate specificity (40).
Our results suggest that most of the existing productive MTBE degraders have degradation kinetics that require relatively high environmental concentrations for the build-up and maintenance of biomass concentrations required for substantial environmental degradation. One may thus predict that evolutionary improvements of enzymatic kinetics are crucial for the formation of substantial concentrations of MTBE-degrading biomass and the reduction of environmental MTBE concentrations. The kinetic limitation of the natural attenuation of MTBE shows a clear analogy to the bioavailability-limited biodegradation (20) of hydrophobic organic compounds in that in both cases low biomass concentrations are sustained, which translates into high apparent contaminant threshold concentrations.
Although a prominent example of an emerging contaminant, MTBE differs in its massive environmental concentrations from other synthetic contaminants such as therapeutics, fragrances, detergents, etc. In terms of the evolution of catabolic pathways, many of these latter compounds have the disadvantage that they are unlikely to become a selective factor for the evolution of microbes. MTBE is thus a more promising driver of natural microbial evolution, and it will be interesting to see if and when nature will solve the pressing problem of MTBE contamination.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Babel, W., U. Brinkmann, and R. H. Müller. 1993. The auxiliary substrate concept: an approach for overcoming limits in microbial performances. Acta Biotechnol. 13:211-249. [Google Scholar]
- 2.Babel, W., and R. H. Müller. 1985. Mixed substrate utilization in microorganisms: biochemical aspects and energetics. J. Gen. Microbiol. 131:39-45. [Google Scholar]
- 3.Babel, W., and R. H. Müller. 1985. Correlation between cell composition and carbon conversion efficiency in microbial growth: a theoretical study. Appl. Microbiol. Biotechnol. 22:201-207. [Google Scholar]
- 4.Baehr, A. I., P. E. Stackelberg, and R. J. Baker. 1999. Evaluation of the atmosphere as a source of volatile organic compounds in shallow groundwater. Water Resource Res. 35:127-136. [Google Scholar]
- 5.Borden, R. C., R. A. Daniel, L. E. LeBrun, IV, and C. W. Davis. 1997. Intrinsic biodegradation of MTBE and BTEX in a gasoline-contaminated aquifer. Water Resource Res. 33:1105-1115. [Google Scholar]
- 6.Bradley, P. M., J. E. Landmeyer, and F. H. Chapelle. 1999. Aerobic mineralization of MTBE and tert-butyl alcohol by surface water sediments. Environ. Sci. Technol. 33:1877-1879. [Google Scholar]
- 7.Bradley, P. M., J. E. Landmeyer, and F. H. Chapelle. 2001. Effect of redox conditions on MTBE biodegradation in surface water sediments. Environ. Sci. Technol. 35:4643-4647. [DOI] [PubMed] [Google Scholar]
- 8.Chauvaux, S., F. Chevalier, C. Le Dantec, F. Fayolle, I. Miras, F. Kunst, and P. Beguin. 2001. Cloning of a genetically unstable cytochrome P-450 gene cluster involved in degradation of the pollutant ethyl tert-butyl ether by Rhodococcus ruber. J. Bacteriol. 183:6551-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesbro, W., T. Evans, and R. Eifert. 1979. Very slow growth of Escherichia coli. J. Bacteriol. 139:625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church, C. D., P. G. Tratnyek, and K. M. Scow. 2000. Pathways for the degradation of MTBE and other fuel oxygenates by isolate PM1. ACS Preprints Extended Abstr. 40:261-263. [Google Scholar]
- 11.Deeb, R. A., K. M. Scow, and L. Alvarez-Cohen. 2000. Aerobic MTBE biodegradation: an examination of past studies, current challenges and future research directions. Biodegradation 11:171-186. [DOI] [PubMed] [Google Scholar]
- 12.Drews, A., and M. Kraume. 2006. On maintenance models in severely and long-term limited membrane bioreactor cultivations. Biotechnol. Bioeng. doi: 10.1002/bit.21211. [DOI] [PubMed]
- 13.Fayolle, F., A. François, L. Garnier, D. Godefroy, H. Mathis, P. Piveteau, and F. Monot. 2003. Limitations in MTBE biodegradation. Oil Gas Sci. Technol. 58:497-504. [Google Scholar]
- 14.Fayolle, F., J.-P. Vandecasteele, and F. Monot. 2001. Microbial degradation and fate in the environment of methyl tert-butyl ether and related fuel oxygenates. Appl. Microbiol. Biotechnol. 56:339-349. [DOI] [PubMed] [Google Scholar]
- 15.Fortin, N. Y., M. Morales, Y. Nakagawa, D. D. Focht, and M. A. Deshusses. 2001. Methyl tert-butyl ether (MTBE) degradation by a microbial consortium. Environ. Microbiol. 3:407-416. [DOI] [PubMed] [Google Scholar]
- 16.François, A., L. Garnier, H. Mathis, F. Fayolle, and F. Monot. 2003. Roles of tert-butyl formate, tert-butyl alcohol, and acetone in the regulation of methyl tert-butyl ether degradation by Mycobacterium austroafricanum IFP 2012. Appl. Microbiol. Biotechnol. 62:256-262. [DOI] [PubMed] [Google Scholar]
- 17.François, A., H. Mathis, D. Godefroy, P. Piveteau, F. Fayolle, and F. Monot. 2002. Biodegradation of methyl tert-butyl ether and other fuel oxygenates by a strain, Mycobacterium austroafricanum IFP 2012. Appl. Environ. Microbiol. 68:2754-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson, J. R., C. E. Ackerman, and K. M. Scow. 1999. Biodegradation of methyl tert-butyl ether by a bacterial pure culture. Appl. Environ. Microbiol. 65:4788-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardison, L. K., S. S. Curry, L. M. Ciuffetti, and M. R. Hyman. 1997. Metabolism of diethyl ether and cometabolism of methyl tert-butyl ether by a filamentous fungus, a Graphium sp. Appl. Environ. Microbiol. 63:3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms, H., and T. N. P. Bosma. 1997. Mass transfer limitation of microbial growth and pollutant degradation. J. Ind. Microbiol. 18:97-105. [Google Scholar]
- 21.Hatzinger, P. B., K. McClay, S. Vainberg, M. Tugusheva, C. W. Condee, and R. J. Steffan. 2001. Biodegradation of methyl tert-butyl ether by a pure bacterial culture. Appl. Environ. Microbiol. 63:5601-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman, M., K. Glover, A. House, E. Johnson, and C. Smith. 2004. Physiological and enzymatic diversity of aerobic MTBE biodegradation processes, p. 39-43. In Proceedings of the Second European Conference on MTBE. CSIC, Barcelona, Spain.
- 23.Hyman, M., P. Kwon, K. Williamson, and K. O'Railly. 1998. Cometabolism of MTBE by alkane-utilizing microorganisms, p. 321-326. In G. B. Wickamanayake and R. E. Hinhee (ed.), Natural attenuation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, OH.
- 24.Janssen, D. B., I. J. T. Dinkla, G. J. Poelarends, and P. Terpstra. 2005. Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities. Environ. Microbiol. 7:1868-1882. [DOI] [PubMed] [Google Scholar]
- 25.Jensen, H. M., and E. Arvin. 1990. Solubility and degradability of the gasoline additive MTBE, methyl tert-butyl-ether and gasoline compounds in water, p. 445-448. In F. Arendt, M. Hinsenveld, and W. J. van den Brink (ed.), Contaminated soil '90. Kluver, Dordrecht, The Netherlands.
- 26.Johnson, E. L., C. A. Smith, K. T. O'Reilly, and M. R. Hyman. 2004. Induction of methyl tertiary butyl ether (MTBE)-oxidizing activity in Mycobacterium vaccae JOB5 by MTBE. Appl. Environ. Microbiol. 70:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane, S. R., H. R. Beller, T. C. Legler, C. J. Koester, H. C. Pinkart, R. U. Halden, and A. M. Happel. 2001. Aerobic biodegradation of methyl tert-butyl ether by aquifer bacteria from leaking underground storage tank sites. Appl. Environ. Microbiol. 67:5824-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharoune, M., A. Pauss, and J. M. Lebeault. 2001. Aerobic biodegradation of an oxygenate mixture: ETBE, MTBE and TAME in an upflow fixed-bed reactor. Water Resource Res. 35:1665-1674. [DOI] [PubMed] [Google Scholar]
- 29.Klinger, J., C. Stiehler, F. Sacher, and H. J. Branch. 2002. MTBE (methyl tertiary-butyl ether) in groundwaters: monitoring results from Germany. J. Environ. Monit. 4:276-279. [DOI] [PubMed] [Google Scholar]
- 30.Konopka, A. 2000. Microbial physiological state at low growth rate in natural and engineered ecosystems. Curr. Opin. Microbiol. 3:244-247. [DOI] [PubMed] [Google Scholar]
- 31.Li, C., K. Akopiants, and K. A. Reynolds. 2006. Identification and disruptional analysis of the Streptomyces cinnamonensis msdA gene, encoding methylmalonic acid semialdehyde dehydrogenase. J. Ind. Microbiol. Biotechnol. 33:75-83. [DOI] [PubMed] [Google Scholar]
- 32.Lopes Ferreira, N., D. Labbé, F. Monot, F. Fayolle-Guichard, and C. W. Greer. 2006. Genes involved in the methyl tert-butyl ether (MTBE) metabolic pathway of Mycobacterium austroafricanum IFP 2012. Microbiology 152:1361-1374. [DOI] [PubMed] [Google Scholar]
- 33.Lopes Ferreira, N., C. Malandain, and F. Fayolle-Guichard. 2006. Enzymes and genes involved in the aerobic biodegradation of MTBE. Appl. Microbiol. Biotechnol. 72:252-262. [DOI] [PubMed] [Google Scholar]
- 34.Mo, K., C. O. Lora, A. E. Wanken, M. Javanmardian, X. Yang, and C. F. Kulpa. 1997. Biodegradation of methyl t-butyl ether by pure bacterial cultures. Appl. Microbiol. Biotechnol. 47:69-72. [DOI] [PubMed] [Google Scholar]
- 35.Müller, R. H., and W. Babel. 1996. Measurement of growth at very low rates (μ ≥ 0), an approach to study the energy requirement for the survival of Alcaligenes eutrophus JMP134. Appl. Environ. Microbiol. 62:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatsu, C. H., K. Hristova, S. Hanada, X.-Y. Meng, J. Hanson, K. M. Scow, and Y. Kamagata. 2006. Methylibium petroleiphilum PM1T gen. nov., sp. nov., a new methyl tert-butyl ether (MTBE) degrading methylotroph of the beta-Proteobacteria. Int. J. Syst. Evol. Microbiol. 56:983-989. [DOI] [PubMed] [Google Scholar]
- 37.Payne, W. J. 1970. Energy yields and growth of heterotrophs. Annu. Rev. Microbiol. 24:17-24. [DOI] [PubMed] [Google Scholar]
- 38.Pirt, S. J. 1982. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch. Microbiol. 133:300-302. [DOI] [PubMed] [Google Scholar]
- 39.Piveteau, P., F. Fayolle, J. P. Vandecasteele, and F. Monot. 2001. Biodegradation of tert-butyl alcohol and related xenobiotics by a methylotrophic bacterial isolate. Appl. Biotechnol. Microbiol. 55:369-373. [DOI] [PubMed] [Google Scholar]
- 40.Ratnatilleke, A., J. W. Vrijbloed, and J. A. Robinson. 1999. Cloning and sequencing of the coenzyme B12-binding domain of isobutyryl-CoA mutase from Streptomyces cinnamonensis, reconstitution of mutase activity, and characterization of the recombinant enzyme produced in Escherichia coli. J. Biol. Chem. 274:31679-31685. [DOI] [PubMed] [Google Scholar]
- 41.Rittmann, B. E. 2004. Definition, objectives, and evaluation of natural attenuation. Biodegradation 15:349-357. [DOI] [PubMed] [Google Scholar]
- 42.Rohwerder. T., U. Breuer, D. Benndorf, U. Lechner, and R. H. Müller. 2006. The alkyl tertiary butyl ether intermediate 2-hydroxyisobutyrate is degraded via a novel cobalamin-dependent mutase pathway. Appl. Environ. Microbiol. 72:4128-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohwerder, T., V. Cenini, C. Held, M. Martienssen, U. Lechner, and R. H. Müller. 2004. Novel MTBE-degrading bacterial isolate from Leuna groundwater (Germany): characterization of the degradation pathway with focus on 2-HIBA oxidation, p. 47-50. In Proceedings of the Second European Conference on MTBE. CSIC, Barcelona, Spain.
- 44.Salanitro, J. P., L. A. Diaz, M. P. Williams, and H. L. Wisniewski. 1994. Isolation of a bacterial culture that degrades methyl t-butyl ether. Appl. Environ. Microbiol. 60:2593-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salanitro, J. P., P. C. Johnson, G. E. Spinnler, P. M. Maner, H. L. Wisniewski, and C. Bruce. 2000. Field-scale demonstration of enhanced MTBE biodegradation through aquifer bioaugmentation and oxygenation. Environ. Sci. Technol. 34:4152-4162. [Google Scholar]
- 46.Schirmer, M., B. J. Butler, C. D. Church, J. K. Barker, and N. Nadarajah. 2003. Laboratory evidence of MTBE biodegradation in Borden aquifer material. J. Contam. Hydrol. 60:229-249. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt, T. C., E. Morgenroth, M. Schirmer, M. Effenberger, and S. B. Haderlein. 2002. Use and occurrence of fuel oxygenates in Europe, p. 58-79. In A. F. Diaz and D. L. Drogos (ed.), Oxygenates in gasoline: environmental aspects. ACS, Washington, DC.
- 48.Schmidt, T. C., M. Schirmer, H. Weiss, and S. B. Haderlein. 2004. Microbial degradation of methyl tert-butyl ether and tert-butyl alcohol in the subsurface. J. Contam. Hydrol. 70:173-203. [DOI] [PubMed] [Google Scholar]
- 49.Scow, K. M., and K. A. Hicks. 2005. Natural attenuation and enhanced bioremediation of organic contaminants in groundwater. Curr. Opin. Biotechnol. 16:246-253. [DOI] [PubMed] [Google Scholar]
- 50.Smith, C. A., K. T. O'Reilly, and M. R. Hyman. 2003. Characterization of the initial reactions during the cometabolic oxidation of methyl tert-butyl ether by propane-grown Mycobacterium vaccae JOB5. Appl. Environ. Microbiol. 69:796-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squillace, P., J. S. Zogorski, W. G. Wilber, and V. C. Price. 1996. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30:1721-1730. [Google Scholar]
- 52.Steffan, R. J., K. McClay, S. Vainberg, C. W. Condee, and D. Zhang. 1997. Biodegradation of the gasoline oxygenates methyl tert-butyl ether, ethyl tert-butyl ether, and tert-amyl methyl ether by propane-oxidizing bacteria. Appl. Environ. Microbiol. 63:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steffan, R. J., S. Vainberg, C. W. Condee, K. McClay, and P. Hatzinger. 2000. Biotreatment of MTBE with a new bacterial isolate, p. 165-173. In G. B. Wickramanayake, A. R. Gaveskar, B. C. Alleman, and V. S. Magar (ed.), Bioremediation and phytoremediation of chlorinated and recalcitrant compounds. Battelle Press, Columbus, OH.
- 54.Stouthamer, A. H. 1973. A theoretical study on the amount of ATP required for the synthesis of microbial cell material. Antonie Leeuwenhoek 39:545-565. [DOI] [PubMed] [Google Scholar]
- 55.Tappe, W., A. Laverman, M. Bohland, M. Braster, S. Rittershaus, J. Groeneweg, and H. W. van Verseveld. 1999. Maintenance energy demand and starvation recovery dynamics of Nitrobacter europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention. Appl. Environ. Microbiol. 65:2471-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tros, M. E., T. N. P. Bosma, G. Schraa, and A. J. B. Zehnder. 1996. Measurement of minimum substrate concentration (Smin) in a recycling fermentor and its prediction from kinetic parameters of Pseudomonas sp. strain B13 from batch and chemostat cultures. Appl. Environ. Microbiol. 62:3655-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vadivelu, V. M., Z. Yuan, C. Fux, and J. Keller. 2006. Stoichiometric and kinetic characterization of Nitrobacter in mixed culture by decoupling the growth and energy generation processes. Biotechnol. Bioeng. 94:1176-1188. [DOI] [PubMed] [Google Scholar]
- 58.Vainberg, S., A. P. Togna, P. M. Sutton, and R. J. Steffan. 2002. Treatment of MTBE-contaminated water in fluidized bed bioreactor. J. Environ. Eng. 128:842-851. [Google Scholar]
- 59.van Beilen, J. B., E. G. Funhoff, A. van Loon, A. Just, L. Kaysser, M. Bouza, R. Holtackers, M. Rothlisberger, Z. Li, and B. Witholt. 2006. Cytochrome P450 alkane hydroxylases of the CYP153 family are common in alkane-degrading eubacteria lacking integral membrane alkane hydroxylases. Appl. Environ. Microbiol. 72:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Beilen, J. B., T. H. Smits, F. F. Roos, T. Brunner, S. B. Balada, M. Rothlisberger, and B. Witholt. 2005. Identification of an amino acid position that determines the substrate range of integral membrane alkane hydroxylases. J. Bacteriol. 187:85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Beilen, J. B., T. H. Smits, L. G. Whyte, S. Schorcht, M. Rothlisberger, T. Plaggemeier, K. H. Engesser, and B. Witholt. 2002. Alkane hydroxylase homologues in gram-positive strains. Environ. Microbiol. 4:676-682. [DOI] [PubMed] [Google Scholar]
- 62.van Dijken, J. P., and W. Harder. 1975. Growth yields of microorganisms on methanol and methane: a theoretical study. Biotechnol. Bioeng. 17:25-30. [Google Scholar]
- 63.Van Verseveld, H. W., W. R. Chesbro, M. Braster, and A. H. Stouthamer. 1984. Eubacteria have 3 growth modes keyed to nutrient flow. Arch. Microbiol. 137:176-184. [DOI] [PubMed] [Google Scholar]
- 64.Wick, L. Y., N. Pasche, S. M. Bernasconi, O. Pelz, and H. Harms. 2003. Characterization of multiple-substrate utilization by anthracene-degrading Mycobacterium frederiksbergense LB501T. Appl. Environ. Microbiol. 69:6133-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zoeckler, J. R., M. A. Widdowson, and J. T. Novak. 2003. Aerobic biodegradation of methyl tert-butyl ether in gasoline-contaminated aquifer sediments. J. Environ. Eng. 129:642-650. [Google Scholar]