Abstract
Conversion of lignocellulosic feedstocks to ethanol requires microorganisms that effectively ferment both hexose and pentose sugars. Towards this goal, recombinant organisms have been developed in which heterologous genes were added to platform organisms such as Saccharomyces cerevisiae, Zymomonas mobilis, and Escherichia coli. Using a novel approach that relies only on native enzymes, we have developed a homoethanologenic alternative, Escherichia coli strain SE2378. This mutant ferments glucose or xylose to ethanol with a yield of 82% under anaerobic conditions. An essential mutation in this mutant was mapped within the pdh operon (pdhR aceEF lpd), which encodes components of the pyruvate dehydrogenase complex. Anaerobic ethanol production by this mutant is apparently the result of a novel pathway that combines the activities of pyruvate dehydrogenase (typically active during aerobic, oxidative metabolism) with the fermentative alcohol dehydrogenase.
Ethanol is an important, renewable transportation fuel that currently replaces a part of our automotive fuel (12, 30). Although ethanol is currently produced in the United States by fermenting glucose from corn starch using Saccharomyces cerevisiae (2), expanding this process to produce a large fraction of our fuel requirement may adversely impact the food and feed industry. Cellulosic biomass is an attractive alternative feedstock that can be fermented to ethanol after appropriate pretreatment without impacting the food and feed supply (31, 32). In contrast to corn starch, biomass contains significant amount of pentose sugars that are recalcitrant to fermentation by Saccharomyces cerevisiae. Ethanologenic Escherichia coli strains containing the pdc and adh genes from Zymomonas mobilis ferment both hexoses and pentoses to ethanol at a high rate and yield (11). Genetic engineering of S. cerevisiae and Z. mobilis by adding genes for pentose utilization has yet to produce a biocatalyst that matches the pentose fermentation characteristics of recombinant ethanologenic E. coli (14, 22). However, the use of a recombinant organism for large-scale fuel production is perceived by some as a barrier to commercialization.
In this report, we describe the development of an ethanologenic E. coli mutant that is devoid of foreign genes. This mutant effectively ferments hexose and pentose sugars to ethanol and represents a new alternative to recombinant biocatalysts for fuel ethanol production, especially from pentoses.
MATERIALS AND METHODS
Bacterial strains.
E. coli K-12 strain W3110 (ATCC 27325) was used as the parent for construction of the ethanologenic strain. The genotypes of the strains used in this study are listed in Table 1. Strain SE2378 is an ethanologenic mutant of strain AH242. Deletion of the genes pflB, adhE, and aceF was done as described by Datsenko et al. (6). An ldhA mutant was constructed after introduction of transposon Tn10 into the ldhA gene followed by selection for fusaric acid resistance (13, 18). Construction of other strains utilized standard genetic and molecular biology techniques (19, 21).
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| W3110 | Wild type | ATCC 27325 |
| AH240 | Δ(focA-pflB)-FRT-Km-FRT | This study |
| AH241 | Δ(ldhA) | This study |
| AH242 | Δ(ldhA) Δ(focA-pflB)-FRT-Km-FRT | This study |
| SE2378 | AH242, anaerobic growth positive | This study |
| YK1 | SE2378; Kms | This study |
| YK29 | AH242; Kms | This study |
| YK87 | YK1; Δ(adhE)-FRT-Km-FRT | This study |
| YK93 | YK1; Δ(aceF)-FRT-Km-FRT | This study |
| YK152 | YK29; Δ(aceF)-FRT-Km-FRT | This study |
| YK153 | W3110; Δ(aceF)-FRT-Km-FRT | This study |
| YK157 | YK152; aceF+ (W3110) | YK152 × P1(W3110) |
| YK158 | YK152; aceF+ (SE2378) | YK152 × P1(SE2378) |
Growth medium and fermentation.
Rich medium (L broth) contained the following (per liter): Trypticase peptone, 10 g; yeast extract, 5 g; and NaCl, 5 g (16). Mineral salts medium was described previously (16). Glucose or xylose was added at the indicated concentrations. Fermentations were conducted at 37°C (10). Broth was maintained at pH 7.0 by automatic addition of KOH. Batch fermentations were conducted in 13- by 100-mm screw-cap tubes filled to the top as previously described (23).
Analytical methods.
Sugars and fermentation products were determined by high-performance liquid chromatography (29). Pyruvate decarboxylase activity was measured in disrupted cell preparations as described previously (27).
Materials.
Inorganic salts, organic chemicals, and medium components were obtained from either Fisher Scientific (Pittsburgh, PA) or Sigma Chemical Co. (St. Louis, MO). Corn steep liquor was purchased from the Grain Processing Corp., Muscatine, IA.
RESULTS AND DISCUSSION
Isolation of ethanologenic E. coli strain SE2378.
Strain AH242 is incapable of anaerobic growth in rich medium containing sugars (Table 2) due to mutations in ldhA and pflB, encoding lactate dehydrogenase (LDH) and pyruvate formate-lyase (PFL), that prevent oxidation of NADH to NAD+ (20), an essential substrate for the key glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase and associated ATP production (4). The absence of LDH eliminated NADH oxidation by the reduction of pyruvate to lactate. Due to the PFL mutation, insufficient acetyl coenzyme A (acetyl-CoA) is available for effective NADH oxidation by native aldehyde and alcohol dehydrogenase activities encoded by adhE. Aerobic growth of strain AH242 was unaffected (Table 2).
TABLE 2.
Growth characteristics of E. coli mutants with mutations in anaerobic pathways
| Strain | Genotype | Specific growth rate (h−1) in mediuma:
|
|||
|---|---|---|---|---|---|
| Aerobic
|
Anaerobic
|
||||
| LB | Minimal | LBG | Minimal | ||
| W3110 | Wild type | 1.31 | 0.97 | 0.98 | 0.51 |
| AH240 | pflB | 1.23 | 0.99 | 0.79 | 0.17 |
| AH241 | ldhA | 1.35 | 0.94 | 0.81 | 0.30 |
| AH242 | pflB ldhA | 1.21 | 0.97 | NG | NG |
| SE2378 | pflB ldhA Ana+ | 1.18 | 0.56 | 0.46 | (0.10; 0.16) |
LB, L broth; Minimal, glucose-minimal medium; LBG, LB plus glucose (1% [wt/vol]). The concentrations of glucose in minimal medium were 0.3% for aerobic growth and 1% (wt/vol) for anaerobic growth. The first value in parentheses represents the growth rate in glucose-minimal medium with acetate (1 mg ml−1), and the second value is the growth rate in the same medium with glutamate added (1 mg ml−1). NG, no growth.
After mutagenesis with ethyl methane sulfonate (5), mutants of AH242 were recovered that grew anaerobically in rich medium with glucose (1%). Thirty-one independent mutants were tested for anaerobic growth and fermentation products. All mutants produced ethanol as the major fermentation product. One mutant, designated SE2378, was selected for further study. Aerobic growth of SE2378 was comparable to that of the wild type E. coli strain W3110 or any of the single or double mutants affected in PFL or LDH when cultured in rich medium (Table 2). In glucose-minimal medium, the aerobic growth rate of SE2378 was only about 50% of the growth rate of the parent, AH242.
Strain SE2378 grew anaerobically in rich medium, but the growth rate was only about 50% of that of AH240 and AH241 (Table 2). However, strain SE2378 did not grow anaerobically in glucose-minimal medium. In contrast to the pflB mutant (AH240) that grew in minimal medium with acetate, the ethanologenic derivative, SE2378, required both acetate and glutamate for comparable anaerobic growth in glucose-minimal medium. Previous studies from our laboratory have shown that ethanologenic E. coli strain KO11 also required glutamate for optimum fermentation of xylose (28). This glutamate requirement by KO11 in 9% xylose medium could be replaced by the addition of the protective osmolyte betaine to the medium. However, the glutamate requirement for anaerobic growth of SE2378 in minimal medium (1% sugar) was not suppressed by betaine, consistent with a biosynthetic deficiency due to limited acetyl-CoA flux to 2-ketoglutarate (precursor of glutamate) rather than an osmotic requirement. Apparently, the high rate of conversion of acetyl-CoA to ethanol by this ethanologenic mutant limited the availability of acetyl-CoA for biosynthesis. With these supplements, the growth rate of SE2378 in minimal medium was equivalent to that of the pflB parent, AH240. Corn steep liquor, a low-cost medium supplement, replaced glutamate for growth of SE2378 in glucose-minimal medium.
Glucose fermentation.
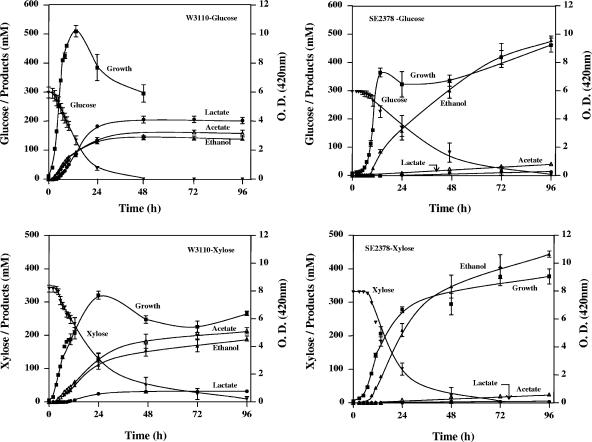
In pH-controlled fermentations with 50 g liter−1 glucose (278 mM), SE2378 grew with a specific growth rate of 0.46 h−1 after a lag of about 6 h and produced ethanol as the primary product (Fig. 1 and Tables 3 and 4). Since the immediate parent, AH242, did not grow anaerobically, the fermentation of SE2378 was compared to that of W3110. Strain W3110 completely fermented the added glucose in about 24 h, producing acetate, ethanol, lactate, formate, and succinate. The ethanologenic mutant SE2378 required about 72 h to ferment the same amount of glucose. This difference in fermentation time could be a result of cell density differences (2.5 mg dry weight ml−1 for the wild type versus 1.7 mg dry weight ml−1 for the mutant). Strain SE2378 produced about 480 mmol liter−1 ethanol (22 g liter−1), 88% of the total products, which included small amounts of acetate, lactate, and succinate. This is in contrast to strain W3110 fermentations, in which ethanol represented only 27% of the products. The maximum specific ethanol productivity (qP) observed for strain SE2378 was 1.34 g h−1 g cells−1 (Table 4), comparable to the value of 1.6 g h−1 g cells−1 reported for batch fermentations with S. cerevisiae (26).
FIG. 1.
Growth and fermentation characteristics of E. coli wild-type strain W3110 and ethanologenic mutant SE2378 in LB medium with glucose or xylose (50 g liter−1) at 37°C and pH 7.0. O.D., optical density.
TABLE 3.
Fermentation characteristics of E. coli mutant strain SE2378 and wild-type strain W3110a
| Strain | Concn of sugar consumed (mM) | Concn of product (mM)
|
Ethanol yieldb | ||||
|---|---|---|---|---|---|---|---|
| Ethanol | Acetate | Formate | Lactate | Succinate | |||
| Glucose fermentation | |||||||
| W3110 | 298 ± 19 | 142 ± 6 | 162 ± 6 | 206 ± 11 | 206 ± 11 | 18 ± 0.7 | 0.24 ± 0.01 |
| SE2378 | 296 ± 4 | 478 ± 15 | 27 ± 2 | 0 | 13 ± 2 | 27 ± 2 | 0.81 ± 0.02 |
| Xylose fermentation | |||||||
| W3110 | 333 ± 8 | 191 ± 7 | 215 ± 10 | 248 ± 53 | 32 ± 3 | 57 ± 1 | 0.34 ± 0.00 |
| SE2378 | 325 ± 2 | 444 ± 9 | 25 ± 2 | 0 | 0 | 33 ± 5 | 0.82 ± 0.01 |
Fermentations were conducted in L broth supplemented with 50 g liter−1 sugar at pH 7.0 and 37°C.
Ethanol yield is shown as a fraction of the theoretical maximum (0.51 g ethanol/g sugar).
TABLE 4.
Growth and ethanol production by E. coli strain SE2378 grown on glucose or xylosea
| Fermentation by strain | μmax | YX/S | QS | QP | YP/S | qS | qP |
|---|---|---|---|---|---|---|---|
| W3110 | |||||||
| Glucose | 0.44 | 0.04 | 2.94 | 0.50 | 0.12 | 4.10 | 0.49 |
| Xylose | 0.37 | 0.04 | 1.58 | 0.36 | 0.18 | 4.93 | 0.89 |
| SE2378 | |||||||
| Glucose | 0.46 | 0.04 | 1.29 | 0.61 | 0.41 | 3.26 | 1.34 |
| Xylose | 0.38 | 0.04 | 1.65 | 0.53 | 0.42 | 5.33 | 2.24 |
Glucose and xylose fermentations by strains W3110 and SE2378 in L broth are presented in Table 3 and Fig. 1. The reported values are calculated maximum values. μmax, specific growth rate h−1; YX/S, g cells g substrate−1; QS, g sugar consumed liter−1 h−1; QP, g ethanol produced liter−1 h−1; YP/S, g ethanol g substrate−1; qS, g sugar consumed g cell dry weight−1 h−1; qP, g ethanol produced g cell dry weight−1 h−1.
Xylose fermentation.
The wild-type strain W3110 and mutant strain SE2378 grew at similar rates during anaerobic fermentation with 50 g liter−1 xylose (333 mM), although strain SE2378 lagged by approximately 8 h (Fig. 1; Table 4). Specific growth rates on xylose were 80% of those with glucose (Table 4), consistent with previous reports (9). Surprisingly, SE2378 fermented xylose more rapidly than the prototrophic W3110 strain (Fig. 1). Approximately 88% of the fermentation products produced by SE2378 was ethanol. The maximum specific productivity of ethanol for SE2378 with xylose of 2.24 g h−1 g cells−1 was higher than that with glucose, in agreement with the higher rate of fermentation of xylose than glucose (qP; Table 4).
It is interesting to note that the specific ethanol productivities of both W3110 and SE2378 were higher with xylose than with glucose, reflecting the higher xylose consumption rate (qS; Table 4). This may reflect the lower energy yield from xylose metabolism (10). For the wild type, the net ATP yield from xylose is only about 1.5 per xylose, compared to 3.0 per glucose due to the ATP requirement for each of the following three steps in xylose metabolism to pyruvate: xylose transport, phosphorylation of xylulose and fructose-6-phosphate. This would require that the cells utilize more xylose to produce the same amount of cell mass. However, the specific rate of xylose consumption by the wild type was only slightly higher than that of glucose (qS, 4.93 versus 4.10 g h−1 g cells−1) (Table 4) accounting for the lower growth rate, cell yield, and longer fermentation time compared to glucose fermentation (Fig. 1). In contrast, SE2378 lacks pyruvate formate-lyase, an enzyme critical for xylose fermentation in minimal medium (10). Although SE2378 produced a small amount of acetate during xylose fermentation, this acetate was produced during the nongrowth, fermentation phase and thus may not be contributing to the overall energetics of the cell. In this ethanologenic mutant, this lower ATP yield from xylose may be compensated for by an increase in xylose flux. The specific productivity of ethanol from xylose of 2.24 g h−1 g cells−1 is higher than the value of 1.6 g h−1 g cells−1 reported for S. cerevisiae on glucose (26) and comparable to the values for glucose or xylose in the ethanologenic E. coli strain KO11 carrying the Z. mobilis pdc and adh genes (about 2 g h−1 g cells−1) (unpublished data).
Pathway for homoethanologenic fermentation using native E. coli genes.
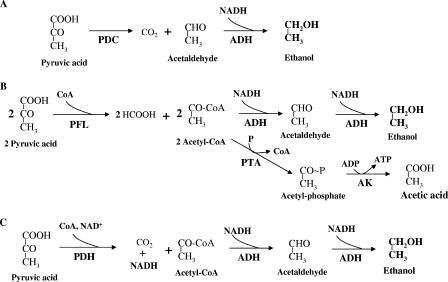
The enzymes of glycolysis convert each mol of glucose into 2 mol of pyruvate plus 2 mol of NADH and a net of 2 mol of ATP. The production of compounds more reduced than pyruvate (ethanol, lactate, etc.) serves as a mechanism to oxidize NADH and regenerate NAD+, essential for glycolysis. In the only known homoethanol pathway that evolved in yeast, plants, and bacteria (i.e., Z. mobilis), pyruvate is decarboxylated to yield carbon dioxide and acetaldehyde by the nonoxidative pyruvate decarboxylase. The resulting acetaldehyde serves as the electron acceptor for NADH oxidation by alcohol dehydrogenase during production of one ethanol from each pyruvate (Fig. 2A). Z. mobilis genes encoding these activities have been used previously to engineer homoethanol pathways in recombinant ethanologenic bacteria (11).
FIG. 2.
Proposed pathway for ethanol production from pyruvate in E. coli SE2378, native E. coli, and other ethanologenic microorganisms. (A) Pathway for ethanologenic organisms (yeast, Z. mobilis, and recombinant ethanologenic E. coli). (B) Native pathway for ethanol production in E. coli. (C) Proposed pathway for ethanol production in E. coli strain SE2378. PDC, pyruvate decarboxylase; ADH, alcohol dehydrogenase E; PFL, pyruvate formate-lyase; PTA, phosphotransacetylase; AK, acetate kinase; PDH, pyruvate dehydrogenase.
A completely different ethanol pathway exists in E. coli in which pyruvate is converted to acetyl-CoA and formate by pyruvate formate-lyase in which reducing equivalents are contained in the formate and dissipated as hydrogen gas (and CO2) by formate hydrogen-lyase. One acetyl-CoA is the electron acceptor for the oxidation of two NADH by adhE-encoded aldehyde-alcohol dehydrogenase (Fig. 2B). Due to the requirement of 2 NADH per ethanol, the second acetyl-CoA from glycolysis of glucose is converted to acetate and an additional ATP. Thus, the native E. coli pathway for ethanol from acetyl-CoA cannot support homoethanol fermentation due to the need for 2 NADH per ethanol produced. Redox balance is preserved by an equal amount of acetate production (Table 3).
Strain AH242 and the ethanologenic derivative SE2378 carry a deletion mutation in pflB and are thus incapable of producing formate and acetyl-CoA from pyruvate, blocking the native route for ethanol production. The production of ethanol as the primary fermentation product by strain SE2378 may have resulted from a mutation or mutations that activated expression of a silent unknown gene whose product has catalytic activity resembling that of pyruvate decarboxylase. However, pyruvate decarboxylase activity was not detected in the extracts of anaerobically grown SE2378. Analysis of the E. coli genome sequence also failed to reveal coding regions resembling pyruvate decarboxylase. A second alternative is the mutational activation of a silent pyruvate formate-lyase gene (pflCD) (1) restoring production of acetyl-CoA. The ethanol yield for SE2378 of 0.8 per pyruvate with an ethanol/acetate ratio of 18 to 1 and the absence of formate in the broth (Table 3) do not support the presence of pyruvate formate-lyase activity in this ethanologen.
A third alternative is the activation of pyruvate dehydrogenase (PDH), an enzyme that normally functions during aerobic growth while pyruvate formate-lyase is inactive. By metabolizing pyruvate with PDH, an additional NADH per pyruvate is made available that can be used to fully reduce each acetyl-CoA to ethanol (Fig. 2C), consistent with the observed high ethanol yield and low acetate/ethanol ratio for strain SE2378. Although genes coding for pyruvate dehydrogenase are typically expressed under both aerobic and anaerobic conditions in E. coli, the activity of this complex during anaerobic growth has been reported to be very low (3, 8). This lack of activity in the anaerobic cell is proposed to result from an inhibition of PDH activity by both NADH and pyruvate (7, 25). It is likely that mutations increasing the activity of this enzyme complex under anaerobic conditions have occurred to produce a homoethanol pathway in SE2378. Since 6 mol of xylose is metabolized to 10 mol of pyruvate and 10 mol of NADH, analogous arguments and yields can be directly applied to the homoethanol pathway used for pentose sugars in this mutant.
Pyruvate dehydrogenase is essential for homoethanol production.
Preliminary genetic analysis of SE2378 revealed that the mutation or mutations responsible for anaerobic growth and homoethanol production by the ldhA pflB double mutant are located in or near the genes coding for PDH complex (pdh locus: pdhR aceEF lpd) (24). To confirm that PDH is required for the ethanologenic phenotype of SE2378, a mutation in the aceF gene (dihydrolipoyl acetyltransferase; E2 enzyme of PDH) was transduced into SE2378 (strain YK152). Anaerobic growth of strain YK152 was defective in all of the media tested (Table 5). The aceF mutation in strain YK152 was transduced to aceF+ by phage P1 with the gene from either W3110 (wild type) or SE2378 (ethanologen), and the transductants were selected for growth in minimal medium under aerobic conditions. Transductants that received the aceF+ gene from the wild-type strain, W3110, grew aerobically in minimal medium but failed to grow anaerobically in any of the media tested due to the presence of ldhA and pflB mutations. On the other hand, all transductants receiving the aceF+ gene from strain SE2378 grew anaerobically and all of the tested transductants produced ethanol as the main fermentation product. Ethanol accounted for about 90% of the total fermentation products produced by YK158 grown in L broth (LB)-glucose medium (data not presented), a value that is similar to that of SE2378 (Table 3). These results show that the ethanologenic phenotype of SE2378 requires PDH activity and are in agreement with the proposed PDH-dependent pathway (Fig. 2C) for ethanol production.
TABLE 5.
Growth characteristics of ethanologenic E. coli strain SE2378 with a mutation in the pdh locus
| Strain | Genotype | Specific growth rate (h−1) on mediuma:
|
|||
|---|---|---|---|---|---|
| Aerobic
|
Anaerobic
|
||||
| LB | Minimal | LB | Minimal | ||
| W3110 | Wild type | 1.31 | 1.05 | 0.98 | 0.51 |
| YK153 | W3110; aceF | 0.46 | NG (0.55) | 1.07 | 0.44 |
| YK152 | pflB ldhA aceF | 0.83 | NG (0.54) | NG | NG |
| YK157 | YK152; aceF+ (W3110) | 1.32 | 0.96 | NG | NG |
| YK158 | YK152; aceF+ (SE2378) | 1.17 | 0.51 | 0.45 | NGb |
Minimal, glucose-minimal medium. Values in parentheses represent the specific growth rate in glucose-minimal medium with acetate (1 mg ml−1) under aerobic conditions. NG, no growth.
In this new pathway for homoethanol production, pyruvate is oxidatively decarboxylated to acetyl-CoA with the conservation of reductant as NADH, allowing both the reduction of acetyl-CoA to acetaldehyde and the reduction of acetaldehyde to ethanol by alcohol dehydrogenase E containing both aldehyde and alcohol dehydrogenase activities (Fig. 2C). Deletion of either aceF (PDH negative; YK93), required for acetyl-CoA production, or adhE (ADH negative; YK87), needed for ethanol production, resulted in loss of anaerobic growth, supporting the essential role of both activities for anaerobic growth of SE2378 lacking fermentative lactate dehydrogenase and pyruvate formate-lyase.
Similar conservation of reductant as NADH during pyruvate decarboxylation to acetyl-CoA has been observed in the ethanologenic anaerobes Thermoanaerobium brockii and Thermoanaerobacter ethanolicus (formerly Clostridium thermohydrosulfuricum). In these anaerobes, pyruvate-ferredoxin oxidoreductase produces acetyl-CoA and reduced ferredoxin from pyruvate. Reductant from the reduced ferredoxin is channeled to NAD+ by ferredoxin-NADH oxidoreductase. Combination of these two enzyme activities produces the needed second NADH for reduction of acetyl-CoA to ethanol (15, 17).
In conclusion, an E. coli mutant has been developed that produces ethanol as the primary fermentation product from both glucose and xylose using only the native genetic repertoire of the E. coli chromosome. The rate of fermentation of xylose by the mutant was higher than that of glucose fermentation, with a specific ethanol productivity comparable to those of other ethanologenic organisms on glucose or xylose. The putative metabolic pathway for conversion of pyruvate to ethanol in this strain involves the pyruvate dehydrogenase complex and alcohol dehydrogenase E instead of pyruvate decarboxylase and alcohol dehydrogenase typical of other homoethanologenic organisms such as S. cerevisiae, Z. mobilis, and recombinant ethanologenic bacteria. Further metabolic engineering is expected to optimize this pathway towards the development of a nonrecombinant ethanologen that can ferment all of the sugars in lignocellulosic biomass. Development of this type of nonrecombinant bacterial biocatalyst may reduce one of the perceived barriers to commercial ethanol production from lignocellulosic substrates.
Acknowledgments
We thank Lee Ann Blalock for performing pyruvate decarboxylase assays.
This work was supported by a grant from the Department of Energy (DE-FG36-04GO14019).
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 2.Bothast, R. J., and M. A. Schlicher. 2005. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 67:19-25. [DOI] [PubMed] [Google Scholar]
- 3.Cassey, B., J. R. Guest, and M. M. Attwood. 1998. Environmental control of pyruvate dehydrogenase complex expression in Escherichia coli. FEMS Microbiol. Lett. 159:325-329. [DOI] [PubMed] [Google Scholar]
- 4.Clark, D. P. 1989. The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 5:223-234. [DOI] [PubMed] [Google Scholar]
- 5.Close, T. J., and K. T. Shanmugam. 1980. Genetic analysis of a pleiotropic mutant of Klebsiella pneumoniae affected in nitrogen metabolism. J. Gen. Microbiol. 116:501-510. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta, A. 1991. Characterization of the inhibition of Escherichia coli pyruvate dehydrogenase complex by pyruvate. Biochem. Biophys. Res. Commun. 176:517-521. [DOI] [PubMed] [Google Scholar]
- 8.de Graef, M. R., S. Alexeeva, J. L. Snoep, and M. J. Teixeira de Mattos. 1999. The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J. Bacteriol. 181:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez, R., H. Tao, K. T. Shanmugam, S. W. York, and L. O. Ingram. 2002. Global gene expression differences associated with changes in glycolytic flux and growth rate in Escherichia coli during fermentation of glucose and xylose. Biotechnol. Prog. 18:6-20. [DOI] [PubMed] [Google Scholar]
- 10.Hasona, A., Y. Kim, F. G. Healy, L. O. Ingram, and K. T. Shanmugam. 2004. Pyruvate formate lyase and acetate kinase are essential for anaerobic growth of Escherichia coli on xylose. J. Bacteriol. 186:7593-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingram, L. O., H. C. Aldrich, A. C. Borges, T. B. Causey, A. Martinez, F. Morales, A. Saleh, S. A. Underwood, L. P. Yomano, S. W. York, J. Zaldivar, and S. Zhou. 1999. Enteric bacterial catalysts for fuel ethanol production. Biotechnol. Prog. 15:855-866. [DOI] [PubMed] [Google Scholar]
- 12.Kheshgi, H. S., R. C. Prince, and G. Marland. 2000. The potential of biomass fuels in the context of global climate change: focus on transportation fuels. Annu. Rev. Energy Env. 25:199-244. [Google Scholar]
- 13.Kleckner, N., J. Bender, and S. Gottesman. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204:139-180. [DOI] [PubMed] [Google Scholar]
- 14.Kuyper, M., M. J. Toirkens, J. A. Diderich, A. A. Winkler, J. P. van Dijken, and J. T. Pronk. 2005. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 5:925-934. [DOI] [PubMed] [Google Scholar]
- 15.Lamed, R., and J. G. Zeikus. 1980. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J. Bacteriol. 144:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, J. H., P. Patel, P. Sankar, and K. T. Shanmugam. 1985. Isolation and characterization of mutant strains of Escherichia coli altered in H2 metabolism. J. Bacteriol. 162:344-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovitt, R. W., G.-J. Shen, and J. G. Zeikus. 1988. Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J. Bacteriol. 170:2809-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloy, S., and W. D. Nunn. 1981. Selection for loss of tetracycline resistance by Escherichia coli. J. Bacteriol. 145:1110-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 20.Mat-Jan, F., K. Y. Alam, and D. P. Clark. 1989. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J. Bacteriol. 171:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 22.Mohagheghi, A., N. Dowe, D. Schell, Y. Chou, C. Eddy, and M. Zhang. 2004. Performance of a newly developed integrant of Zymomonas mobilis for ethanol production on corn stover hydrolysate. Biotechnol. Lett. 26:321-325. [DOI] [PubMed] [Google Scholar]
- 23.Patel, M. A., M. S. Ou, R. Harbrucker, H. C. Aldrich, M. L. Buszko, L. O. Ingram, and K. T. Shanmugam. 2006. Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl. Environ. Microbiol. 72:3228-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quail, M. A., D. J. Hayden, and J. R. Guest. 1994. The pdhR-aceEF-lpd operon of Escherichia coli expresses the pyruvate dehydrogensae complex. Mol. Microbiol. 12:95-104. [DOI] [PubMed] [Google Scholar]
- 25.Shen, L. C., and D. E. Atkinson. 1970. Regulation of pyruvate dehydrogenase from Escherichia coli. Interactions of adenylate energy charge and other regulatory parameters. J. Biol. Chem. 245:5974-5978. [PubMed] [Google Scholar]
- 26.Smits, H. P., J. Hauf, S. Muller, T. J. Hobley, F. K. Zimmermann, B. Hahn-Hagerdal, J. Nielsen, and L. Olsson. 2000. Simultaneous overexpression of enzymes of the lower part of glycolysis can enhance the fermentative capacity of Saccharomyces cerevisiae. Yeast 16:1325-1334. [DOI] [PubMed] [Google Scholar]
- 27.Talarico, L. A., L. O. Ingram, and J. A. Maupin-Furlow. 2001. Production of the Gram-positive Sarcina ventriculi pyruvate decarboxylase in Escherichia coli. Microbiology 147:2425-2435. [DOI] [PubMed] [Google Scholar]
- 28.Underwood, S. A., M. L. Buszko, K. T. Shanmugam, and L. O. Ingram. 2004. Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 70:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underwood, S. A., S. Zhou, T. B. Causey, L. P. Yomano, K. T. Shanmugam, and L. O. Ingram. 2002. Genetic changes to optimize carbon partitioning between ethanol and biosynthesis in ethanologenic Escherichia coli. Appl. Environ. Microbiol. 68:6263-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wooley, R., M. Ruth, D. Glassner, and J. Sheehan. 1999. Process design and costing of bioethanol technology: a tool for determining the status and direction of research and development. Biotechnol. Prog. 15:794-803. [DOI] [PubMed] [Google Scholar]
- 31.Wyman, C. E. 2003. Potential synergies and challenges in refining cellulosic biomass to fuels, chemicals, and power. Biotechnol. Prog. 19:254-262. [DOI] [PubMed] [Google Scholar]
- 32.Zaldivar, J., J. Nielsen, and L. Olsson. 2001. Fuel ethanol production from lignocellulose: a challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 56:17-34. [DOI] [PubMed] [Google Scholar]