Abstract
The ability of bacteriophage to persist in the phyllosphere for extended periods is limited by many factors, including sunlight irradiation, especially in the UV zone, temperature, desiccation, and exposure to copper bactericides. The effects of these factors on persistence of phage and formulated phage (phage mixed with skim milk) were evaluated. In field studies, copper caused significant phage reduction if applied on the day of phage application but not if applied 4 or 7 days in advance. Sunlight UV was evaluated for detrimental effects on phage survival on tomato foliage in the field. Phage was applied in the early morning, midmorning, early afternoon, and late evening, while UVA plus UVB irradiation and phage populations were monitored. The intensity of UV irradiation positively correlated with phage population decline. The protective formulation reduced the UV effect. In order to demonstrate direct effects of UV, phage suspensions were exposed to UV irradiation and assayed for effectiveness against bacterial spot of tomato. UV significantly reduced phage ability to control bacterial spot. Ambient temperature had a pronounced effect on nonformulated phage but not on formulated phages. The effects of desiccation and fluorescent light illumination on phage were investigated. Desiccation caused a significant but only slight reduction in phage populations after 60 days, whereas fluorescent light eliminated phages within 2 weeks. The protective formulation eliminated the reduction caused by both of these factors. Phage persistence was dramatically affected by UV, while the other factors had less pronounced effects. Formulated phage reduced deleterious effects of the studied environmental factors.
Phages have attracted an increased interest as natural antimicrobial agents to fight bacterial diseases in plants. They have been used as part of integrated disease management because of ease of application, can be used in combination with other bactericides or as an alternative to other bactericides, and have a relatively low cost (27). Moreover, because phages are generally quite specific for their host bacterial species, they can be specially targeted towards the pathogen without affecting other members of the bacterial community (16). Although there has been considerable doubt that phages can be used as effective biological control agents (28, 41), phage therapy also has been used successfully against bacterial blotch of mushrooms (25), bacterial leaf spot of mung bean (7), bacterial spot of peach (8, 9, 10, 32, 33), soft rot incited by Erwinia spp. (12), and fire blight of pear and apple (17, 34, 35). Moreover, phages have been used to disinfest Streptomyces scabies-infected seed potatoes (23). Bacteriophages (21) have been used against bacterial blight of geraniums (14, 15) and bacterial spot of tomatoes (4) caused by Xanthomonas hortorum pv. pelargonii and X. perforans, respectively. In greenhouse and field studies, bacteriophages reduced the severity of bacterial spot of tomato to levels equal or lower than those obtained by use of copper bactericides (5, 14, 27). However, the effectiveness of phages for biological control depends not only on the susceptibility of the target bacterium but also on environmental factors that affect phage survival. In general, the survival of microorganisms in the phyllosphere depends on the presence of the limited resources available in this habitat (20, 24) and the ability of organisms to cope with varied environmental stress conditions, including fluctuating water availability, heat, osmotic stress, and desiccation (2).
Survival is most influenced by exposure to solar UV radiation (30). The daily influx of solar UV includes UVA (320 to 400 nm), UVB (280 to 320 nm), and UVC (100 to 280 nm). UVC is absorbed by the atmospheric ozone, whereas high-energy UVB is particularly detrimental to organisms and causes direct DNA damage by inducing lesions in cellular DNA, including the production of cyclobutane pyrimidine dimers and pyrimidine(6-4) pyrimidinone photoproducts (30). These DNA lesions result in the blockage of DNA replication and RNA transcription; the accumulation of photoproducts, in the absence of efficient cellular mechanisms for their removal, can be fatal (24). UVC could prevent phage adsorption to host cells (19). UVC and UVA plus UVB (UVA+B) damage bacteriophage by different mechanisms, with UVC altering biological systems in general.
Agricultural plants are frequently treated with chemical pesticides. While phages are compatible with many agricultural chemicals (4, 33), copper bactericides have been shown to reduce phage populations in vitro (4).
Phage formulations that increased phage longevity on tomato foliage and improved disease control efficacy have been developed (3). In that study, it was determined that the best application time is the evening and that formulated phages provided better disease control than did the standard copper-mancozeb treatment (5). Despite recent interest in phage therapy for the control of bacterial diseases (4, 5, 14, 15, 16), the interactions between phages, bacteria, plants, and environmental factors are still not well understood. The aim of this study was to better understand the fate of phages during phage therapy on leaf surfaces when exposed to various physical parameters. Hence, the objectives of this study were to evaluate (i) the effect of natural sunlight UV on the survival of phages in the tomato canopy at different times of the year; (ii) the effect of artificial UV irradiation on disease control efficacy achieved with phages; (iii) the effect of temperature on phage survival in growth chamber conditions; (iv) the effect of desiccation and fluorescent light irradiation on phage longevity in vitro; (v) the effect of copper on phage persistence on tomato leaf surfaces under field and greenhouse conditions, and (vi) the level of protection the skim milk (M) formulations provided against these harmful factors.
MATERIALS AND METHODS
Bacteriophage preparation.
Phages ΦXV3-16 and ΦXacm 2004-16 (Fig. 1) were propagated on X. perforans strain 97-2 and on X. axonopodis pv. citrumelo strain Xacm2003-6, respectively. First, the phage, originating from a single plaque, was mass streaked on the lawn of the propagating bacterium on soft nutrient yeast agar (0.6%) (5) medium by use of a sterile toothpick. Following overnight incubation at 28°C, the phages were eluted with 5 ml sterilized tap water, and the resulting phage suspensions (∼109 PFU/ml) were used to prepare larger volumes of high-titer suspensions as follows. One liter nutrient agar medium (5) was inoculated with the propagating bacterium to 108 CFU/ml final concentration and with 107 PFU/ml (multiplicity of infection, 0.1) phage. After a 15-min preincubation, the cultures were placed a rotary shaker (150 rpm) for overnight incubation at 28°C. The lysates were chloroform sterilized (10%, 15 min), and then the bacterial debris was eliminated following centrifugation (10,000 × g, 10 min). The resulting phage suspensions had titers of 2 × 1010 to 5 × 1010 PFU/ml.
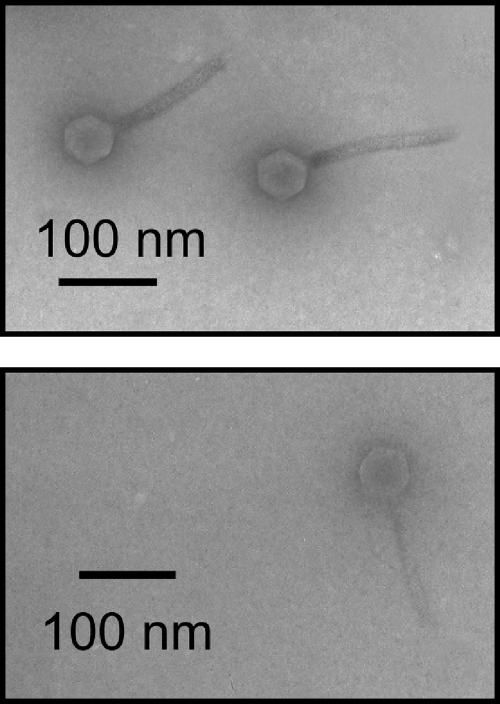
FIG. 1.
Electron micrographs of phages ΦXacm 2004-16 (top) and ΦXV3-16 (bottom).
Quantification of phage titer.
Bacteriophages were eluted from the leaf samples by adding 100 ml deionized water to each sample and shaking for 30 min. Phages were enumerated from the eluate by serial dilution plating method as previously described (4).
Relationship between UV and phage survival.
Two field experiments were conducted in the spring and fall growing seasons in 2005 at the Plant Science Unit of the University of Florida in Citra, FL, where tomato plants of cultivar FL47 were transplanted on 25 March and 8 September, respectively. The first experiment (for UV evaluations in May and June) consisted of three treatments and four replications (blocks) arranged in a randomized block design. Plants within rows were 0.5 m apart, and there were 6-m distances between blocks and plots within blocks. Each plot consisted of two rows of 10 plants with rows 1 m apart. The second experiment (for UV evaluations in October and December) consisted of the same three treatments and four replications, but this time there was only one row of 10 plants per plot. Plots within blocks and between blocks were separated by 6 m. Tomato plants were maintained under standard cultivation methods with drip irrigation and plastic mulch.
UVA+B data were collected every second by use of a PMA 1107-WP UVA+B detector (Solar Light Co. Inc., Glenside, PA) along with a CR10 data logger (Campbell Scientific, Inc.). The data logger was programmed to record averages every 15 min. The data logger and batteries were hooked up and placed inside a weatherproof compartment specifically designed for this purpose. The sensor was mounted on a 2-m-long angled aluminum pole driven into the ground close to the first block of the tomato experimental plot. Data were downloaded with a Palm One handheld computer.
ΦXacm 2004-16 phage (2 × 108 PFU/ml) was applied (i) formulated with skim milk (7.5 g/liter) and sucrose (2.5 g/liter) (M+S), (ii) formulated with skim milk only, or (iii) without the addition of skim milk or sucrose. In order to insure that the phage was not multiplying on the leaf surface, phage ΦXacm 2004-16 was used because it is specific to X. axonopodis pv. citrumelo strain Xacm 2003-6 and cannot infect Xanthomonas strains pathogenic to tomato. Furthermore, the bacterium X. axonopodis pv. citrumelo is not pathogenic to tomato and does not colonize the tomato leaf surface. Phage was applied four times during the day: at 6:00 a.m., at 10:00 a.m., at 2:00 p.m., and at 7 p.m. Leaf samples were collected from each treatment directly after phage application and then every 2 h until 9:00 p.m. Each plot was sampled again at 8:30 a.m. the next morning. Samples consisted of five leaflets that were collected in Ziploc bags and processed as previously described (4). Phage population data were expressed as PFU per g leaf tissue.
Effect of in vitro UV irradiation of phage on efficacy of disease control.
The effect of in vitro UV irradiation on inactivation of phage and subsequent reduction in efficacy in the control of bacterial spot was examined in the following manner. The phage suspension was adjusted to a concentration which in preliminary experiments reduced bacterial spot severity in greenhouse experiments on tomato seedlings inoculated with Xanthomonas campestris pv. vesicatoria strain 75-3R by approximately 100%. The phage suspension in petri dish bases was irradiated with different doses of UV by use of a “UV cross-linker” (XL-1000 Spectrolinker; Spectronics Corporation). The irradiated phage suspension was then spray inoculated, using a hand-trigger sprayer, onto the upper and lower leaf surfaces of four replicate 6-week-old tomato seedlings (cv. Agriset 761) which had just been inoculated with a ∼108-CFU/ml suspension of 75-3R. Control plants were inoculated with sterile deionized water with no phage. Plants were placed in a randomized arrangement in a plastic tent in which high relative humidity was maintained using an ultrasonic humidifier. Plants were incubated until lesions were evident on the control leaves. The number of lesions per leaflet was determined on 10 leaflets per replicate plant. The experiment was conducted three times. In the first experiment, the UV dose applied ranged from 2 to 100 mJ/cm2, while in the subsequent two experiments the UV dose applied ranged from 4 to 8 mJ/cm2. Percentage reduction in disease severity compared to that for the pathogen-only/no-phage control was determined by subtracting the mean number of lesions for a given treatment from the number of lesions for the pathogen-only control and expressing this as a percentage of the number of lesions for the pathogen-only control. Mean numbers of lesions per leaflet at each dose and mean percentage reductions in disease severity at each dose were determined using the data from the three experiments.
Effect of copper on phage survival.
In greenhouse experiments, the effect of copper-mancozeb was evaluated on nonformulated and skim milk-plus-sucrose-formulated phage populations. Tomato plants were sprayed with copper-mancozeb (3.6 g/liter Kocide 2000 [DuPont, Wilmington, DE] and 2.5 g/liter Manzate 75DF [Griffin Corp., Valdosta, GA]) 8 h before phage application in the late afternoon. Leaflets were harvested the next morning, and phage populations were determined as described above.
Two field experiments were conducted in spring and fall growing seasons in 2005 at the Plant Science Unit of the University of Florida in Citra, FL, where tomato plants of cultivar FL 47 were transplanted on 25 March and 8 September, respectively. The effect of copper on phage survival was evaluated three times each growing season. There were four treatments and four replications per treatment. Tomato plants were sprayed with the copper-mancozeb bactericide 7, 4, and 0 days before phage application or sprayed with water for the control treatment. Phage spray application (ΦXacm 2004-16, 2 × 108 PFU/ml) was done in the evenings just before sundown. The following morning, 10 leaflets per treatment were collected and processed as previously described (5). The experiment was done six times.
Effect of temperature on phage longevity.
Bonny Best seedlings were grown in 10-cm pots in Terra-Lite agricultural mix (The Scotts Co., Marysville, OH). Plants were grown in the greenhouse at 23 to 28°C, fertilized, and watered as needed until they reached the three- or four-leaf stage. They were moved to the growth chambers (32, 28, and 15°C), and phage treatments were applied as sprays. Two different phages (ΦXacm 2004-16 and ΦXV3-16) were used. Phages were applied with or without the skim milk-plus-sucrose formulation. Each treatment consisted of three replications with two plants per replication. Samples were collected immediately after application and then every 48 h. Three leaflets from each replication were placed in 3- by 4-cm Ziploc plastic bags. Bags were weighted and, following the addition of 3 ml distilled water, shaken on a junior orbit shaker (Labline Instruments, Inc., Melrose Park, IL) for 20 min. After that, 1 ml rinsate was taken in 1.5-ml microcentrifuge tubes containing 20 μl of chloroform and stored in a refrigerator for further dilution plating as previously described. The experiment was done twice.
Effect of desiccation and fluorescent light irradiation on phage survival in vitro.
Individual wells in microtiter plates were treated with 10% skim milk and dried in a flow hood for 3 h to minimize nonspecific binding of phage before applying the treatments. There were two treatments; 36 cells per treatment were treated with 30 μl of the Xacm 2004-16 phage suspension (2 × 106 PFU/ml) either formulated (skim milk plus sucrose) or nonformulated. The following day, the plates were placed inside a desiccation jar containing Drierite (W.A. Hammond). The jar was stored at room temperature either in the dark or exposed to 16-h/8-h fluorescent light/dark conditions for 2 months. At day 1 and then every 5 days up to day 55, three wells per treatment were individually assayed for phage by adding 300 μl of sterile tap water to each well. Rinsates from each well were then placed in a 0.5-ml microcentrifuge tube and stored at 4°C until the following day for titer determination.
RESULTS
Relationship between sunlight UV irradiation and phage survival.
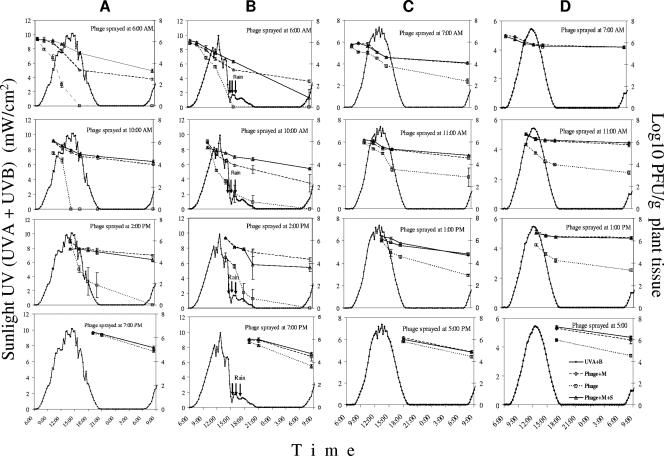
The relationship between sunlight UV irradiation and bacteriophage survival on tomato foliage when bacteriophage was applied without formulation, with M, or with M+S at four different times of the day and four times of the year was evaluated in field experiments. In these experiments, changes in sunlight UV (UVA and UVB) irradiation and in residual phage populations were monitored. In the experiment of 25 May, the maximum sunlight UV (UVA+B) was 10.13 mW/cm2 (at 2:30 p.m.) (Fig. 2A). Nonformulated phage sprayed at 6:00 a.m. dropped 6 log units in the following 10 h and so completely disappeared by 4:00 p.m. On the other hand M+S- and M-formulated phages dropped only 1 and 2.7 log units, respectively, during the same time period. Nonformulated phage sprayed at 10:00 a.m. declined from 6 to 0 log units in 4 h (by 2:00 p.m.), whereas formulated phage dropped only 0.8 log units in the same time period. Nonformulated phage sprayed at 2:00 p.m. dropped 5 log units by 9:00 p.m., while both formulated phages dropped only 1.5 log units during the same time. There was no significant difference in changes of phage populations among the three phage treatments when sprayed at 7:00 p.m. (Fig. 2A). When the population data for the early morning phage application were plotted against UV dose, regression equations were calculated for the three phage populations (Fig. 3A). The slope for phage decline was much lower for the formulated phage applications than for the nonformulated phage applications, indicating that formulated phage persisted for longer periods at higher levels at fairly high doses whereas the nonformulated phage populations dropped precipitously.
FIG. 2.
Effect of sunlight UV (UVA+B) irradiation on survival of phage ΦXacm 2004-16 in the field. Phage suspensions applied in water (Phage), with 0.75% skim milk powder (Phage+M), or with 0.75% skim milk powder plus 0.5% sucrose (Phage+M+S) were sprayed on tomato leaves at four different times during the day on 25 May (A), 8 June (B), 19 October (C), and 1 December (D). Sunlight UVA+B data were collected every second using a PMA 1107-WP UVA+B detector (Solar Light Co. Inc., Glenside, PA).
FIG. 3.
Relationship between changes in ΦXacm 2004-16 phage population and UV dose when sprayed on tomato foliage at Citra, FL, in 2005. Phage was sprayed in the absence of host bacteria on 5 May (A), 8 June (B), 19 October (C), and 1 December (D) at 6:30 a.m. (the first two dates), 7:30 a.m., and 11:00 a.m., respectively. Sunlight UVA+B data were collected every second using a PMA 1107-WP UVA+B detector (Solar Light Co. Inc., Glenside, PA). While the CR10 data logger (Campbell Scientific, Inc.) recorded averages every 15 min, tomato leaflets samples were taken at 0, 2, 4, 6, 8, and 10 h after application to evaluate phage populations. Phage suspensions were applied in water (Phage), with 0.75% skim milk powder (Phage+M), or with 0.75% skim milk powder plus 0.5% sucrose (Phage+M+S).
On 9 June (Fig. 2B), the sunlight UV peaked at 9.88 mW/cm2 at 12:45 p.m., but soon afterwards (2:30 p.m.) the sky became partially cloudy and then completely cloudy by 3:00 p.m. (UV = 2 mW/cm2), and 15 min of rain followed. Cloudy conditions remained for the rest of the day. Nonformulated phage sprayed at 6:00 a.m. persisted at a rate similar to that seen for the experiment on 25 May; however, the ones sprayed at 10:00 a.m. persisted longer in spite of the rain. In this particular case, the M+S-formulated phage population was significantly (1 log unit) lower than the M-formulated one by the next day, except when sprayed at 10:00 a.m. The nonformulated phage populations were significantly lower than the formulated ones in all cases. In the comparison of UV doses and phage persistence, trends similar to those from the May experiment were found, in that the nonformulated phage populations dropped precipitously and the formulated phages were much less affected by dose, as evidenced by the smaller slopes (Fig. 3B).
The maximum sunlight UV levels for the 19 October (Fig. 2C) and 1 December (Fig. 2D) experiments were 7.37 and 5.44 mW/cm2, respectively. Although nonformulated phage persisted at much higher concentrations than in the May and June experiments, formulated phage populations were still significantly higher than nonformulated ones. Higher phage populations were recovered on 1 December than on 19 October. Slightly lower sunlight UV and lower temperature might have influenced these results. In general, there was a strong positive correlation between the rate of decline in the phage populations and the intensity of sunlight UV irradiation. There were very few differences among treatments in the phage reduction-UV dose relationship in October and December (Fig. 3C and D). The slope of decline of the nonformulated phages was small compared to that from the May and June experiments, while the slope of decline of formulated phages stayed at previous levels. Examination of the results of the four sampling dates revealed that the same UV dose incited higher phage reduction when the UV intensity was higher. Based on the slope of the regression curves, a 100-J/cm2 UV dose caused 5.2-, 3.7-, 1.3-, and 2.2-log declines in nonformulated phage populations in May, June, October, and December, respectively.
Effect of in vitro UV irradiation of phage on disease control efficacy.
Phage suspensions were subjected to different doses of UV irradiation in vitro and then used for controlling tomato bacterial spot. Disease severity (mean number of lesions per leaflet) increased with growing UV doses in the range of 4 to 8 mJ/cm2 (Fig. 4A). A dose of 20 mJ/cm2 completely eliminated the protective effect of the phage (data not shown). The mean percentage reduction in disease severity (i.e., efficacy of the phage suspension) decreased rapidly with UV doses greater than 4 mJ/cm2 (Fig. 4B).
FIG. 4.
Effect of in vitro UV irradiation of phage on disease control efficacy. A mixture of phages active against Xanthomonas campestris pv. vesicatoria was subjected to different levels of UV irradiation in vitro and then used for treating tomato plants before inoculation with Xanthomonas campestris pv. vesicatoria strain 73-5R. (A) Disease severity as measured by average number of lesions per leaflet. Error bars indicate the standard error. (B) Reduction in disease severity by the phage treatment compared to that by the non-phage-treated control.
Effect of copper on phage survival.
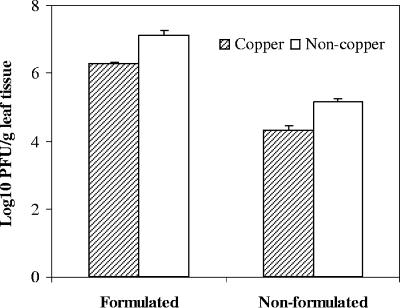
In greenhouse experiments, the effect of copper-mancozeb on nonformulated and skim milk-plus-sucrose-formulated phage populations was evaluated. Tomato plants were sprayed with copper-mancozeb 8 h before phage application, and the phage populations were determined the next day. The presence of copper caused significant reductions in both phage populations; however, the reduction was smaller for the formulated phage population than for the nonformulated one (Fig. 5). Additionally, the population size of formulated phage was higher than the population size of nonformulated phage on both copper-treated and nontreated leaves.
FIG. 5.
Effect of residual copper-mancozeb and the use of protective formulation on persistence of ΦXacm 2004-16 phage on tomato foliage in the greenhouse. Copper-mancozeb was sprayed in the morning, phage was sprayed in the evening of same day in water (nonformulated) or with 0.75% skim milk powder plus 0.5% sucrose (formulated), and phage populations were assessed the following morning. Error bars indicate the standard error.
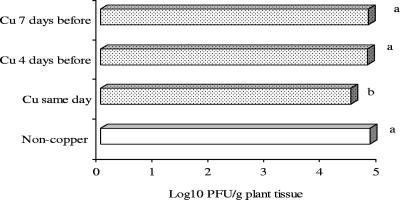
In field experiments, copper-mancozeb was applied on tomato foliage 7 days before, 4 days before, or on the day of application of formulated ΦXacm 2004-16. Control plants were sprayed with water on the day of phage application. Phage populations significantly declined when copper-mancozeb was applied in the morning and phage was applied in the evening of the same day (Fig. 6). Phage population on tomato plants sprayed with copper 4 or 7 days before phage application was not significantly different from the phage population on plants sprayed with water (Fig. 6).
FIG. 6.
Effect of residual copper-mancozeb on persistence of formulated ΦXacm 2004-16 phage on tomato foliage in the field. Copper-mancozeb was applied 0, 4, or 7 days prior to phage application. Bars followed by the same letter are not significantly different according to the Waller-Duncan K-ratio t test at a P value of 0.05.
Effect of temperature on phage survival.
To investigate the effect of temperature on phage persistence, tomato plants were sprayed with phages ΦXV3-16 and ΦXacm 2004-16 with or without formulations and were kept in growth chambers set to 15, 28, and 32°C. Phage populations were determined 0, 2, 4, 6, 8, and 10 days after application. Temperature did not have much effect on formulated ΦXacm 2004-16, which survived for over 10 days at all three temperatures. Nonformulated ΦXacm 2004-16 completely disappeared from the leaves by days 6 and 8 at 32 and 15°C, respectively, whereas a setting of 28°C had less of a detrimental effect on phage survival (Fig. 7A). Lower temperature favored phage survival in the case of ΦXV3-16, since nonformulated phage survived up to 10 days at 15°C (Fig. 7B) but only up to 4 and 6 days at 32 and 28°C, respectively. The same trend was observed for formulated (M+S) phage, but M+S-treated phage was consistently recovered at significantly higher levels. Although there were different responses to temperature with different phages, phage populations always persisted at higher levels on tomato leaves when applied with formulation.
FIG. 7.
Effect of temperature on survival of formulated (M+S) or nonformulated ΦXacm 2004-16 phage (A) or ΦXV3-16 (B) sprayed on tomato plants and maintained at a constant 32, 28, or 15°C for 10 days. The experiment was done twice with similar results. Results from one of two repetitions are shown.
Effect of desiccation and fluorescent light irradiation on phage survival in vitro.
In order to investigate the effect of desiccation on phage survival, formulated and nonformulated ΦXacm 2004-16 phage suspensions were dried on preblocked enzyme-linked immunosorbent assay plate wells. The plates were incubated either in complete darkness or in a 16-h-light/8-h-dark cycle at room temperature in 0% relative humidity, and phages were recovered and enumerated periodically for 60 days. The nonformulated phage population dropped to nondetectable levels 5 days after being subjected to desiccation and fluorescent light illumination (Fig. 8A, upper panel). The formulated phage (M+S) population diminished only 1.62 log units after 60 days. When the phages were kept in constant darkness, the reductions were only 2.06 and 0.82 log units for the nonformulated phage and the formulated phage, respectively, by the 60th day (Fig. 8, lower panel). The experiment was done twice with similar results.
FIG. 8.
Effect of desiccation, fluorescent light irradiation, and use of protective formulation (M+S) on survival of phage ΦXacm 2004-16 (8 × 107 PFU/ml). Phage aliquots (20 μl) were spotted in enzyme-linked immunosorbent assay plate wells, kept in desiccation jars, and exposed to a cycle of 16 h of light (fluorescent lamps) and 8 h of darkness (A) or constant (24-h) darkness (B) at room temperature for 60 days.
DISCUSSION
There are distinct advantages to using bacteriophages for biological control. These include (i) their high specificity, which allows safe, targeted elimination of target bacterium without altering the surrounding microflora; (ii) the fact that they are natural components of the environment and are readily degradable and so can be used in sustainable, environment-friendly agricultural systems; and (iii) their ability not only to destroy the target bacterium but also to reproduce on it, which allows them to move into protected places inside the plant and to have a greater control effect when there is greater disease pressure. However, phages are clearly affected by the surrounding environment, just as their target bacteria are, and their efficacy of control depends not only on the susceptibility of the target bacterium but also on the environmental factors that affect their own survival. Those environmental factors that have been implicated in significantly affecting phage survival on plant foliage are sunlight irradiation, especially in the UV range, desiccation, temperature, precipitation, relative humidity, and presence of residual copper compounds. Some formulations that give protection to phages applied to plant foliage, contributing to longer persistence, have been developed. In this study, we assessed the relative contributions of these environmental factors towards inactivation of phage populations and investigated the effect and the mode of action of the skim milk-plus-sucrose protective formulation.
In vitro irradiation of the phage suspension prior to application onto tomato plants significantly reduced the ability of the phage to reduce bacterial spot severity. This effect is presumably due to phage population reduction that is caused by inactivation of phage virions by the UV irradiation. A dose of 20 mJ/cm2 completely eliminated the protective effect of the phage (Fig. 4). A graph of the mean percentage reduction in disease severity suggests that the phage suspensions tolerate up to 4 μJ/cm2 with minimal loss of efficacy, whereas above this dose the phage is rapidly inactivated and efficacy is lost. The data suggest that a dose of 8 mJ/cm2 is sufficient to reduce the efficacy of the phage suspension by ∼80% (Fig. 4).
We determined that there was a strong negative correlation between phage persistence and UV dose. Both formulated and nonformulated phage populations survived longer following evening applications or when UV intensity was less than 2 mW/cm2. We also noted that intensity at a given point is more critical in terms of phage stability on leaf surfaces, since the phage populations plummeted to much lower levels at the same dose in May and June than in October and December (Fig. 3). This in all likelihood is the result of the UV intensity observed in May and June being higher than that in October and December (Fig. 2). Another supporting evidence for the importance of UV intensity is that in laboratory studies, where the UV dose was administered to the phages with high intensity, the UV doses that significantly affected phage populations were more than a thousandfold smaller than those in field studies (several millijoules in the lab versus tens of joules in the field). Although copper, air temperature, and to a lesser extent desiccation were deleterious to phage persistence, UV irradiation was the most important factor. Deleterious effects of UV irradiation to microorganisms are widely known and have been analyzed in various microbial ecology studies (19, 26, 29). In those studies, it was concluded that UVB has a negative impact on individual microbial species as well as on complex microbial communities. On the other hand, it has also been reported that differential resistance to UVB (18, 36, 37), DNA repair capabilities (38, 16), and photoreactivation as well as UV avoidance have contributed to survival of bacteria (39). Furthermore, increased microbial populations on abaxial leaf surfaces as a UV avoidance mechanism have been reported (6, 37, 42).
We consistently observed that the protective formulation consisting of skim milk and sucrose developed by Balogh et al. (5) effectively protected the phage from detrimental effects of UV irradiation and other factors associated with survival on leaf surfaces in the absence of the host bacterium. The high content of protein and sugars in milk may be favorable for virion survival. Knowledge of the beneficial properties of milk for phage preservation dates back to 1953 with the use of whey filtrate (31). Likewise, dextrose and tryptone were reported (11, 13) to protect phages. For instance, when coliphages were aerosolized with a 0.1 M dextrose solution, the aerosol decay rate of coliphage at 50% relative humidity was 3.6% per min, compared with 8.5% per min for coliphage disseminated without the additive. In the same study, however, there was no significant difference at 85% relative humidity. Furthermore, Ehrlich et al. (13) tested a range of relative humidities to evaluate the survival of airborne T3 coliphage and observed that the coliphage survival increased with an increase in humidity. Humidity below 70% significantly reduced aerosol survival. In our growth chamber studies, phage survived at higher concentrations at 32°C when relative humidity was maintained at ≥70% (data not shown). However, survival of phages at different temperatures and levels of relative humidity has been shown to vary with phage and conditions being studied (1). We consistently observed better phage survival in growth chamber experiments regardless of the temperature when phage was applied formulated with skim milk and sucrose. On the other hand, air temperature had detrimental effects on the survival of nonformulated phage, which would not survive more than 5 to 10 days. We observed that high temperature (32°C) and low humidity (≥40%) had a negative effect on persistence of nonformulated phage. However, there appears to be significant variation depending on the phage being studied and the initial phage concentration used. In agreement with our growth chamber results, we recovered the highest concentration of ΦXacm 2004-16 in the field in December (temperature was approximately 15°C). In the field, the low UV intensity might have been the primary explanation for these results.
The fact that formulated phage persisted for 6 or more days on leaf surface in the growth room compared to ≥24 h in the field is evidence that air temperature is not as detrimental as UV for short-term (2- or 3-day) phage survival in the field. Desiccation had minimal effect on short-term (2- or 3-day) phage survival. These observations are in agreement with the results of Prouty (31), who evaluated the viability of dried specimens of lactic acid streptococci's bacteriophage every six months and at 0, 12, 25, and 32°C. The desiccated viruses remained viable at 0°C for 42 months and at 37°C for 72 months. At 12 and 25°C, phage continued to remain viable after 78 months. Prouty used sterile squares of filter paper saturated with whey filtrate. In our studies, there was strong evidence that fluorescent light is detrimental for nonformulated phage, in contrast to dark conditions, under which both formulated and nonformulated phage survived for over 60 days.
In conclusion, the present study suggests that UV irradiation is a major obstacle in the persistence of phages on tomato leaf surfaces. Furthermore, we provide evidence that previously developed phage formulations (5) can protect phage from sunlight UV and other detrimental environmental factors. However, because of the diversity of bacteria and their phages (35, 17), extrapolation of phage therapy practices from one pathogen system to another will not always be possible (17). Nonetheless, the genotypic diversity that occurs in natural populations of biocontrol agents provides a great resource for improving biological control of plant diseases (19, 22, 40) and can be used to select for better biocontrol agents. It may be possible to identify phages that are superior with respect to phyllosphere survival (i.e., more resistant to UV) and more effective in infecting and eliminating the targeted bacterial pathogens.
Acknowledgments
This work was supported by funding from the USDA T-STAR to M. T. Momol, J. B. Jones, and S. M. Olson (USDA 2003-34135-14077).
We thank Donna Williams for her work on the electron micrographs.
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Abad, F. X., R. M. Pinto, and A. Bosch. 1994. Survival of enteric viruses on environmental fomites. Appl. Environ. Microbiol. 60:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York, NY.
- 3.Balogh, B. 2002. Strategies for improving the efficacy of bacteriophages for controlling bacterial spot of tomato. M.S. thesis. University of Florida, Gainesville.
- 4.Balogh, B., J. B. Jones, M. T. Momol, and S. M. Olson. 2005. Persistence of bacteriophages as biocontrol agents in the tomato canopy, p. 299-302. In M. T. Momol, P. Ji, and J. B. Jones (ed.), Proc. 1st Int. Symp. Tomato Dis., 2005. International Society for Horticultural Science, Acta Hortic. 695.
- 5.Balogh, B., J. B. Jones, M. T. Momol, S. M. Olson, A. Obradovic, P. King, and L. E. Jackson. 2003. Improved efficacy of newly formulated bacteriophages for management of bacterial spot of tomato. Plant Dis. 87:949-954. [DOI] [PubMed] [Google Scholar]
- 6.Beattie, G. A., and S. E. Lindow. 1995. The secret life of foliar bacterial pathogens on leaves. Annu. Rev. Phytopathol. 33:145-172. [DOI] [PubMed] [Google Scholar]
- 7.Borah, P. K., J. K. Jindal, and J. P. Verma. 2000. Integrated management of bacterial leaf spot of mungbean with bacteriophages of Xav and chemicals. J. Mycol. Plant Pathol. 30:19-21. [Google Scholar]
- 8.Civerolo, E. L., and H. L. Kiel. 1969. Inhibition of bacterial spot of peach foliage by Xanthomonas pruni bacteriophage. Phytopathology 59:1966-1967. [Google Scholar]
- 9.Civerolo, E. L. 1973. Relationships of Xanthomonas pruni bacteriophages to bacterial spot disease in prunus. Phytopathology 63:1279-1284. [Google Scholar]
- 10.Civerolo, E. L. 1974. Temperature effects on the relationships between Xanthomonas pruni and its virulent phages. Phytopathology 64:1248-1255. [Google Scholar]
- 11.Dubovi, J. E., and G. A. Thomas. 1970. Airborne stability of tailless bacterial viruses S-13 and MS-2. Appl. Microbiol. 19:624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eayre, C. G., D. E. Concelmo, and J. A. Bartz. 1990. Control of soft rot Erwinias with bacteriophages. Phytopathology 80:994. (Abstract.) [Google Scholar]
- 13.Ehrlich, R., S. Miller., and L. S. Idoine. 1964. Effects of environmental factors on the survival of airborne T-3 coliphage. Appl. Microbiol. 12:479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty, J. E., J. B. Jones, B. K. Harbaugh, G. C. Somodi, and L. E. Jackson. 2000. Control of bacterial spot on tomato in the greenhouse and field with h-mutant bacteriophages. HortScience 35:882-884. [Google Scholar]
- 15.Flaherty, J. E., J. B. Jones, B. K. Harbaugh, G. C. Somodi, and L. E. Jackson. 2001. H-mutant bacteriophages as a potential biocontrol of bacterial blight of geranium. HortScience 36:98-100. [Google Scholar]
- 16.Gill, J. J., and T. S. Abedon. 2003. Bacteriophage ecology and plants. APSnet Feature. http://www.apsnet.org/online/feature/phages/abedon.pdf.
- 17.Gill, J. J., A. M. Svircev, R. Smith, and A. J. Castle. 2003. Bacteriophages of Erwinia amylovora. Appl. Environ. Microbiol. 69:2133-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunasekera, T. S., N. D. Paul, and P. G. Ayres. 1997. The effects of ultraviolet-B (UV-B: 290-320 nm) radiation on blister blight disease of tea (Camellia sinensis). Plant Pathol. 46:179-185. [Google Scholar]
- 19.Handelsman, J., and E. V. Stabb. 1996. Biocontrol of soilborne plant pathogens. Plant Cell 8:1855-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson, L. E. May. 1989. Bacteriophage prevention and control of harmful plant bacteria. U.S. patent 4,828,999.
- 22.Jensen, C. E., H. S. Schrader, B. Rieland, T. L. Thompson, K. W. Lee, K. W. Nickerson, and T. A. Kokjohn. 1998. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 64:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna, F., K. A. El-Tarabily, K. A. Hardy, G. E. S. J. Hardy, and B. Dell. 2001. Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes. Plant Pathol. 50:666-675. [Google Scholar]
- 24.Mercier, J., and S. E. Lindow. 2000. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 66:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munsch, P., and J. M. Olivier. 1995. Biocontrol of bacterial blotch of the cultivated mushroom with lytic phages: some practical considerations, p. 595-602. In T. J. Elliott (ed.), Science and cultivation of edible fungi. A. A. Balkema, Rotterdam, The Netherlands.
- 26.Newsham, K. K., M. N. R. Low, A. R. McLeod, P. D. Greenslade, and B. A. Emmett. 1997. Ultraviolet-B radiation influences the abundance and distribution of phylloplane fungi on pedunculate oak (Quercus robur). New Phytol. 138:287-297. [Google Scholar]
- 27.Obradovic, A., J. B. Jones, M. T. Momol, S. M. Olson, L. E. Jackson, B. Balogh, K. Guven, and F. B. Iriarte. 2005. Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis. 89:712-716. [DOI] [PubMed] [Google Scholar]
- 28.Okabe, N., and M. Goto. 1963. Bacteriophages and plant pathogens. Annu. Rev. Phytopathol. 1:397-418. [Google Scholar]
- 29.Paul, N. D., S. Rasanayagam, S. A. Moody, P. E. Hatcher, and P. G. Ayres. 1997. The role of interactions between trophic levels in determining the effects of UV-B on terrestrial ecosystems. Plant Ecol. 128:296-308. [Google Scholar]
- 30.Pfeifer, G. P. 1997. Formation and processing of UV photoproducts: effects of DNA sequence and chromatin environment. Photochem. Photobiol. 65:270-283. [DOI] [PubMed] [Google Scholar]
- 31.Prouty, C. C. 1953. Storage of the bacteriophage of the lactic acid streptococci in the desiccated state with observations on longevity. Appl. Microbiol. 1:250-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randhawa, P. S., and E. L. Civerolo. 1984. Inhibition of Xanthomonas campestris pathovar pruni by bacteria and pruniphage on detached peach leaves. Phytopathology 74:864. (Abstract.) [Google Scholar]
- 33.Saccardi, A., E. Gambin, M. Zaccardelli, G. Barone, and U. Mazzucchi. 1993. Xanthomonas campestris pv. pruni control trials with phage treatments on peaches in the orchard. Phytopathol. Mediterr. 32:206-210. [Google Scholar]
- 34.Schnabel, E. L., and A. L. Jones. 2001. Isolation and characterization of five Erwinia amylovora bacteriophages and assessment of phage resistance in strains of Erwinia amylovora. Appl. Environ. Microbiol. 67:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnabel, E. L., W. G. D. Fernando, L. L. Jackson, M. P. Meyer, and A. L. Jones. 1998. Bacteriophages of Erwinia amylovora and their potential for biocontrol. Acta Hortic. 489:649-654. [Google Scholar]
- 36.Sinton, L. W., C. H. Hall, P. A. Lynch, and R. J. Davies-Colley. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundin, G. W., and J. L. Jacobs. 1999. Ultraviolet radiation (UVR) sensitivity analysis and UVR survival strategies of a bacterial community from the phyllosphere of field-grown peanut (Arachis hypogeae L.). Microb. Ecol. 38:27-38. [DOI] [PubMed] [Google Scholar]
- 38.Sundin, G. W., S. P. Kidambi, M. Ullrich, and C. L. Bender. 1996. Resistance to ultraviolet light in Pseudomonas syringae: sequence and functional analysis of the plasmid-encoded rulAB genes. Gene 177:77-81. [DOI] [PubMed] [Google Scholar]
- 39.Sundin, G. W., S. P. Kidambi, M. Ullrich, and C. L. Bender. 1999. Functional analysis of the Pseudomonas syringae rulAB determinant in tolerance to ultraviolet B (290-320 nm) radiation and distribution of rulAB among P. syringae pathovars. Environ. Microbiol. 1:75-87. [DOI] [PubMed] [Google Scholar]
- 40.Thomashow, L. S., and D. M. Weller. 1996. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites, p. 187-236. In G. Stacey, and N. T. Keen (ed.), Plant-microbe interactions, vol. 1. Chapman & Hall, New York, NY. [Google Scholar]
- 41.Vidaver, A. K. 1976. Prospects for control of phytopathogenic bacteria by bacteriophages and bacteriocins. Annu. Rev. Phytopathol. 14:451-465. [Google Scholar]
- 42.Wilson, M., S. S. Hirano, and S. E. Lindow. 1999. Location and survival of leaf-associated bacteria in relation to pathogenicity and potential for growth within the leaf. Appl. Environ. Microbiol. 65:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]