Abstract
Research was undertaken to characterize Escherichia coli isolates in interstitial water samples of a sandy beach on the southeastern shore of Lake Huron, Ontario, Canada. A survey of the beach area revealed the highest abundance of E. coli in interstitial water of the foreshore beach sand next to the swash zone. Higher concentrations of E. coli (up to 1.6 × 106 CFU/100 ml of water) were observed in the interstitial water from the sampling holes on the beach itself compared to lake water and sediment. Repetitive extragenic palindromic PCR (REP-PCR) was used to characterize the genetic diversity of E. coli isolates from interstitial water samples on the beach. E. coli isolates from the same sampling location frequently exhibited the same REP-PCR pattern or were highly similar to each other. In contrast, E. coli isolates from different sampling locations represented populations distinct from each other. This study has identified a unique ecological niche within the foreshore area of the beach where E. coli may survive and possibly multiply outside of host organisms. The results are of interest as increasing concentrations of E. coli in recreational waters are often considered to be an indication of recent fecal pollution.
Recent studies have assumed a direct link between the presence of Escherichia coli in recreational waters and the originating source(s). Many studies have limited their focus to well-known sources such as agriculture, sewage treatment plants, and combined sewer overflows (8, 21) and assumed limited bacterial survival between the sources and surface waters. However, high bacterial counts in surface waters along shorelines may also be a result of bacterial survival in beach sand in the absence of fecal input (2, 14). Environmental survival of E. coli outside of animal hosts has been reported in tropical (5) and temperate soils (13). Shoreline sand may also be a suitable environment for survival of E. coli (12, 26). However, little is known about the mechanism(s) by which E. coli survives in such an environment.
It is unknown if the sources of increased bacterial indicator counts represent recent bacterial input or survival and periodic release from sand and/or sediments. Thus, characterization of continuous, localized sources, including environmental sources, of microbial indicators is essential to complement current water-monitoring strategies and standards. Differentiation between freshly introduced and resident E. coli strains at the shore could assist in understanding the microbial ecology of the beach environment and water quality.
Repetitive extragenic palindromic PCR (REP-PCR) has been used to characterize the composition of microbial communities in environmental samples (15, 16). In this research, REP-PCR was used to evaluate E. coli isolates obtained from interstitial water on the beach. Ashfield Township Park beach, on the southeastern shore of Lake Huron, Canada, was chosen as a study site because it has been periodically posted as unsafe for swimming because of high E. coli concentrations in the water. This beach is representative of the area and has all of the features that are characteristic of southeastern Lake Huron such as sandy beaches backed by clay cliffs (the Huron Fringe), followed by gentle slope plains in the direction of the lake (Huron Slopes) and an abundance of small tributaries discharging into the lake (10).
The objectives of this research were to survey E. coli in the beach sand of Ashfield Township Park beach and to characterize the genetic diversity of the isolates obtained.
MATERIALS AND METHODS
Study area.
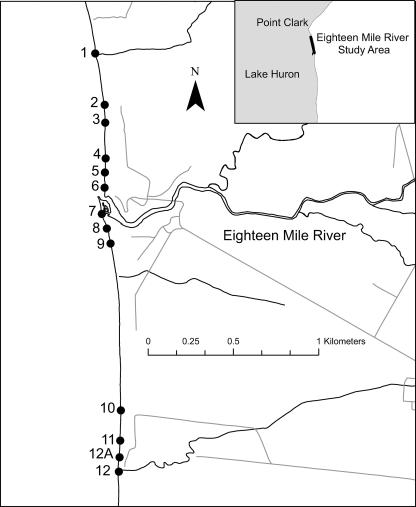
The study area encompasses a 5-km section of a sandy beach at the mouth of Eighteen Mile River on the southeastern shore of Lake Huron (see Fig. 1). The land use within the Eighteen Mile River watershed is predominantly agricultural, with the major focus on livestock farming (23). The beach includes a public beach (Ashfield Township Park beach) and privately owned land.
FIG. 1.
Map of study area and 12 sampling sites.
Sampling approach.
There were three components to this study. The first was a survey for the presence of E. coli in the lake (water and sediment) adjacent to the study area, as well as from Eighteen Mile River at a site approximately 500 m upstream from the mouth of the river. Weekly sampling occurred at the mouth of the river and at five sampling stations in the lake at a 1-m depth. Between May and November 2005, five more extensive surveys were conducted in which water and sediment samples were collected from the river, near the shore of the lake, and up to 4 km offshore in the lake. Samples were collected and analyzed for E. coli as described below.
The second component was to examine the presence of E. coli in interstitial water within the foreshore sand on the beach. On 10 August 2005, samples of interstitial water were collected from the shore at six locations (sites 1, 5, 6, 7, 8, and 9 in Fig. 1). Two sampling holes were excavated with a shovel to the water table at each sampling station as described below. The lakeside edge of the first hole was approximately 25 cm from the furthest landward extent of the swash zone. For this study, the swash zone was defined as the portion of the beach face that is alternately being covered by incoming waves and exposed by the backrush. The edge of the second hole was 1 m from the shoreside edge of the first hole. The average diameter of each hole was 40 cm. A total of 13 interstitial water samples were collected and analyzed for E. coli on the following day as described below. Subsequently, on 31 August 2005, 12 holes were excavated along the beach (sites 1 to 12, excluding 12A, in Fig. 1), all approximately 25 cm from the furthest landward extent of the observed swash zone. Finally, as part of this component, samples were collected in the same manner on 23 December 2005 after the nearshore part of the lake had frozen and the beach was under snow. These samples were collected from sites 1, 3, 5, and 6 (Fig. 1). Observations of shorebirds and algae were noted during each sampling excursion.
The final component was a comparison of E. coli isolates obtained from interstitial water in holes excavated along the beach. To accomplish this, more isolates were collected from each sampling location on 4 July 2006. Four sampling holes at sites 9, 11, 12A, and 12 (Fig. 1) were excavated, as described below, at 25 cm from the furthest landward extent of the observed swash zone, and interstitial water was collected. Samples were analyzed for E. coli within 24 h as described below. Twenty-four colonies from each sample were isolated, confirmed as E. coli, and subjected to REP-PCR analysis as described below. Resulting DNA fingerprints of each isolate were compared for similarity to observe genetic diversity among isolates from the same sampling location.
For all components, each sampling station had its own unique identifier and coordinates determined by global positioning system.
Sample collection.
Surface water (lake and river) samples were collected in sterile 300-ml bottles, leaving at least a 2.5-cm air space in each bottle (17). Lake sediments were collected with a mini-Ponar dredge, which was disinfected with ethanol and allowed to air dry between samples, and placed into sterile Whirl-Pak bags with a sterile plastic scoop (18). Interstitial water was collected by first excavating a hole on the beach with a disinfected shovel. The shovel was disinfected with alcohol and allowed to air dry between sampling holes. Care was taken to ensure that minimal sediment particles were included in the water. In the event that a hole collapsed, it was abandoned and a new hole was excavated. Exposed interstitial water was collected from the holes into a sterile 300-ml bottle. Sediments were collected from the bottom of holes with sterile plastic scoops and placed into sterile Whirl-Pak bags with a sterile plastic scoop (18). Sediment and surface and interstitial water samples from all sampling locations were transported to the laboratory on ice at a temperature below 10°C and analyzed for E. coli within 24 h.
Isolation of E. coli from water and sediment samples.
Sediment and surface and interstitial water samples were analyzed for E. coli as previously described (17, 18). Surface and interstitial water samples were passed through a sterile 47-mm diameter cellulose ester disk filter (average pore size, 0.45 μm; PALL Life Sciences, Mississauga, Ontario, Canada). Filters were placed on mFC-BCIG agar plates (Difco, Sparks, MD) containing 10.0 g of tryptose, 5.0 g of proteose peptone, 3. 0 g of yeast extract, 1.5 g of bile salts, 5.0 g of sodium chloride, 0.1 g of BCIG (5-bromo-4-chloro-3-indoxyl-β-d-glucuronide), and 15.0 g of agar per liter and incubated at 44.5 ± 0.5°C for 24 ± 2 h. mFC-BCIG medium allowed the selection of colonies that have β-galactosidase and β-glucuronidase activities. β-Glucuronidase activity, which is specific for E. coli among members of the thermotolerant coliform group, was assessed by the conversion of BCIG and the production of a blue color. Blue colonies (putative E. coli) were picked and restreaked on BHI agar (EMD Chemicals, Gibbstown, NJ) for isolated colonies. Individual isolates were confirmed as E. coli on ChromCult agar (Merck, Darmstadt, Germany), which, in addition to confirming β-galactosidase and β-glucuronidase activities, contains tryptophan, which improves the indole reaction. All isolates were positive for indole formation upon addition of Kovacs' indole reagent to a colony. The isolates were stored at −20°C on Microbank beads (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada) in accordance with the manufacturer's instructions for future analysis.
In total, isolates from 300 water and 96 sediment samples (five colonies from each sample or fewer if five were not available) were confirmed as E. coli on mFC-BCIG agar. The isolates were stored at −20°C on Microbank beads (Pro-Lab Diagnostics, Richmond Hill, Ontario, Canada) as already described.
REP-PCR DNA fingerprinting.
Genomic DNA from individual pure cultures of E. coli isolates was extracted by the procedures of Dombek et al. (7) and Sambrook and Russell (22). Cells were suspended in 200 μl of Tris-EDTA lysis buffer with proteinase K (0.5 mg/ml) and lysed for 1 h at 37°C, followed by incubation for 10 min at 80°C. Cell debris was pelleted by centrifugation for 10 min at 10,000 × g, and 1 μl of supernatant was used for PCR amplification with the BOX1AR primer (5′-CTACGGCAAGGCGACGCTGACG-3′) (7). Amplification was performed with a PCR thermal cycler (Barloworld Scientific) with the following program: 35 cycles of 94°C for 20 s, 60°C for 20 s, and 65°C for 5 min with initial denaturation at 94°C for 2 min and a final extension at 65°C for 5 min. PCR products were separated on a 1% agarose gel in Tris-acetate-EDTA buffer and visualized under UV transillumination after staining with ethidium bromide. A 100-bp (100 to 3,000 bp) DNA ladder (Fermentas, Burlington, Ontario, Canada) was used as the standard. Gel images were captured and stored electronically with GeneSnap software (Syngene, Cambridge, United Kingdom).
REP-PCR fingerprint analysis was performed with Bionumerics v 3.5 software (Applied Maths, Sint-Martens-Latem, Belgium). The dendrograms for fingerprint similarities were generated by the unweighted-pair group method using average linkages clustering algorithm.
RESULTS
E. coli concentrations in interstitial water on the beach.
In early August 2005, it was observed that the concentrations of E. coli at the beach increased 24-fold from 3 to 4 August (based on Huron County Health Unit beach monitoring on 3 and 4 August; unpublished data). The only visible change in the conditions observed at the beach was a higher intensity of the wave action on the shore on 4 August. Water samples taken on 4 August from Eighteen Mile River, a tributary discharging into the lake adjacent to Ashfield Township Park beach, suggested that there was little input of E. coli from the river (16 to 50 CFU/100 ml). On 3 August, E. coli was not detected in any samples taken up to 3 km offshore, and the counts were below the Ontario Provincial water quality objective of 100 CFU/100 ml (19) near shore. Sediment samples taken at a 1-m water depth also contained low concentrations of E. coli (2 to 22 CFU/g, wet weight). These results implied that there was little input of E. coli from either the river or sediment from the lake bottom.
The results showed the highest concentration of E. coli in the interstitial water from the beach itself and not in the lake water or sediment adjacent to the beach (Table 1). Interstitial water was collected 1 week after surface water from the lake. These sampling dates were compiled together in Table 1 to show overall trends recognizing the potential for changes in environmental conditions over the intervening time. However, the water level in Eighteen Mile River was stable over the period, suggesting little change in the watershed upstream from the shoreline.
TABLE 1.
E. coli concentrations on the beach on 10 August 2005a
| Site |
E. coli concn in:
|
|||||
|---|---|---|---|---|---|---|
| Sampling hole 1
|
Sampling hole 2
|
Lake
|
||||
| Water (CFU/100 ml) | Sediment (CFU/g) | Water (CFU/100 ml) | Sediment (CFU/g) | Water (CFU/100 ml) | Sediment (CFU/g) | |
| 1 | 8,800 | 4,712 | 1,700 | 5 | 68 | 22 |
| 5 | 300 | <1 | 40 | 1 | 58 | 2 |
| 6 | 780 | 3 | 2,400 | 4 | 76 | NDb |
| 7 | 600,000 | 5,207 | 5,900 | 31 | 220 | 4 |
| 8 | 400,000 | 1,905 | 100,000 | 372 | 180 | ND |
| 9 | 210,000 | 1,428 | 9,000 | 42 | 110 | 16 |
| Geometric mean | 21,678 | 242 | 3,088 | 15 | 105 | 6 |
Lake water and sediment samples were collected on 2 and 3 August, respectively.
ND, not determined (sediment was not collected because of the rocky lake bottom).
There were variations in E. coli counts in interstitial water between sampling locations. E. coli counts were high at sites where numerous seagulls and Canada geese were observed on the day of sampling. E. coli counts were generally higher in interstitial water than in the lake water samples. All sampling holes closest to the lake consistently contained higher E. coli concentrations than the holes 1 m from the lake.
On 31 August 2005, only one hole per site was sampled at each location and more locations were chosen to cover more distance across the beach. Results from these samples (Table 2) were similar to 10 August 2005 in that E. coli concentrations were higher in the foreshore sand and interstitial water than in the lake. On 31 August 2005, there was no direct discharge from the river to the lake because of a sand bar across the mouth of the river. E. coli levels in the river were low preceding the sampling of the shoreline holes on 30 August 2005. In the river directly upstream from the lake, there were 14 and 50 CFU/100 ml and 500 m further upstream there were 180 CFU/100 ml. Surface drains to the shoreline, which can be sources of E. coli contamination, were visually monitored, and no surface water discharge was observed during periodic surveys in August 2005. There were no shorebirds observed on 31 August 2005.
TABLE 2.
E. coli concentrations on the beach on 31 August 2005a
| Site |
E. coli concn in:
|
|||
|---|---|---|---|---|
| Sampling holes
|
Lake
|
|||
| Water (CFU/100 ml) | Sediment (CFU/g) | Water (CFU/100 ml) | Sediment (CFU/g) | |
| 1 | 40,000 | 1,169 | 110 | 9 |
| 2 | 420,000 | 1 | 140 | NDb |
| 3 | 11,000 | 45 | 140 | ND |
| 4 | 180,000 | 89 | 120 | 1 |
| 5 | 9,400 | 51 | 120 | 4 |
| 6 | 240,000 | 537 | 160 | 1 |
| 7 | 2,900 | 15 | 220 | 12 |
| 8 | 600 | 5 | 170 | 1 |
| 9 | 100 | 1 | 240 | 1 |
| 10 | 400,000 | 919 | 240 | 1 |
| 11 | 700 | 1 | 280 | ND |
| 12 | 550,000 | 577 | 320 | 8 |
| Geometric mean | 18,849 | 36 | 177 | 2 |
Lake water samples were collected on 30 August, and lake sediment was collected on 1 September.
ND, not determined (sediment was not collected because of the rocky lake bottom).
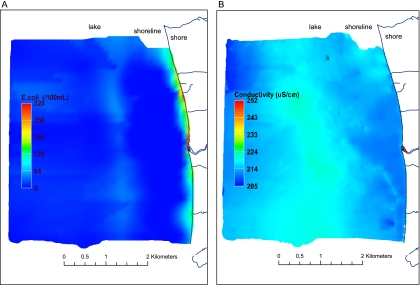
Concurrent with lake water sampling, we measured the electrical conductivity of lake water. Conductivity can be measured in a field setting and is frequently used to identify mixing of water sources with differing ionic compositions by determining spatial patterns in conductivity over an area of mixing. Mapping of lake water conductivity (Fig. 2B) in the field on 30 August 2005 indicated a negligible influence of runoff from the shoreline on water quality. However, the levels of E. coli in lake water samples taken along the immediate shoreline were elevated (up to 320 CFU/100 ml) on 30 August (Fig. 2A). Levels of E. coli in surface sediment from the bed of the lake were low (0 to 12 CFU/g, wet weight), as observed earlier in August.
FIG. 2.
(A) Surface map of E. coli levels in lake water. (B) Surface map of conductivity of lake water over the Eighteen Mile River study area on 30 August 2005.
During the winter sampling on 23 December 2005, E. coli levels in interstitial water in the sand ranged from 40 to 2.6 × 103 CFU/100 ml. The lake was frozen, and there was 50 cm of snow on the ground. There were no shorebirds or any wildlife observed.
Concentrations of E. coli in interstitial water in the summer of 2006 were comparable to summer 2005 results and ranged from 260 to 4.8 × 105 CFU/100 ml. No shorebirds were observed on the beach during sampling.
REP-PCR DNA fingerprinting of E. coli isolated from interstitial water on the beach.
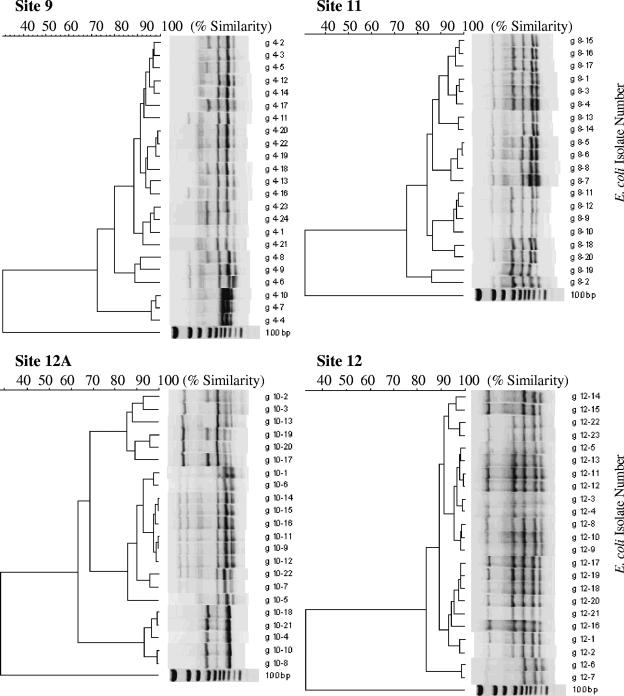
E. coli isolated from the same sampling location frequently exhibited the same REP-PCR pattern or were highly similar to each other. Dendrogram analysis revealed that many of the isolates from the same hole were more than 90% similar (Fig. 3). Each hole contained clusters of isolates with fingerprints exhibiting 90% or greater similarity. Sites 9, 11, and 12A had three major clusters of isolates with greater than 90% similarity to each other, and at site 12 there were two major clusters of isolates with greater than 90% similarity (Fig. 3). The dominant strain for each hole accounted for between 40 and 56% of the total number of isolates from each sampling hole. Greater than 85% similarity was observed between clusters of DNA fingerprints of isolates from the same hole. The levels of similarity between isolates taken from different sampling holes ranged from 65 to 85% (data not shown), which demonstrated that different locations on the beach have different sets of prevalent E. coli strains.
FIG. 3.
Dendrogram of REP-PCR DNA fingerprints of E. coli isolates obtained from interstitial water. The code beside each fingerprint on the right-side y axis is the unique identifier of each isolate. The bottom lane of each dendrogram shows the 100-bp molecular size markers. The scale on the top x axis shows percent similarity between the isolates as determined by Bionumerics software.
DISCUSSION
Field site characteristics.
Ashfield Township Park beach is located on the southeastern shore of Lake Huron and has an ongoing problem of periodically high E. coli counts in the lake water during the summer season. In August 2005, there was infrequent rainfall and little apparent runoff from the shoreline to the lake in the study area. Microbiological water quality measurements at the beach in August 2005 occasionally exceeded the Ontario Provincial water quality objective of 100 CFU E. coli/100 ml (19). A contributing source of indicator bacteria may have been the beach sand which is resuspended because of increased wave action on the shore during windy weather. Palmateer and Huber (20) reported that indicator bacterial levels in Lake Huron are higher when wave action is higher than normal.
The porosity of the matrix has an important role in the adaptation of bacteria to the subsurface environment. Gounot (9) summarized the studies in the field and reported that in aquifers, bacteria are generally present in higher concentrations in sandy sediments than in sediments rich in clay. Ashfield Township Park beach is a sand dune beach.
Abundance and distribution of E. coli bacteria in beach sand.
Foreshore sand and interstitial water consistently contained higher counts of E. coli than lake water samples at adjacent sites. Counts of E. coli were considerably higher in the presence of shore birds. The high counts on the shore, even at sites not apparently influenced by birds, suggested that E. coli survival is prolonged in this environment. These results were supported by winter sampling of the interstitial waters on the beach when E. coli counts were 40 to 2.6 × 103 CFU/100 ml of interstitial water.
The elevated E. coli concentrations observed in nearshore lake water on 30 August 2005 may have been due in part to water erosion and resuspension of material from the shore itself. Spring and event runoff from upstream in combination with sources along the shoreline may inoculate foreshore sand, allowing the persistence of E. coli in and near the lake for undetermined periods over the summer. The presence of E. coli in interstitial water on 23 December 2005 suggests that some strains of E. coli survived winter conditions. E. coli associated with the foreshore sand may have survived under the local conditions over a longer period of time.
Genetic relatedness of E. coli isolates as assessed by REP-PCR fingerprinting.
REP-PCR has been used to characterize the genetic relatedness of E. coli isolates from environmental samples (6). REP-PCR analysis of E. coli found in interstitial water on the beach revealed that sampling holes contained dominant strains with multiple isolates exhibiting identical (100%) or highly similar (greater than 90%) DNA fingerprints. Different clusters of dominant strains from the same sampling hole were also closely related (greater than 85%). This may have resulted from deposition of E. coli from a single source. There were no rain or storm events prior to the sampling of 4 July 2006. There was no observed runoff from surface drains to the shoreline. E. coli counts in the river water were low (46 CFU/100 ml), and the river was disconnected from the lake by a sand bar. The lack of rainfall and observable runoff suggests that there was no recent input of bacteria prior to sampling. Furthermore, genetic diversity of E. coli directly from fecal sources is known to be broad, necessitating the construction of large libraries for microbial source tracking undertakings (4). Contamination from a single fecal source would likely result in greater diversity between isolates.
Other possible reasons for the presence of related strains are the growth and survival of E. coli in the environment. Survival of E. coli has been reported in tropical-zone soil (5) and temperate soils (13). It was reported that some E. coli strains were more persistent than others in waters and sediments in the subtropics (3). The abundance of E. coli in interstitial water during the winter supports these findings. A recent study by Alm et al. (1) demonstrated the growth of E. coli in shoreline sand under both laboratory and field conditions. Our results support these findings. The presence of dominant isolates from interstitial water may reflect the ability of some strains to persist and multiply in wet sand and interstitial water. Each sampled site had certain prevalent strains that were distinct from isolates from different sites on a beach (with similarities as low as 65%). Environmental replication may produce localized clusters of highly similar strains that differ from strains from a different location.
The interstitial water of the beach is characterized by a constant interaction between the beach sand and water. This interaction may create an environment that allows survival and growth of E. coli on the beach. It is possible that only some strains adapt and preferentially survive in this ecological niche. The mechanisms of such survival under less-than-optimal conditions are poorly understood. There is a need to assess the survival and overwintering of E. coli strains in the environment.
The E. coli isolates collected may be further characterized in the laboratory and used in research to investigate the physiology of strains that survive in the beach environment. Various features such as motility (9) and the ability to live in low-temperature and/or low-nutrient environments (11) are possibly involved in bacterial adaptation to life in interstitial water of the sandy beach. These and other characteristics may reveal possible mechanisms of E. coli survival outside of the intestinal tract of an animal.
E. coli is commonly used as an indicator to assess recreational-water quality worldwide (19, 24, 25, 27). The presence of E. coli at the beach in the absence of obvious fecal input confounds water quality analysis and public health decisions. Improved understanding of E. coli survival in a beach environment will provide information on microbial ecology which may allow more accurate decisions to be formed regarding public health.
Acknowledgments
We gratefully acknowledge financial support by the Best in Science Program of the Ontario Ministry of the Environment. J.T.T. and H.L. acknowledge infrastructure and equipment support from the Canadian Foundation for Innovation and the Ontario Innovation Trust.
We also thank Ontario Ministry of Environment Microbiology staff Rhonda Schop, Paul Vyse, Matthew Butchart, Janet Chow, Lilijana Lukic, and Svetlana Dermicheva for membrane filtration and enumeration of E. coli bacteria in lake samples; summer students Faye Randle and Sheila Rono for helping us to analyze the samples; and a dedicated field crew of the Environmental Monitoring and Reporting Branch, Greg Hobson, John Thibeau, Wendy Page, Emil Bandelj, Dave Supper, and Lance Boyce, for support of this project and sampling the lake throughout the study period.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Alm, E. W., J. Burke, and E. Hagan. 2006. Persistence and potential growth of the fecal indicator bacteria, Escherichia coli, in shoreline sand at Lake Huron. J. Great Lakes Res. 32:401-405. [Google Scholar]
- 2.Alm, E. W., J. Burke, and A. Spain. 2003. Fecal indicator bacteria are abundant in wet sand at freshwater beaches. Water Res. 37:2978-2982. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, K. L., J. E. Whitlock, and V. J. Hardwood. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, M. A., J. E. Whitlock, and V. J. Hardwood. 2006. Diversity and distribution of Escherichia coli genotypes and antibiotic resistance phenotypes in feces of humans, cattle, and horses. Appl. Environ. Microbiol. 72:6914-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byappanahalli, M. N., and R. S. Fujioka. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171-174. [Google Scholar]
- 6.Byappanahalli, M. N., D. A. Shively, M. B. Nevers, M. J. Sadowsky, and R. L. Whitman. 2003. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 46:203-211. [DOI] [PubMed] [Google Scholar]
- 7.Dombek, P. E., L. K. Johnson, S. T. Zimmerley, and M. J. Sadowsky. 2000. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 66:2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George, I., A. Anzil, and P. Servais. 2004. Quantification of fecal coliform inputs to aquatic systems through soil leaching. Water Res. 38:611-618. [DOI] [PubMed] [Google Scholar]
- 9.Gounot, A. M. 1994. Microbial ecology of ground waters, 189-215. In J. Gibert, D. L. Danielopol, and J. A. Stanford (ed.), Groundwater ecology. Academic Press, San Diego, CA.
- 10.Howell, T., S. Abernathy, M. Charleton, A. Crowe, T. Edge, H. House, C. Lofranco, J. Milne, P. Scharfe, R. Steele, S. Sweeney, S. Watson, S. Weir, A. M. Weselan, and M. Veliz. 2005. Sources and mechanisms of delivery of E. coli (bacteria) pollution to the Lake Huron shoreline of Huron County. A report from the Lake Huron Science Committee to the Ontario Ministry of the Environment. http://www.ene.gov.on.ca/envision/techdocs/5077e.pdf.
- 11.Ihssen, J., and T. Egli. 2005. Global physiological analysis of carbon- and energy-limited growing Escherichia coli confirms a high degree of catabolic flexibility and preparedness for mixed substrate utilization. Environ. Microbiol. 10:1568-1581. [DOI] [PubMed] [Google Scholar]
- 12.Ishii, S., W. B. Ksoll, D. L. Hansen, R. E. Hicks, and M. J. Sadowsky. 2006. Source tracking of Escherichia coli at the Duluth Boat Club Beach: near-shore sand acts as a temporal source and sink of this fecal indicator bacterium, abstr. Q-213, p. 508. In Abstr. 106th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 13.Ishii, S., W. B. Ksoll, R. E. Hicks, and M. J. Sadowsky. 2006. Presence and growth of naturalized Escherichia coli in temperate soils from Lake Superior watersheds. Appl. Environ. Microbiol. 72:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLellan, S. L., and A. K. Salmore. 2003. Evidence for localized bacterial loading as the cause of chronic beach closings in a freshwater marina. Water Res. 37:2700-2708. [DOI] [PubMed] [Google Scholar]
- 15.McLellan, S. L., L. D. Daniels, and A. K. Salmore. 2003. Genetic characterization of Escherichia coli populations from host sources of fecal pollution by using DNA fingerprinting. Appl. Environ. Microbiol. 69:2587-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ontario Ministry of the Environment. 2004. MFMICRO-E3371. A membrane filtration method for the detection and enumeration of total coliform, Escherichia coli, Pseudomonas aeruginosa and fecal streptococci in environmental samples. Laboratory Services Branch, Quality Management Unit, Etobicoke, Ontario, Canada.
- 18.Ontario Ministry of the Environment. 2004. MICROBIO-E3433. Isolation, detection and enumeration of Escherichia coli in biosolids. Laboratory Services Branch, Quality Management Unit, Etobicoke, Ontario, Canada.
- 19.Ontario Ministry of the Environment and Energy. 1994. Water management, policies, guidelines, provincial water quality objectives of the Ministry of the Environment and Energy. Publication no. 3303. Ontario Ministry of the Environment and Energy, Toronto, Ontario, Canada.
- 20.Palmateer, G., and D. Huber. 1984. Lake Huron beaches—factors affecting microbiological water quality in 1984 summary report. Southwest Region, Ontario Ministry of the Environment, London, Ontario, Canada.
- 21.Saini, R., L. J. Halverson, and J. C. Lorimor. 2003. Rainfall timing and frequency influence on leaching of Escherichia coli RS2G through soil following manure application. J. Environ. Qual. 32:1865-1872. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Statistics Canada. 2001. Agricultural census report. Statistics Canada, Ottawa, Ontario, Canada.
- 24.U.S. Environmental Protection Agency. 2003. Bacterial water quality standards for recreational waters (freshwater and marine) status report. EPA-823-R-03-008. U.S. Environmental Protection Agency, Washington, DC.
- 25.U.S. Environmental Protection Agency. 2000. Improved enumeration methods for the recreational water quality indicators: enterococci and Escherichia coli. EPA/821/R-97/004. Office of Science and Technology, U.S. Environmental Protection Agency, Washington, DC.
- 26.Whitman, R. L., and M. B. Nevers. 2003. Foreshore sand as a source of Escherichia coli in nearshore water of a Lake Michigan beach. Appl. Environ. Microbiol. 69:5555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. 2003. Guidelines for safe recreational water environments, volume 1, coastal and fresh waters. World Health Organization, Geneva, Switzerland.