Abstract
A novel β-transaminase gene was cloned from Mesorhizobium sp. strain LUK. By using N-terminal sequence and an internal protein sequence, a digoxigenin-labeled probe was made for nonradioactive hybridization, and a 2.5-kb gene fragment was obtained by colony hybridization of a cosmid library. Through Southern blotting and sequence analysis of the selected cosmid clone, the structural gene of the enzyme (1,335 bp) was identified, which encodes a protein of 47,244 Da with a theoretical pI of 6.2. The deduced amino acid sequence of the β-transaminase showed the highest sequence similarity with glutamate-1-semialdehyde aminomutase of transaminase subgroup II. The β-transaminase showed higher activities toward d-β-aminocarboxylic acids such as 3-aminobutyric acid, 3-amino-5-methylhexanoic acid, and 3-amino-3-phenylpropionic acid. The β-transaminase has an unusually broad specificity for amino acceptors such as pyruvate and α-ketoglutarate/oxaloacetate. The enantioselectivity of the enzyme suggested that the recognition mode of β-aminocarboxylic acids in the active site is reversed relative to that of α-amino acids. After comparison of its primary structure with transaminase subgroup II enzymes, it was proposed that R43 interacts with the carboxylate group of the β-aminocarboxylic acids and the carboxylate group on the side chain of dicarboxylic α-keto acids such as α-ketoglutarate and oxaloacetate. R404 is another conserved residue, which interacts with the α-carboxylate group of the α-amino acids and α-keto acids. The β-transaminase was used for the asymmetric synthesis of enantiomerically pure β-aminocarboxylic acids. (3S)-Amino-3-phenylpropionic acid was produced from the ketocarboxylic acid ester substrate by coupled reaction with a lipase using 3-aminobutyric acid as amino donor.
β-Amino acids occur naturally in diverse forms. They have been found as free forms of metabolites, such as β-alanine and β-aminobutyric acid, in mammals and lower organisms (19). They are also found as a component of peptidic or nonpeptidic molecules (28). β-Amino acid-containing molecules have interesting pharmacological roles such as antibiotics (51), antitumor agents (14, 36, 53), or antifungal agents (15). β-Lactams and taxoids are such examples of the important molecules further developed from the natural compounds with β-amino acid moiety (26, 38). The higher stability of β-amino acid peptides against peptidases also has drawn great interest in peptide chemistry (16). The significance of β-amino acids and their related molecules attracts increasing attention for the synthesis of β-amino acids (5, 37, 38, 41), especially for the enantioselective synthesis of β-amino acids (26, 28, 30, 40). Although recent reviews of the biocatalytic synthesis of β-amino acids elaborated a wide range of approaches, most of the methods dealt with the chiral resolution of precursors using hydrolytic enzymes (29). As an alternative, we have focused on the study of transaminases for the synthesis of versatile chiral amine compounds including unnatural amino acids.
Transaminases have been studied for the production of chiral amino acids because they generally show rapid reaction rates, broad substrate specificity, and no requirement for external cofactor regeneration (44, 49, 54). Moreover, transaminases allow asymmetric synthesis from prochiral ketone compounds depending upon the properties of target chemical compounds (1, 4, 10, 11, 17, 49, 50). Though transaminases are not widespread, we have reported an ω-transaminase of Alcaligenes denitrificans which can catalyze mainly the transamination between aliphatic β-amino acids and pyruvate (56). Other examples were recently introduced for the transamination of aliphatic and aromatic β-amino acids by Alcaligenes eutrophus and Variovorax paradoxus (6).
Recently, we reported screening of a transaminase having activity toward a β-amino acid and its N-terminal amino acid sequence (27). We have tried to clone the gene of this enzyme by PCR using the degenerative primers of consensus transaminase sequences (56). However, due to the large population of the homologous transaminases in the screened organism, we found a few transaminases without any activities for β-amino acids, even though we used genuine N-terminal amino acid sequence to make one of the degenerative primers. Due to the low recovery of the purified protein, our attempts to obtain any internal peptide sequences had been also unsuccessful. The present study illustrates the easy isolation of enzyme with higher recovery, molecular cloning, sequencing, heterologous expression of the gene encoding the new β-transaminase in Escherichia coli, and its characterization. In this article, we report a novel β-transaminase from Mesorhizobium sp. strain LUK showing a high activity for β-aminocarboxylic acids. In addition, the asymmetric synthesis of a β-aminocarboxylic acid is presented along with a short discussion on the substrate recognition mode of β-transaminase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Escherichia coli XL1-Blue MRF, DH5α, and BL21(DE3) were used as hosts for the construction of a genomic library, proliferation of cloned genes, and heterologous expression of protein, respectively. The plasmids pOJ446 (7), pGEM-T (Promega, WI), pET24ma (donated by David Sourdive, Pasteur Institute, France), and pET28a (Novagen, WI) were used for DNA cloning and cloned gene expression. Mesorhizobium sp. strain LUK (KCCM-10752P) was previously isolated by enrichment culture with a limited nitrogen source and grown as reported before (27).
Enzyme assay and analytical methods.
One unit of enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 μmol of l-alanine from 10 mM racemic 3-amino-3-phenylpropionic acid and 10 mM pyruvate for 1 min in 100 mM phosphate buffer (pH 7.0) at 37°C. Quantitative chiral analysis of 3-amino-3-phenylpropionic acid was performed using a C18 Symmetry column (4.6 × 150 mm; Waters, MA) with a Waters high-pressure liquid chromatography system after the derivatization of sample with 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate (24). Separation of alanine and each enantiomer of 3-amino-3-phenylpropionic acid was achieved with an isocratic elution using 20 mM of phosphate buffer (pH 6.4) and acetonitrile (66:34, vol/vol) with a flow rate of 1 ml/min, observed at 250 nm. Kinetic constants were obtained from the transamination reactions performed by varying the concentrations of 3-amino-3-phenylpropionic acid and pyruvate. The reactions were started by adding 0.0006 U of the purified enzyme at 37°C. Substrate specificity was investigated by transamination reactions between different sets of amino donors and amino acceptors (see Table 2). To determine the optimum reaction pH, initial enzyme activity was measured by analyzing the amount of 3-amino-3-phenylpropionic acid consumed within a pH range of 5.3 to 9.0. The buffer systems used were 50 mM sodium acetate buffer (pH 5.3), 50 mM sodium phosphate buffer (pH 6.0 to 7.6), and 50 mM borate buffer (pH 8.0 to 9.0).
TABLE 2.
Substrate specificity of the β-transaminase from Mesorhizobium sp. strain LUK
| Amino donor or acceptor | Relative activity (%) |
|---|---|
| Donorsa | |
| 3-Amino-3-phenylpropionic acid | 100 (D,S) |
| 3-Amino-5-methylhexanoic acid | 185 (D,R) |
| 3-Aminobutyric acid | 208 (D,R) |
| 1-Phenylethylamine | 14.3 (S) |
| Phenylpropylamine | 11.0 |
| l-Valine | 9.6 |
| dl-Phenylalanine | 8.4 (L,S) |
| l-Asparagine | 7.9 |
| l-Tyrosine | 6.5 |
| l-Leucine | 5.8 |
| l-Glutamic acid | 4.0 |
| l-Lysine | 2.2 |
| l-Aspartic acid | 1.0 |
| d-Glutamic acid | NRc |
| d-Aspartic acid | NR |
| l-Phenylglycine | NR |
| Acceptorsb | |
| Pyruvate | 100 |
| 3-Phenylpyruvate | NR |
| Oxaloacetate | 37 |
| 2-Ketoglutarate | 111 |
| Pyruvate methyl ester | 90 |
| Pyruvate ethyl ester | 101 |
| Propioraldehyde | 4 |
| Acetophenone | NR |
| Butylaldehyde | NR |
| Benzoformate | NR |
| Benzaldehyde | NR |
Pyruvate (10 mM) and 5 mM each amino donor were used at pH 7.6. The initial reaction rate was measured by analyzing the amount of substrate consumed by the GITC (2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate) derivatization of each amino donor. The activity for 3-amino-3-phenylpropionic acid, corresponding to 1.6 U/mg, was taken as 100%. For racemic amino donor, the concentration of each substrate was 10 mM.
Racemic 3-amino-3-phenylpropionic acid (10 mM) and 10 mM each amino acceptor were used at pH 7.6, and the initial reaction rate was measured by analyzing the decrease of 3-amino-3-phenylpropionic acid. The activity for pyruvate, corresponding to 1.6 U/mg, was taken as 100%.
NR, not reactive.
Rapid partial purification of β-transaminase using native gel electrophoresis.
Frozen cell pellets (15 g, wet weight) were resuspended in 75 ml of lysis buffer containing 100 mM potassium phosphate buffer (pH 7.0), 0.2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 0.01% (vol/vol) 2-mercaptoethanol. Cells were disrupted by two passages through a French press at 18,000 lb/in2. The supernatant obtained after centrifugation (17,000 × g, 20 min) at 4°C was used as cell extract. Solid ammonium sulfate was added carefully to the cell extract with gentle stirring until 30% saturation was reached. After equilibration for 30 min, the supernatant after centrifugation was taken and ammonium sulfate was added again to 38% saturation. The solution was allowed to equilibrate for 30 min, and the resulting precipitate was collected by centrifugation. The precipitate was dissolved in 100 mM potassium phosphate buffer (pH 7.0) and dialyzed overnight against dialysis buffer containing 20 mM Tris-HCl buffer (pH 7.4) and 20 μM pyridoxal 5′-phosphate (PLP). The dialyzed ammonium sulfate fraction was loaded onto a Q-Sepharose FF column (40 ml, 1.6 cm by 16 cm; Pharmacia, Uppsala, Sweden) preequilibrated with 20 mM Tris-HCl buffer (pH 7.4). After the column was washed with the same buffer, elution was carried out with a linear gradient from 0 to 20% of elution buffer containing 20 mM Tris-HCl (pH 7.4) and 1 M KCl. The active fractions were collected and dialyzed against 50 mM potassium phosphate buffer (pH 7.0) containing 20 μM PLP. Partially purified fractions from the Q-Sepharose FF column were run on a 4 to 21% gradient native polyacrylamide gel for 3 h with an 80-mA constant current at 4°C. The gel was stained with native gel activity stain solution containing 15 mM racemic 3-amino-3-phenylpropionic acid, 10 mM pyruvate, 1 mM NAD+, 0.4 mM phenazine methosulfate, 0.5 mM nitroblue tetrazolium, and 20 U of alanine dehydrogenase in 50 mM sodium borate buffer (pH 9.0). The fraction of gel stained as a purple band (42) was sliced and crushed into small pieces in order to use the crushed gel as a loading sample onto a sodium dodecyl sulfate (SDS)-polyacrylamide gel (10%). After the separation by SDS-polyacrylamide gel electrophoresis (PAGE), major bands were used for analysis to get partial sequences of protein by matrix-assisted laser desorption ionization (MALDI)-mass spectrometry (MS).
Determination of partial peptide sequence of β-transaminase.
N-terminal amino acid sequence was previously determined by Edman degradation with a Procise 492 cLC protein sequencer (Applied Biosystems, CA) at the Institute of Korea Basic Science Institute (Seoul Center, Korea) (27). For internal peptide sequencing, a major protein band of ca. 47 kDa was isolated and digested with sequencing-grade trypsin (Promega, WI) with iodoacetamide modification on Cys residues to obtain tryptic fragments for mass analysis. Internal sequences were determined by MALDI-time of flight/time of flight-mass spectrometry with a Voyager system (Applied Biosystems, CA) at Yonsei Proteomics Research Center (Seoul, Korea).
Construction of genomic DNA library of Mesorhizobium sp. strain LUK.
DNA manipulations, including preparation of chromosomal DNA and plasmids, restriction enzyme digestion and ligation, transformation of E. coli, Southern hybridization, and colony hybridization, followed the methods of Sambrook et al. (46). Purified chromosomal DNA of Mesorhizobium sp. strain LUK was partially digested with Sau3AI to achieve DNA fragments of 10 to 20 kb. The fragments were ligated into the cosmid vector pOJ446 cut with BamHI and HpaI. The ligation mixture was packaged in vitro by using a lambda packaging system (Stratagene, CA) and transfected into E. coli XL1-Blue MRF. The colonies were selected on an LB agar plate containing apramycin (100 μg/ml).
Construction of the probe for colony hybridization.
Degenerated PCR primers were designed according to the partial amino acid sequences from purified protein. The first eight amino acids of the N-terminal sequence (MNEPIGEP), which had no unidentified sequence gap, were used to generate the forward degenerated primer, Pr1N (TTATGAAYGARCCIATHGGIGARCC; Y = CT, R = AG, I = inosine, and H = ATC). Consistent sequences of seven amino acids (FFFHM[I or L]R) were selected from the five candidate internal sequences, and Pr2r (TTCKIAICATRTGRAARAARAA; K = GT) was synthesized as reverse degenerated primer. PCR was performed with the primers using the genomic DNA of Mesorhizobium sp. strain LUK as a template. The PCR product was ligated into pGEM-T vector, and the DNA sequence was analyzed.
Another set of primers was designed using the partially determined internal DNA sequence for the preparation of probe for colony hybridization and Southern blot analysis. Using the pGEM-T vector clone as template, PCR product was obtained with primers Pr3f (forward primer, 5′-TCGACGAGGTGATGACCTC-3′) and Pr4r (reverse primer, 5′-TCCTTGAGTTGCTGGTCGG-3′), and digoxigenin-labeled random-primed DNA probe was prepared using a DIG High Prime DNA Labeling and Detection Starter Kit I (Roche Applied Science, Germany).
Isolation of β-transaminase gene.
Colony hybridization was performed using the DIG High Prime DNA Labeling and Detection Starter Kit I on a positively charged nylon membrane. Positive clones were confirmed by PCR with a primer set of Pr3f and Pr4r. Cosmid plasmid obtained from the candidate clone was digested with various restriction enzymes, and a fragment of ca. 2.5 kb containing the structural gene of β-transaminase was obtained from Southern blot analysis by digestion with EcoRI and PstI. This fragment was cloned into pUC18, and the nucleotide sequence was determined with an ABI3100 DNA sequencer (Perkin-Elmer, MA). Sequence alignments were performed with ClustalX (25) using a BLOSUM45 matrix with a gap opening penalty of 1.0 and a gap extension penalty of 0.10. A phylogenic tree was generated using the NJPlot function in ClustalX. Graphic presentations of the alignments were made by ESPript (18).
Heterologous expression of β-transaminase in E. coli.
To express the enzyme without excessive flanking parts, the coding region of the β-transaminase gene was amplified by PCR using Pr5f (5′-TTAACCATGGGCAACGAGCCGATTGGAGAACCTGG-3′; underlining indicates a restriction site on the primer) and Pr6r (5′-TCGAGAATTCACATCAGCAAGGCGC-3′) primers. The fragment was digested with NcoI and EcoRI and inserted into the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression vector pET28a. The plasmid was transformed into E. coli BL21(DE3), and the transformant was grown in LB broth containing 50 μg/ml of kanamycin at 37°C. When the optical density at 600 nm reached 0.5, IPTG was added to 0.5 mM. After 6 h of induction, the cells were harvested and disrupted by sonication. After centrifugation, the enzyme activity of the cell extract was measured to evaluate expression efficiency. β-Transaminase was purified from the E. coli expression clone by following the method of a previous report (27) using crude extract obtained by ultrasonic disruption with 50 ml of resuspended cell pellet harvested from 1 liter of E. coli culture broth.
Two other transaminases were cloned in order to compare their activities with that of newly found β-transaminase. The transaminase gene of Polaromonas sp. strain JS666 (Geninfo identifier [gi], 91787361) was amplified by PCR (forward primer, 5′-TTAACATATGAACAAGCCGTCCACGTCTTCC-3′; reverse primer, 5′-TTAACTCGAGTCAACCTGCAACGGGCAACAG-3′), cloned into NdeI/XhoI-cleaved pET24ma, and expressed in E. coli BL21(DE3). The glutamate-semialdehyde aminomutase (GSA) gene of E. coli K-12 (gi, 1786349) was cloned into BamHI/XhoI-cleaved pET24ma (forward primer, 5′-TCGCGGATCCATGAGTAAGTCTGAAAATCTTTA-3′; reverse primer, 5′-TCGCCTCGAGTCACAACTTCGCAAACACCCGAC-3′) and expressed in E. coli BL21(DE3).
Asymmetric synthesis of enantiomerically pure (3S)-amino-3-phenylpropionic acid by coupled enzyme reaction.
Enantiomerically pure (3S)-amino-3-phenylpropionic acid was synthesized using a coupled enzyme reaction with β-transaminase and lipase. A 10 mM concentration of ethylbenzoylacetate and a 20 mM concentration of racemic 3-aminobutyric acid were mixed in 1 ml of 50 mM sodium phosphate buffer (pH 7.5) containing 100 μM PLP. The reaction was started by adding 100 U of Candida rugosa lipase (Sigma, MO) and 0.8 mg of β-transaminase, and the mixture was incubated at 37°C for 24 h.
Nucleotide and protein sequence accession numbers.
The nucleotide and protein sequences of the β-transaminase of Mesorhizobium sp. strain LUK were deposited in the GenBank database under accession numbers EF127643 and ABL74379, respectively.
RESULTS
Partial purification and determination of internal peptide sequence.
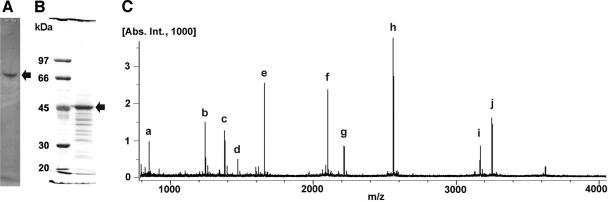
Mesorhizobium sp. strain LUK was previously selected for β-transaminase activity specific for d-β-aminocarboxylic acid, and the corresponding enzyme has been successfully purified (27). The sequence of 23 amino acid residues (MNEPIGEPXRSPAXDTAEKAQXI) was obtained earlier from the N-terminal sequencing of the partially purified transaminase (27). However, a BLAST search showed no similar sequence of known proteins having any significant homology with the determined N-terminal sequence. To obtain more sequence information, the protein was partially purified using native PAGE. Previously, the single band of proteins had been obtained after fractionation with ammonium sulfate precipitation and two successive column chromatographic procedures (27). However, the recovery yield of the proteins dramatically decreased at the last step of purification with hydrophobic affinity chromatography (from 45% to 1.1%). To prevent such loss of the proteins, we carried out native PAGE after fractionation with an anion-exchange column (Q-Sepharose FF). Active fraction from the anion-exchange column was resolved by successive gel electrophoresis with native PAGE (Fig. 1A) and SDS-PAGE (Fig. 1B). A single major band was observed at a previously determined molecular mass (47 kDa). The major band from SDS-PAGE was subjected to internal peptide sequencing. Five candidate peptide sequences were obtained from the molecular ion peak of 1,239.57 m/z (M + H), (i) KNFFFHMIR, (ii) KGGFFFHMLR, (iii) NKFFFHMIR, (iv) LEFFFHMLR, and (v) ELFFFHMLR, resulting in a defined peptide sequence of XXFFFHM(I/L)R (peak b of Fig. 1C).
FIG. 1.
(A) Native PAGE of partially purified protein from Mesorhizobium sp. strain LUK showing the stained activity band (arrow). (B) SDS-PAGE of partially purified protein from native PAGE. The protein band of ca. 47 kDa (arrow) was analyzed further by mass spectrometry. (C) MALDI spectrum of the in-gel tryptic-digested 47-kDa protein from SDS-PAGE. One hundred images were collected to draw the cumulative chromatogram. Twenty peaks were selected from the identically observed peaks from the three independent experiments. The 10 highest peaks correspond to the signals for the peptides of residues 234 to 240 (a; AFLDLLR; M + H/z, 847.5036; calculated, 847.56), residues 396 to 404 (b; ELFFFHMLR; M + H/z, 1,239.6343; calculated, 1,239.57), residues 281 to 294 (c; YIGGGMSFGAFGGR; M + H/z, 1,376.6415; calculated, 1,376.53), residues 413 to 426 (d; GMYALSLEIADAGR; M + H/z, 1,466.7307; calculated, 1,466.61), residues 427 to 441 (e; DAFAEALADFIGEQR; M + H/z, 1,652.7914; calculated, 1,652.64), residues 55 to 73 (f; SILFHRPFPLVIAQGTGSR; M + H/z, 2,096.1763; calculated, 2,096.05), residues 141 to 162 (g; FTNSGTEANLMALATATAITGR; M + H/z, 2,211.1074; calculated, 2,211.00), residues 355 to 378 (h; IAVENQAPLQFTGLGSLGTIHFSR; M + H/z, 2,556.3568; calculated, 2,556.26), residues 306 to 335 (i; DGAFAHAGTFNNNILTMSAGHAALTQIYTR; M + H/z, 3,163.5377; calculated, 3,163.32), and residues 74 to 102 (j; FQDVDGHAYVNFLGEYTAGLFGHSHPVIR; M + H/z, 3,246.5755; calculated, 3,246.38). Peak b was analyzed by MALDI-MS/MS to get the internal peptide sequence.
Determination of nucleotide sequence of β-transaminase gene.
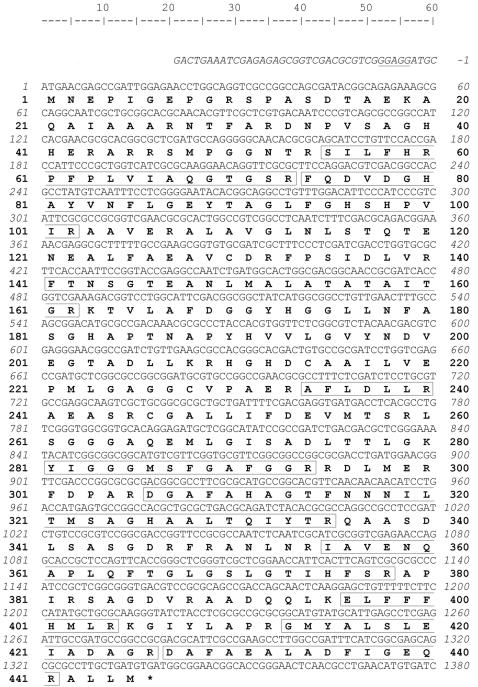
Using the degenerate primers based on the N-terminal sequence and partial peptide sequence obtained above, a 1,217-bp DNA fragment was obtained from the PCR using the genomic DNA of Mesorhizobium sp. strain LUK as the template, and the fragment was cloned into pGEM-T vector. The translated peptide sequence of the fragment indicated that 9 out of 10 peptide fragments found from the highest peak of MALDI mass signal were well matched with the deduced peptide sequence. However, the fragment lacked the partial sequence information encoding ca. 4.2 kDa of the C-terminal fragment of the protein. We tried to find the genomic DNA fragment containing this partial fragment by hybridization. The pGEM-T partial clone was used as a template to make a probe for colony hybridization to screen the genomic cosmid library of Mesorhizobium sp. strain LUK. A 433-bp partial gene fragment was generated by PCR using primer set Pr3f/Pr4r, and this fragment was used to make the probe for further analysis. By colony hybridization, 12 out of 850 colonies showed blue spots. Among the four colonies picked, one of the clones showed the same size as the PCR product with Pr3f and Pr4r primers. By analyzing the plasmid of the clone, a 2.5-kb nucleotide fragment was found with the complete structural nucleotide sequence of the β-transaminase. This 2.5-kb fragment was cloned into pUC18 and subjected to DNA sequencing. The nucleotide sequence of β-transaminase of Mesorhizobium sp. strain LUK and its flanking region is shown in Fig. 2 with deduced amino acid sequence. The open reading frame of 1,335 bp was composed of 445 amino acid residues with a theoretical pI of 6.2 and a calculated molecular mass of 47,244 Da with a putative ribosomal binding site, GGAGG, at 4 bp upstream of the start codon, ATG (Fig. 2).
FIG. 2.
The nucleotide and deduced amino acid sequences of the β-transaminase gene. Numbering starts at the ATG starting codon. The putative ribosomal binding site GGAGG is underlined. The peptide fragments observed by MALDI mass spectrometry are presented in boxes. The stop codon TGA is marked with an asterisk at 1,336 bp.
Sequence comparison with other transaminases.
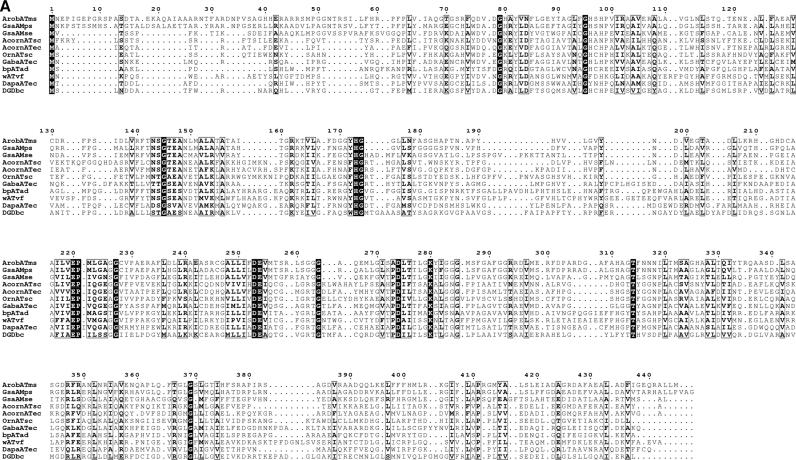
Due to the unique transaminase activity toward β-aminocarboxylic acids, it is quite interesting to compare the amino acid sequence with other transaminases. The β-transaminase of Mesorhizobium sp. strain LUK showed the highest identity (53%) and similarity (66%) with a glutamate-1-semialdehyde 2,1-aminomutase of Polaromonas sp. strain JS666. GSA belongs to transaminase subgroup II, which includes acetylornithine transaminase, ornithine transaminase, 4-aminobutyrate transaminase, 2,2-dialkylglycine decarboxylase, 7,8-diaminopelargonic acid synthase, ω-transaminase, and β-alanine-pyruvate transaminase (12). To verify the transaminase subgroup to which the newly found β-transaminase belongs and to obtain structural and functional insights into the β-transaminase, the primary structure of the β-transaminase was aligned with several sequences of transaminase group II proteins (Fig. 3A). Sequence alignment showed that the β-transaminase shares the same consensus amino acid residues found in transaminase subgroup II with several invariant amino acid residues (34). K280 is the lysine residue anchoring PLP via Schiff base formation in the active site, which supports the PLP-dependent transamination mechanism. G227 and D253 are also invariant amino residues, which are located at the domain interface and form a salt bridge and a hydrogen bond to PLP, respectively (35). Pairwise sequence alignment with a known glutamate-1-semialdehyde 2,1-aminomutase having structural information (Protein Data Bank entry 2GSA [22]) showed that the sequence from positions 148 to 153 of 2GSA with the conserved sequences of GxYHGxx is also well preserved in the sequence (positions 170 to 176, GGYHGGL) and consists of a putative PLP binding site of the cloned β-transaminase. The sequence from positions 154 to 182 of 2GSA whose mobility is affected by the PLP binding (22) is partly absent in the sequences of the newly found β-transaminase.
FIG. 3.
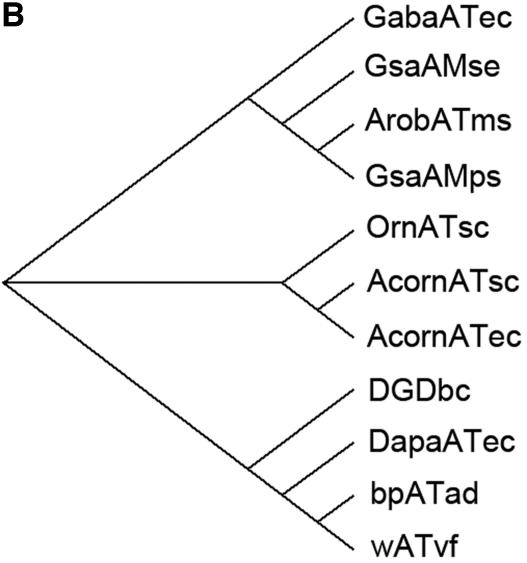
Sequence comparison with transaminase subgroup II enzymes. (A) Sequence alignment of the β-transaminase and other related proteins. Highly conserved residues are highlighted in black, and less strongly conserved residues are in gray boxes. Proteins: ArobATms, β-transaminase in this study; GsaAMps, glutamate-1-semialdehyde 2,1-aminomutase of Polaromonas sp. strain JS666 (gi, 91787361); GsaAMse, glutamate-1-semialdehyde 2,1-aminomutase of Synechococcus elongatus (gi, 581789); AcornATec, acetylornithine transaminase of Escherichia coli K-12 (gi, 16131238); AcornATsc, acetylornithine transaminase of Saccharomyces cerevisiae (gi, 6324432); OrnATsc, ornithine transaminase of Saccharomyces cerevisiae (gi, 6323470); GabaATec, 4-aminobutyrate transaminase of Escherichia coli K-12 (gi, 16130576); bpATad, β-alanine:pyruvate transaminase of Alcaligenes denitrificans (gi, 33086798); wATvf, ω-transaminase of Vibrio fluvialis (47); DapaATec, 7,8-diaminopelargonic acid synthase of Escherichia coli K-12 (gi, 16128742); DGDbc, 2,2-dialkylglycine decarboxylase of Burkholderia cepacia (gi, 729318). (B) A molecular phylogenetic tree. Abbreviations are as defined for panel A.
A brief molecular phylogenetic tree drawn using the neighbor-joining method (Fig. 3B) shows that the sequence of the cloned β-transaminase is close to the glutamate-1-semialdehyde 2,1-aminomutase, 4-aminobutyrate transaminase, dialkylglycine decarboxylase, 7,8-diaminopelargonic acid synthase, ω-transaminase, β-alanine-pyruvate transaminase, acetylornithine transaminase, and ornithine transaminase in decreasing order. Although the sequence homology with the GSA gave us some insights into the classification of this protein, there is no information about whether or not the GSA has similar β-aminocarboxylic acid transaminase activity. To confirm whether other homologs could have such an activity, we cloned two transaminases, one from Polaromonas sp. strain JS666 showing the highest sequence similarity and the other from E. coli K-12. However, neither of these enzymes showed an activity for the β-aminocarboxylic transamination using 3-amino-3-phenylpropionic acid and pyruvate as substrates.
Overproduction and purification of the β-transaminase in E. coli.
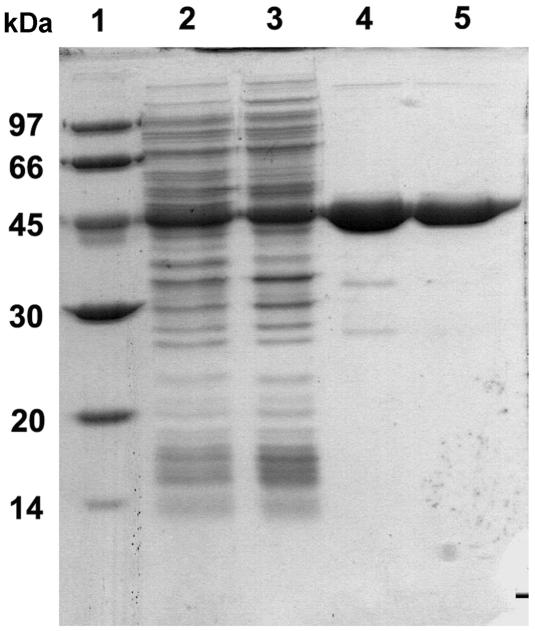
The recombinant β-transaminase was overexpressed in E. coli BL21(DE3). The total enzyme activity from the recombinant E. coli was ca. 27-fold higher than that of the wild-type Mesorhizobium sp. strain LUK. The recovery of the β-transaminase from the recombinant E. coli was ninefold higher than that from the wild type with the same purification procedures (Table 1). We used affinity purification as the last step, although there was some activity loss in that step, to ensure that the homogeneous protein preparation was uncontaminated. The purified β-transaminase gave a single protein band on SDS-PAGE (Fig. 4) with a specific activity of 1.27 U/mg at pH 7 (Table 1).
TABLE 1.
Purification of the cloned β-transaminase from E. coli BL21(DE3)
| Purification step | Total protein (mg) | Sp act (U mg−1) | Total activity (U) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|
| Cell extract | 242 | 0.082 | 19.8 | 100 | |
| Ammonium sulfate (28 to 42%) | 64.6 | 0.192 | 12.4 | 62 | 2.3 |
| Q-Sepharose FF column | 3.24 | 1.43 | 4.65 | 23 | 17.5 |
| HiTrap Phenyl HP column | 1.41 | 1.27 | 1.79 | 9.1 | 15.5 |
FIG. 4.
SDS-PAGE of β-transaminase expressed in E. coli at different stages of purification. Proteins were separated on a 10% polyacrylamide gel in the presence of 1% SDS. Lane 1, molecular mass marker (sizes of marker proteins are designated on the left side in kDa); lane 2, cell extract of E. coli BL21(DE3); lane 3, ammonium sulfate fraction (28 to 42%); lane 4, after Q-Sepharose FF column chromatography; lane 5, after HiTrap Phenyl HP column chromatography.
Characterization of the β-transaminase from Mesorhizobium sp. strain LUK.
Though all the purification procedures were performed at pH 7, brief optimization of its reaction pH showed an optimum pH of β-transaminase at pH 7.6 (data not shown), and this was used for further characterization of the β-transaminase. In terms of the substrate specificity of the purified enzyme (Table 2), it showed higher activity toward β-aminocarboxylic acids than toward α-amino acids. 3-Amino-5-methylhexanoic acid and 3-aminobutyric acid showed ca.-twofold-higher reactivities than 3-amino-3-phenylpropionic acid, whereas the phenylalanine showed ca.-10-fold-lower reactivity. In the case of the amino acceptor, 2-ketoglutarate, pyruvate ethyl ester, pyruvate, and pyruvate methyl ester showed similar reactivities, while oxaloacetate showed slightly lower reactivity. It is quite interesting that this enzyme has no striking differences in the specificities toward different amino acceptors, because transaminases generally show a clear preference either for pyruvate or for 2-ketoglutarate/oxaloacetate (33, 47, 48, 52, 56). However, considering the fact that this enzyme shows a high sequence similarity with the GSA which does not need any second substrate and catalyzes the intramolecular isomerization, it is possible that the unique amino acceptor specificity could be due to different structural reasons with other transaminases. In terms of the enantioselectivity, the enzyme showed a high enantioselectivity toward the d-β-amino acids (Table 2) and produced l-alanine with pyruvate (27). It also showed enantioselectivity for l-α-amino acids with much lower activity (Table 2).
The relationships between reaction rates and each substrate concentration showed a typical substrate inhibition mode of the reaction (55). The apparent Km and kcat for 3-amino-3-phenylpropionic acid were 1.2 mM and 513 min−1, respectively, with an apparent substrate inhibition constant (Ki) of 3.2 mM in the presence of 10 mM pyruvate. In the presence of 10 mM 3-amino-3-phenylpropionic acid, the apparent Km and kcat for pyruvate were 3.9 mM and 228 min−1, respectively, with an apparent Ki of 177 mM. The substrate inhibition by pyruvate has been examined with several different ω-transaminases screened from soil samples (47). Compared to those results, the β-transaminase showed a slight decrease in the reaction rate up to 500 mM pyruvate, which is reaching half of its maximum reaction rate. Considering the low aqueous solubility of 3-amino-3-phenylpropionic acid, the β-transaminase maintained about 35% of its activity at the concentration of half of its solubility (i.e., ca. 50 mM at room temperature).
Asymmetric synthesis of enantiomerically pure β-aminocarboxylic acids.
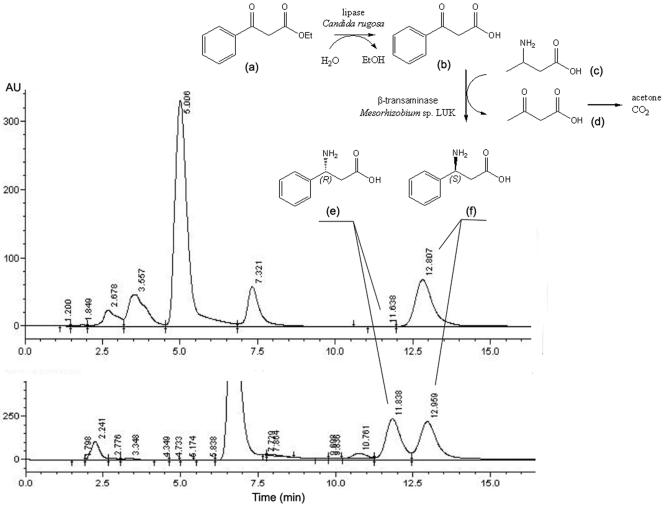
The asymmetric synthesis of β-aminocarboxylic acids from their ketocarboxylic acid precursors is quite challenging, because the β-ketocarboxylic acids spontaneously lose their carboxyl moiety through the decarboxylation reaction (27). Instead of using labile β-ketocarboxylic acid (compound b in Fig. 5) for the asymmetric reaction, we tried to use more stable β-ketocarboxylic acid ester (compound a in Fig. 5). Using the ester compound, we could not observe the formation of corresponding β-aminocarboxylic acid ester as the product for transamination. A possible explanation would be the substrate recognition mechanism of the transaminase subgroup II enzymes. In general, the substrate in the active site is recognized by the two substrate binding pockets around the PLP-lysine Schiff base (9, 23). The main roles of the binding pockets are to recognize the side chain of the substrate and to anchor the carboxylate groups of the substrate (9, 39). Consequently, it can be assumed that the β-ketocarboxylic acid ester cannot be used as its amino acceptor due to the absence of the carboxylate group. According to the amino acceptor specificity data in Table 2, all the aldehydes were nonreactive as amino acceptors, while α-ketocarboxylic acids served well as amino acceptors, suggesting that in this case the prior key determinant of the substrate would be the existence of the carboxylate group.
FIG. 5.
Reaction scheme for the asymmetric synthesis of enantiomerically pure β-aminocarboxylic acid and the chiral analysis of asymmetrically synthesized (3S)-amino-3-phenylpropionic acid using the coupled enzyme reaction with a lipase and the β-transaminase. The lower chromatogram was obtained with racemic 3-amino-3-phenylpropionic acid for comparison. (a) Ethylbenzoylacetate; (b) benzoylacetatic acid; (c) 3-aminobutyric acid; (d) acetoacetic acid; (e) (3R)-amino-3-phenylpropionic acid; (f) (3S)-amino-3-phenylpropionic acid.
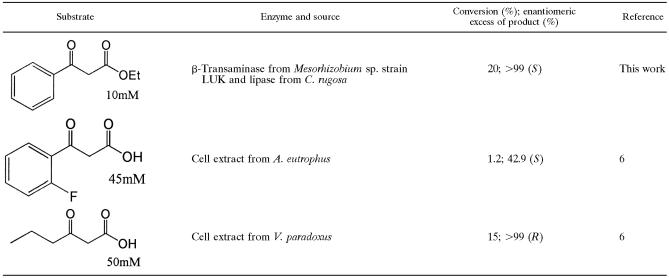
Therefore, we tried to couple the reaction with lipase, which has activity for the hydrolysis of the β-ketocarboxylic acid ester to its acid form. Using the coupled lipase reaction, we expected to be able to control the release of labile β-ketocarboxylic acid from the stable ester substrate. Among the lipases examined, the one from Candida rugosa showed activity for the hydrolysis of β-ketocarboxylic acid ester (data not shown). In the lipase-transaminase coupled reaction, the formation of 3-amino-3-phenylpropionic acid was observed only using 3-aminobutyric acid (compound c in Fig. 5) as an amino donor. The reaction scheme could be figured as the hydrolysis of keto acid ester followed by transamination, as no reactions were observed without the addition of lipase. Chirality analysis of the product showed only (3S)-amino-3-phenylpropionic acid (compound f in Fig. 5) with 20% yield (Table 3; Fig. 5).
TABLE 3.
Comparison of the efficiencies of known transaminases for the asymmetric synthesis of β-aminocarboxylic acid enantiomers
DISCUSSION
As one of the promising biocatalysts for the preparation of enantiomerically pure β-aminocarboxylic acids, a novel β-transaminase was cloned from previously isolated Mesorhizobium sp. strain LUK, which shows β-transaminase activity for the transamination between β-aminocarboxylic acids and keto acids (27). The cloned enzyme shows high sequence similarity with GSA and belongs to subgroup II transaminase. It is interesting that the cloned β-transaminase shared significant sequence similarity with GSA, because it is not known yet whether GSA shows any intermolecular transaminase activity, although the GSA shared a common PLP-dependent catalytic mechanism with the β-transaminase. Our analysis of the two other selected GSAs showed no activities for the β-transaminase reaction. This result suggests a hypothesis that this enzyme is somewhat structurally evolved from GSA to obtain a new function of β-transaminase activity and has achieved quite successful adaptation for the new function (13, 21, 43).
So far, several β-transaminases have been isolated and their substrate specificities toward amino donors and acceptors were measured (6, 56). These enzymes showed high activity toward specific β-aminocarboxylic acids, mainly aliphatic ones such as β-alanine and β-aminobutyric acid, while they showed low activity toward aromatic β-aminocarboxylic acids such as 3-amino-3-phenylpropionic acid. However, the β-transaminase isolated from Mesorhizobium sp. strain LUK shows high activity toward the aromatic β-aminocarboxylic acids. Therefore, its unique substrate specificity makes this enzyme a candidate for the asymmetric synthesis of chiral β-aminocarboxylic acids as described here. Another unexpected characteristic of its substrate specificity was that this enzyme has no striking differences in the specificities toward amino acceptors like the other transaminases do, which generally showed a clear preference either for pyruvate or for 2-ketoglutarate/oxaloacetate. However, the reason for this is not clear from the given information.
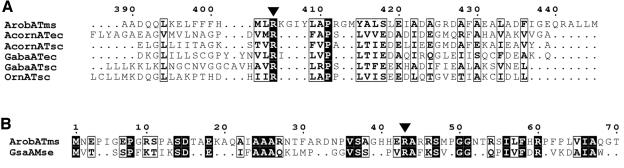
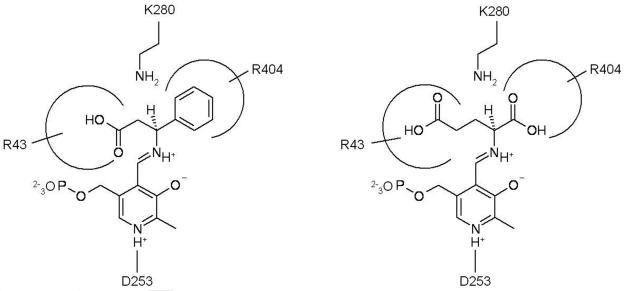
We tried several sequence alignments with different sets of sequences to find more sequence-level information. One of the results showed R404 of β-transaminase as the analogue of R398 of 4-aminobutyrate transaminase of Escherichia coli (Fig. 6A). This is noteworthy because this arginine residue is conserved among all the transaminases and is known to bind the α-carboxylate group of α-amino acids and α-keto acids (31). A pairwise alignment with known GSA showed that E406 in the 2GSA is not conserved in this β-transaminase (data not shown); E406 is an important residue positioned to repel the α-carboxylate group (22). These results suggest that the β-transaminase is able to recognize the α-carboxylate group of α-amino acids and α-keto acids via R404 without repelling or masking by the glutamate residue (31). The pairwise alignment with 2GSA also showed the R43 in β-transaminase conserved with R32 of 2GSA (Fig. 6B) which participates in recognizing the carboxylate group of gabaculine (22). Considering the position of R43 relative to the N-terminal sequence, this result suggests the possible role of R43 as a residue recognizing the β-carboxylate group of the substrates. According to the enantioselectivity data in Table 2, d-β-aminocarboxylic acids are preferred over the l form, while l-α-amino acids are preferred over the d form. Therefore, we can expect that β-aminocarboxylic acid and 2-ketoglutarate bind to the active site through their α-carboxylate groups, forming a hydrogen bond and a salt bridge to R32 and R404, respectively. At the given orientation of two arginine residues (Fig. 7), β-hydrogen of 3-amino-3-phenylpropionic acid is toward the opposite side (si) face at C4′ of the conjugated π system of the external aldimine complex and abstraction of the β-proton occurs on the si face. In the same manner, a proton should be added from the si face into α-ketoglutarate during the amination step to give l-glutamate.
FIG. 6.
(A) Partial alignment of β-transaminase sequence with group II transaminases. (B) Partial alignment of β-transaminase sequence with GsaAMse (gi, 581789). See the Fig. 3A legend for definitions of abbreviations and boxes.
FIG. 7.
Proposed mechanism of substrate recognition in the active site of β-transaminase from Mesorhizobium sp. strain LUK. The structure shows the external aldimine intermediate for transamination of (3S)-d-amino-3-phenylpropionic acid (left) and α-ketoglutarate (right).
For the successful asymmetric synthesis of the unnatural amino acids using transaminase, it is important to select a proper amino donor in many cases. In the case of the transamination catalyzed by α-transaminase, l-aspartate was often used as the amine donor to overcome the reversible nature of the enzyme reaction, as the use of l-aspartate often accelerates the reaction equilibrium shift by the spontaneous decarboxylation of the resulting oxaloacetate (8, 54). l-Alanine and l-glutamate are the other examples of amine donors in the asymmetric synthesis (8). However, frequent observation of product inhibition against the corresponding keto acids requires an additional coupled enzyme reaction to remove the keto acid product (3, 8, 45, 54, 56). In several previous studies, l-lysine was successfully used as an alternative amino donor that was transformed into self-degrading keto acid (2, 32). Here, we found that 3-aminobutyric acid is also a promising amino donor for the asymmetric synthesis of chiral amine compounds using transaminases, as this molecule is changed into acetoacetic acid (compound d in Fig. 5) which is decomposed spontaneously into acetone and carbon dioxide (20), leading to easy removal from the reaction mixture at the end of the reaction (Fig. 5).
Conversely, the β-ketocarboxylic acid has some limitations as an amino acceptor in the asymmetric synthesis reaction owing to substrate inhibition and its spontaneous decarboxylation under the reaction conditions. Here, using the coupled lipase reaction, we successfully controlled the release of labile β-ketocarboxylic acid from the stable ester substrate. Consequently, the limitation caused by the labile substrate could be easily overcome by the simple coupled enzyme reaction with more-stable substrate. In this study, optically pure β-aminocarboxylic acid was asymmetrically synthesized using the newly characterized β-transaminase from Mesorhizobium sp. strain LUK. When our results are compared with the data filed in the recent patent by Banerjee et al. (2005) (6), the β-transaminase of Mesorhizobium sp. strain LUK appears to be a very promising biocatalyst for the preparation of chiral β-aminocarboxylic acids.
Acknowledgments
We appreciate the help of Eun-Mi Kim in the analysis of MALDI and of Eun-Jung Kim in the genetic work.
This work was partly supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2005-005-J16002), the 21C Frontier Microbial Genomics and Application Center Program, Ministry of Science & Technology (M105KK000048-06K1101-04811), and the ERC program of MOST/KOSEF (R11-2000-075-03001-0) of the Republic of Korea.
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Ager, D. J., I. G. Fotheringham, S. A. Laneman, D. P. Pantaleone, and P. P. Taylor. 1997. The large scale synthesis of unnatural amino acids. Chim. Oggi 15:11-14. [Google Scholar]
- 2.Ager, D. J., I. G. Fotheringham, T. Li, D. P. Pantaleone, and R. F. Senkpeil. 2000. The large scale synthesis of “unnatural” amino acids. Enantiomer 5:235-243. [PubMed] [Google Scholar]
- 3.Ager, D. J., T. Li, D. P. Pantaleone, R. F. Senkpeil, P. P. Taylor, and I. G. Fotheringham. 2001. Novel biosynthetic routes to non-proteinogenic amino acids as chiral pharmaceutical intermediates. J. Mol. Catal. B Enzym. 11:199-205. [Google Scholar]
- 4.Alexeeva, M., A. Enright, M. J. Dawson, M. Mahmoudian, and N. J. Turner. 2002. Deracemization of α-methylbenzylamine using an enzyme obtained by in vitro evolution. Angew. Chem. Int. Ed. Engl. 41:3177-3180. [DOI] [PubMed] [Google Scholar]
- 5.Angelaud, R., Y. L. Zhong, P. Maligres, J. Lee, and D. Askin. 2005. Synthesis of a β-amino acid pharmacophore via a β-lactam intermediate. J. Org. Chem. 70:1949-1952. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, A., M. Chase, A. Calyton, and B. Landis. January. 2005. Methods for the stereoselective synthesis and enantiomeric enrichment of β-amino acids. PCT international application patent WO2005005633.
- 7.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of dna from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 8.Cho, B. K., H. J. Cho, S. H. Park, H. Yun, and B. G. Kim. 2003. Simultaneous synthesis of enantiomerically pure (S)-amino acids and (R)-amines using coupled transaminase reactions. Biotechnol. Bioeng. 81:783-789. [DOI] [PubMed] [Google Scholar]
- 9.Cho, B. K., H. Y. Park, J. H. Seo, K. Kinnera, B. S. Lee, and B. G. Kim. 2004. Enzymatic resolution for the preparation of enantiomerically enriched D-β-heterocyclic alanine derivatives using Escherichia coli aromatic L-amino acid transaminase. Biotechnol. Bioeng. 88:512-519. [DOI] [PubMed] [Google Scholar]
- 10.Cho, B. K., J. H. Seo, T. J. Kang, J. Kim, H. Y. Park, B. S. Lee, and B. G. Kim. 2006. Engineering aromatic L-amino acid transaminase for the asymmetric synthesis of constrained analogs of L-phenylalanine. Biotechnol. Bioeng. 94:842-850. [DOI] [PubMed] [Google Scholar]
- 11.Cho, B. K., J. H. Seo, T. W. Kang, and B. G. Kim. 2003. Asymmetric synthesis of L-homophenylalanine by equilibrium-shift using recombinant aromatic L-amino acid transaminase. Biotechnol. Bioeng. 83:226-234. [DOI] [PubMed] [Google Scholar]
- 12.Christen, P., and P. K. Mehta. 2001. From cofactor to enzymes. The molecular evolution of pyridoxal-5′-phosphate-dependent enzymes. Chem. Rec. 1:436-447. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, A. J. L., S. A. Bruschi, and M. W. Anders. 2002. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem. Pharmacol. 64:553-564. [DOI] [PubMed] [Google Scholar]
- 14.Corbett, T. H., F. A. Valeriote, L. Demchik, N. Lowichik, L. Polin, C. Panchapor, S. Pugh, K. White, J. Kushner, J. Rake, M. Wentland, T. Golakoti, C. Hetzel, J. Ogino, G. Patterson, and R. Moore. 1997. Discovery of cryptophycin-1 and BCN-183577: examples of strategies and problems in the detection of antitumor activity in mice. Investig. New Drugs 15:207-218. [DOI] [PubMed] [Google Scholar]
- 15.Crews, P., L. V. Manes, and M. Boehler. 1986. Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett. 27:2797-2800. [Google Scholar]
- 16.DeGrado, W. F., J. P. Schneider, and Y. Hamuro. 1999. The twists and turns of β-peptides. J. Pept. Res. 54:206-217. [DOI] [PubMed] [Google Scholar]
- 17.Fotheringham, I. G., N. Grinter, D. P. Pantaleone, R. F. Senkpeil, and P. P. Taylor. 1999. Engineering of a novel biochemical pathway for the biosynthesis of L-2-aminobutyric acid in Escherichia coli K12. Bioorg. Med. Chem. 7:2209-2213. [DOI] [PubMed] [Google Scholar]
- 18.Gouet, P., E. Courcelle, D. I. Stuart, and F. Metoz. 1999. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15:305-308. [DOI] [PubMed] [Google Scholar]
- 19.Griffith, O. W. 1986. β-Amino acids—mammalian metabolism and utility as α-amino-acid analogs. Annu. Rev. Biochem. 55:855-878. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie, J. P. 2002. Uncatalyzed and amine catalyzed decarboxylation of acetoacetic acid: an examination in terms of no barrier theory. Bioorg. Chem. 30:32-52. [DOI] [PubMed] [Google Scholar]
- 21.Han, Q., J. M. Fang, and J. Y. Li. 2001. Kynurenine aminotransferase and glutamine transaminase K of Escherichia coli: identity with aspartate aminotransferase. Biochem. J. 360:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennig, M., B. Grimm, R. Contestabile, R. A. John, and J. N. Jansonius. 1997. Crystal structure of glutamate-1-semialdehyde aminomutase: an α-dimeric vitamin B6-dependent enzyme with asymmetry in structure and active site reactivity. Proc. Natl. Acad. Sci. USA 94:4866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirotsu, K., M. Goto, A. Okamoto, and I. Miyahara. 2005. Dual substrate recognition of aminotransferases. Chem. Rec. 5:160-172. [DOI] [PubMed] [Google Scholar]
- 24.Ito, S., A. Ota, K. Yamamoto, and Y. Kawashima. 1992. Resolution of the enantiomers of thiol compounds by reversed-phase liquid chromatography using chiral derivatization with 2,3,4,6-tetra-O-acetyl-β-image-glucopyranosyl isothiocyanate. J. Chromatogr. 626:187-196. [Google Scholar]
- 25.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal x. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 26.Juaristi, E. 1997. Enantioselective synthesis of β-amino acids. Wiley-VCH, New York, NY.
- 27.Kim, J., D. Kyung, H. Yun, B. K. Cho, and B. G. Kim. 2006. Screening and purification of a novel transaminase catalyzing the transamination of aryl β-amino acid from Mesorhizobium sp. LUK. J. Microbiol. Biotechnol. 16:1832-1836. [Google Scholar]
- 28.Lelais, G., and D. Seebach. 2004. β2-Amino acids—syntheses, occurrence in natural products, and components of β-peptides. Biopolymers 76:206-243. [DOI] [PubMed] [Google Scholar]
- 29.Liljeblad, A., and L. T. Kanerva. 2006. Biocatalysis as a profound tool in the preparation of highly enantiopure β-amino acids. Tetrahedron 62:5831-5854. [Google Scholar]
- 30.Liu, M., and M. P. Sibi. 2002. Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 58:7991-8035. [Google Scholar]
- 31.Liu, W. S., P. E. Peterson, R. J. Carter, X. Z. Zhou, J. A. Langston, A. J. Fisher, and M. D. Toney. 2004. Crystal structures of unbound and aminooxyacetate-bound Escherichia coli γ-aminobutyrate aminotransferase. Biochemistry 43:10896-10905. [DOI] [PubMed] [Google Scholar]
- 32.Lo, H. H., S. K. Hsu, W. D. Lin, N. L. Chan, and W. H. Hsu. 2005. Asymmetrical synthesis of L-homophenylalanine using engineered Escherichia coli aspartate aminotransferase. Biotechnol. Prog. 21:411-415. [DOI] [PubMed] [Google Scholar]
- 33.Lowe, P. N., and A. F. Rowe. 1985. Aspartate-2-oxoglutarate aminotransferase from Trichomonas vaginalis—identity of aspartate-aminotransferase and aromatic amino-acid aminotransferase. Biochem. J. 232:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta, P. K., and P. Christen. 2000. The molecular evolution of pyridoxal-5′-phosphate-dependent enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 74:129-184. [DOI] [PubMed] [Google Scholar]
- 35.Mehta, P. K., T. I. Hale, and P. Christen. 1993. Aminotransferases—demonstration of homology and division into evolutionary subgroups. Eur. J. Biochem. 214:549-561. [DOI] [PubMed] [Google Scholar]
- 36.Miura, K., T. Sawa, T. Takeuchi, and H. Umezawa. 1986. Effects of enzyme-inhibitors in inhibiting the growth and inducing the differentiation of human promyelocytic leukemia-cells, HL-60. J. Antibiot. 39:734-735. [DOI] [PubMed] [Google Scholar]
- 37.Murahashi, S., Y. Imada, T. Kawakami, K. Harada, Y. Yonemushi, and N. Tomita. 2002. Enantioselective addition of ketene silyl acetals to nitrones catalyzed by chiral titanium complexes. Synthesis of optically active β-amino acids. J. Am. Chem. Soc. 124:2888-2889. [DOI] [PubMed] [Google Scholar]
- 38.Ojima, I., S. N. Lin, and T. Wang. 1999. Recent advances in the medicinal chemistry of taxoids with novel β-amino acid side chains. Curr. Med. Chem. 6:927-954. [PubMed] [Google Scholar]
- 39.Okamoto, A., Y. Nakai, H. Hayashi, K. Hirotsu, and H. Kagamiyama. 1998. Crystal structures of Paracoccus denitrificans aromatic amino acid aminotransferase: A substrate recognition site constructed by rearrangement of hydrogen bond network. J. Mol. Biol. 280:443-461. [DOI] [PubMed] [Google Scholar]
- 40.Palko, M., L. Kiss, and F. Fulop. 2005. Syntheses of hydroxylated cyclic β-amino acid derivatives. Curr. Med. Chem. 12:3063-3083. [DOI] [PubMed] [Google Scholar]
- 41.Palomo, C., J. M. Aizpurua, I. Ganboa, and M. Oiarbide. 1999. From β-lactams to α- and β-amino acid derived peptides. Amino Acids 16:321-343. [DOI] [PubMed] [Google Scholar]
- 42.Pedraza, R. O., A. Ramirez-Mata, M. L. Xiqui, and B. E. Baca. 2004. Aromatic amino acid amino transferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol. Lett. 233:15-21. [DOI] [PubMed] [Google Scholar]
- 43.Percudani, R., and A. Peracchi. 2003. A genomic overview of pyridoxal-phosphate dependent enzymes. EMBO Rep. 4:850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozzell, J. D. May 1985. Production of amino acids by transamination. U.S. patent 4,518,692.
- 45.Rozzell, J. D. May 1989. Production of amino acids using coupled aminotransferases. U.S. patent 4,826,766.
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 47.Shin, J. S., and B. G. Kim. 2001. Comparison of the ω-transaminases from different microorganisms and application to production of chiral amines. Biosci. Biotechnol. Biochem. 65:1782-1788. [DOI] [PubMed] [Google Scholar]
- 48.Shin, J. S., H. Yun, J. W. Jang, I. Park, and B. G. Kim. 2003. Purification, characterization, and molecular cloning of a novel amine: pyruvate transaminase from Vibrio fluvialis JS17. Appl. Microbiol. Biotechnol. 61:463-471. [DOI] [PubMed] [Google Scholar]
- 49.Stewart, J. D. 2001. Dehydrogenases and transaminases in asymmetric synthesis. Curr. Opin. Chem. Biol. 5:120-129. [DOI] [PubMed] [Google Scholar]
- 50.Stirling, D. I., A. L. Zeitlin, G. W. Matcham, and J. D. Rozzell, Jr. December 1992. Enantiomeric enrichment and stereoselective synthesis of chiral amines. U.S. patent 5,169,780.
- 51.Sugawara, T., A. Tanaka, K. Tanaka, K. Nagai, K. Suzuki, and T. Suzuki. 1998. YM-170320, a novel lipopeptide antibiotic inducing morphological change of colonies in a mutant of Candida tropicalis pK233. J. Antibiot. 51:435-438. [DOI] [PubMed] [Google Scholar]
- 52.Sung, M. H., K. Tanizawa, H. Tanaka, S. Kuramitsu, H. Kagamiyama, and K. Soda. 1990. Purification and characterization of thermostable aspartate aminotransferase from a thermophilic Bacillus species. J. Bacteriol. 172:1345-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki, K., M. Yamaizumi, S. Tateishi, Y. Monnai, and M. Uyeda. 1999. Topostatin, a novel inhibitor of topoisomerases I and II produced by Thermomonospora alba strain no. 1520. III. Inhibitory properties. J. Antibiot. 52:460-465. [DOI] [PubMed] [Google Scholar]
- 54.Taylor, P. P., D. P. Pantaleone, R. F. Senkpeil, and I. G. Fotheringham. 1998. Novel biosynthetic approaches to the production of unnatural amino acids using transaminases. Trends Biotechnol. 16:412-418. [DOI] [PubMed] [Google Scholar]
- 55.Tipton, K. F. 1996. Patterns of enzyme inhibition, p. 115-174. In P. C. Engel (ed.), Enzymology. LABFAX. Academic Press, San Diego, CA.
- 56.Yun, H., S. Lim, B. -K. Cho, and B. -G. Kim. 2004. ω-Amino acid:pyruvate transaminase from Alcaligenes denitrificans Y2k-2: a new catalyst for kinetic resolution of β-amino acids and amines. Appl. Environ. Microbiol. 70:2529-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]