Abstract
Pseudomonas sp. strain 7-6, isolated from active sludge obtained from a wastewater facility, utilized a quaternary ammonium surfactant, n-dodecyltrimethylammonium chloride (DTAC), as its sole carbon, nitrogen, and energy source. When initially grown in the presence of 10 mM DTAC medium, the isolate was unable to degrade DTAC. The strain was cultivated in gradually increasing concentrations of the surfactant until continuous exposure led to high tolerance and biodegradation of the compound. Based on the identification of five metabolites by gas chromatography-mass spectrometry analysis, two possible pathways for DTAC metabolism were proposed. In pathway 1, DTAC is converted to lauric acid via n-dodecanal with the release of trimethylamine; in pathway 2, DTAC is converted to lauric acid via n-dodecyldimethylamine and then n-dodecanal with the release of dimethylamine. Among the identified metabolites, the strain precultivated on DTAC medium could utilize n-dodecanal and lauric acid as sole carbon sources and trimethylamine and dimethylamine as sole nitrogen sources, but it could not efficiently utilize n-dodecyldimethylamine. These results indicated pathway 1 is the main pathway for the degradation of DTAC.
Quaternary ammonium compounds (QACs) containing a long-chain alkyl group or a benzyl group are cationic surfactants that are widely used in several applications, including as antistatic agents, emulsifiers-dispersants, dye auxiliaries, surface treatment agents, osmotic agents, and hair rinses (8). QACs are also contained in synthetic detergents to reduce static electricity in clothing and improve fabric suppleness. In addition, the bactericidal and fungicidal properties of these compounds, as well as their ability to damage cell membranes and to denature cell proteins, have favored their widespread use in domestic cleaning products (1, 6, 7). Since most of the above-mentioned products are released into the environment through routine disposal wastewater, the accumulation and aquatic toxicity of quaternary-ammonium-based surfactants have been the focus of several studies (4, 20).
Several researchers have reported adaptation to QACs by aquatic organisms through their repeated exposure to these compounds (19) and the biodegradation of QACs by pure cultures of bacteria (18). McBain et al. showed that repeated exposure of pure cultures, especially Ralstonia sp., altered their susceptibility to QACs (11). In addition, a mixture of Pseudomonas sp. and Xanthomonas sp. isolated from soil and sewage grew well on medium containing decyltrimethylammonium salt as the sole carbon source. Xanthomonas sp. oxidized the terminal carbon of the alkyl chain of QAC (2). Pseudomonas sp. strain B1, isolated from activated sludge, grew well on hexadecyltrimethylammonium chloride (the C16 alkyl QAC in this report), using the compound as a carbon and energy source (17). However, strain B1 could not utilize the intermediate, trimethylamine, as a nitrogen source. Although these findings indicate the metabolic fates of QACs in an aquatic environment, little is known about the complete metabolic steps necessary for QACs for mineralization by pure cultures.
The present study examined the adaptation of strain 7-6, isolated from active sludge, to high concentrations of n-dodecyltrimethylammonium chloride (DTAC). Dual pathways for the metabolism of DTAC were proposed based on identification of the main metabolites by gas chromatography-mass spectrometry (GC-MS) analysis and utilization of the identified compounds for growth. In the isolate, combination of the major and miner metabolic pathways for DTAC results in effective metabolism of the surfactant.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
DTAC-assimilating bacteria were enriched from wastewater samples from effluent treatment facilities in Osaka and Hyogo Prefecture. Basal medium (DTAC medium) containing 0.005% (wt/vol) DTAC and 0.05% (wt/vol) NH4NO3 was used for the isolation. Solutions A and B were prepared separately and mixed under sterile conditions to yield the DTAC medium. Solution A contained 0.02 g of KH2PO4, 1.04 g of K2HPO4, 0.1 g of NaCl, 0.04 g of yeast extract, 0.01 g DTAC, 0.10 g of NH4NO3, and deionized water in a total volume of 170 ml, with the final pH adjusted to 7.0. Solution B contained 1 g of MgSO4 · 7H2O, 1 mg each of CaCl2 · 2H2O, CuSO4 · 5H2O, ZnCl2, and FeSO4 · 7H2O, and deionized water to total volume of 30 ml. Solution A was sterilized by filtration, and solution B was sterilized by autoclaving. The bacteria in the present study were cultivated in a test tube containing 7 ml of medium.
Sixteen bacteria able to degrade DTAC (final concentration, 0.05%) during growth were isolated. To allow the isolates to adapt to high concentrations of DTAC and to enhance their biodegradative capability, the concentration of DTAC in the basal medium was gradually increased in steps of 0.05% (wt/vol). After the isolate grew well and degraded the compound almost completely, the culture was inoculated with medium containing a much higher concentration of DTAC. Of the 16 isolates, strain 7-6 grew best on medium containing 0.4% (wt/vol) DTAC (15 mM) and 0.05% (wt/vol) NH4NO3 and completely degraded the surfactant in 2 days.
Identification of strain 7-6.
Strain 7-6 was identified according to morphological and biochemical criteria, such as the Gram reaction, flagellum type, catalase activity, oxidase activity, oxidation-fermentation test, and the production of diffusible fluorescent pigment, using methods described previously (5) and by analysis of the 16S rRNA gene, which was amplified following the methods of Edward et al. (3). The partial nucleotide sequence of the 16S rRNA gene of strain 7-6 reported here was deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB278070.
Isolation and quantification of the metabolites of DTAC.
Strain 7-6 was cultivated on DTAC medium containing 0.1% (wt/vol) DTAC (3.8 mM) as a sole carbon, nitrogen, and energy source and without NH4NO3. Fifty tubes were used, with cultures taken from five randomly chosen tubes every 7 h. After centrifugation, the supernatant of the culture was divided into four parts and processed as follows.
(i) The supernatant (3 ml) was adjusted to pH 3. The metabolites were extracted with 0.5 ml of ethyl acetate. The upper layer (sample 1) contained n-dodecanal, n-dodecyldimethylammonium, and lauric acid (see Fig. 4). These compounds were identified by GC and GC-MS as described below.
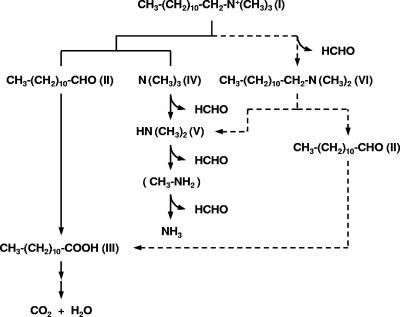
FIG. 4.
Proposed pathways of n-dodecyltrimethylammonium metabolism in Pseudomonas sp. strain 7-6. I, n-dodecyltrimethylammonium; II, n-dodecanal; III, lauric acid; IV, trimethylamine; V, dimethylamine; VI, n-dodecyldimethylamine.
(ii) The supernatant (3 ml) was concentrated with an evaporator and then adjusted to pH 11. The metabolites were extracted with toluene. The upper layer (sample 2) contained the metabolite trimethylamine (see Fig. 4), which was identified by GC and GC-MS as described below.
(iii) The supernatant (3 ml) was concentrated to dryness. The residues were dissolved in 3 ml of methanol containing 1.5 mg of pentafluorophenylhydrazine and 1 drop of 1 N HCl. After a 1-h incubation, the hydrazone derivative (sample 3) was analyzed by GC and GC-MS as described below.
(iv) The supernatant (10 ml) was mixed with 1 ml of benzenesulfonylchloride and 1 ml of 10 N NaOH, and the volume was brought up to 100 ml with distilled water. After a 30-min incubation, 5 ml of 10 N NaOH was added to the mixture, and the solution was incubated in boiling water for 30 min. The solution was adjusted to pH 7.0 and then concentrated to 20 ml with a rotary evaporator. The upper layer was concentrated to dryness, and the residue was dissolved in ethyl acetate. The ethyl acetate solution (sample 4) was analyzed by GC and GC-MS as described below.
Effects of various factors on growth and the degradation of DTAC.
Strain 7-6 was precultured in a test tube with 7 ml of DTAC medium containing 0.4% (wt/vol) DTAC (15 mM) and 0.05% (wt/vol) NH4NO3 at 30°C for 48 h with shaking. DTAC test medium containing 0.4% (wt/vol) DTAC (15 mM) and 0.05% (wt/vol) NH4NO3 was then inoculated with 150 μl of the preculture. The growth of strain 7-6 and the remaining concentration of DTAC were measured. All experiments were done in triplicate, and average values are shown.
Effects of aeration.
After 48 h of precultivation, 150 μl, 7 ml, or 21 ml of preculture was added to 7 ml (in test tube), 70 ml (in 500 ml-shaking flask), or 400 ml (in a 3-liter shaking flask), respectively, of fresh test medium.
Effects of carbon source.
The test medium also contained one of eight sugars (d-glucose, lactose, l-arabinose, d-mannose, d-galactose, fructose, sucrose, or maltose) or one of five organic acids (sodium acetate, disodium succinate, d,l-maleic acid, or trisodium citrate) as a supplement.
Effects of nitrogen source.
The test medium also contained sodium nitrate, ammonium chloride, urea, or one of 20 amino acids instead of ammonium nitrate.
Effects of the concentration of DTAC.
The test medium contained DTAC at a concentration ranging between 0.01% (wt/vol) (0.38 mM) and 3.0% (wt/vol) (114 mM).
Analytical methods.
QACs, including DTAC, were measured spectrophotometrically with Phloxine B (22). Metabolites of DTAC were analyzed by a Hitachi G-3900 gas chromatograph or a Hitachi M-2500 mass spectrometer with electron-impact ionization (70 eV) coupled to a Hitachi G-3000 gas chromatograph. A TC-1 fused silica capillary column (0.25 mm by 30 m; GL Science, Tokyo, Japan) was used. Helium gas was the carrier at a linear velocity of 35 ml/min. The column temperature was held at 125°C for 1 min and was then increased stepwise from 125 to 240°C at a rate of 3°C/min. Total carbon in the culture supernatant and in the cells was measured by using the modified Walkley-Black method (13). NH4+ in the culture fluid was measured by the indophenol blue method (21). Total nitrogen in the culture supernatant and the cells was determined by Kjeldahl digestion and steam distillation according to the instructions provided by the manufacturer (BÜCHI Labortechnik AG, Zurich, Switzerland). In this procedure, the amino nitrogen of organic materials and free ammonia are converted to NH4+ by Kjeldahl digestion unit K-424 (BÜCHI Labortechnik AG). The converted NH4+ is distilled from the digested solution and absorbed in boric acid solution by using distillation unit K-342 (BÜCHI Labortechnik AG). NH4+ was potentiometrically measured by pH and a conductivity combination meter HM-25R (DKK-TOA Corp., Tokyo, Japan). The dry weight of the bacteria was measured as follows: washed cells from the culture (1 ml) were dried in an oven at 105°C for 5 h, and the weight of the dried biomass was determined.
Chemicals.
QACs (see Table 1) including DTAC (molecular weight = 263.89) were purchased from Tokyo Kasie Kogyo (Tokyo, Japan). Phloxine B was from Nakarai Tesque (Kyoto, Japan). Benzenesulfonylchloride was from Wako Pure Chemicals (Osaka, Japan).
TABLE 1.
Growth of Pseudomonas sp. strain 7-6 on QACs
| Concn (mM) | Growth (OD660), % degradation (cultivation time in days) on growth substratea:
|
||||||
|---|---|---|---|---|---|---|---|
| C6 | C8 | C10 | C12 | C14 | C16 | C18 | |
| 5 | 0.31, 20 (12) | 0 | 1.9, 100 (8) | 0.99, 100 (8) | |||
| 10 | 0.45, 17 (12) | 0 | 3.2, 100 (12) | 3.9, 100 (2) | 2.5, 100 (10) | ||
| 25 | 0.57, 22 (18) | 0 | 3.3, 50 (18) | 4.8, 100 (5) | 4.4, 100 (6) | 5.9, 100 (7) | 2.6, 100 (12) |
| 50 | 0 | 0 | 4.9, 100 (5) | 4.4, 100 (10) | 6.0, 100 (12) | 2.0, 64 (18) | |
| 100 | 0 | 0 | 3.2, 30 (14) | 4.9, 24 (14) | 5.8, 33 (14) | 0 | |
| 200 | 2.4, 12 (14) | 3.6, 8 (14) | 4.5, 20 (14) | ||||
| 400 | 2.5, 8 (14) | 2.2, 4 (10) | 4.2, 36 (14) | ||||
| 600 | 1.2, 1.5 (14) | 1.3, 1.0 (10) | 0 | ||||
| 800 | 0 | 0 | |||||
The growth substrate specificity of strain 7-6 was examined using QACs with a long-chain alkyl group (CnH2n + 1), such as hexyltrimethylammonium chloride (C6), octyltrimethylammonium chloride (C8), decyltrimethylammonium chloride (C10), dodecyltrimethylammonium chloride (C12, DTAC), tetradecyltrimethylammonium chloride (C14), hexadecyltrimethylammonium chloride (C16), and octadecyltrimethyl ammonium chloride (C18). Each QAC was added to DTAC basal medium containing 0.05% (wt/vol) NH4NO3 instead of DTAC. The concentration of QAC was measured by using Phloxine B as described in the text.
RESULTS
Identification of strain 7-6.
Strain 7-6 is a motile rod of 0.6 to 1.2 by 1.5 to 5.0 μm with polar flagella. It is aerobic, gram negative, non-spore-forming, and catalase and oxidase positive. The bacterium produces acids oxidatively from d-glucose, arabinose, mannitol, and fructose and a green fluorescent pigment on King A, King B, and Pseudomonas P agar. The nucleotide sequence of the 16S rRNA gene of strain 7-6 (1,532 bp; accession number AB278070) was 98.9 and 99.5% identical to that of Pseudomonas fluorescens strain Pf-5 (CP000076) and Pseudomonas fluorescence strain CHA0 (AJ278812), respectively. Thus, strain 7-6 was assigned to the genus of Pseudomonas.
Adaptation to high concentrations of DTAC.
The nonacclimated isolates, including strain 7-6, retained the ability to grow on medium containing DTAC at concentrations ranging between 0.005 and 0.05% without frequent transfer to fresh DTAC medium; however, these strains could not grow on 0.1% DTAC medium. Strains maintained on nutrient agar medium for 1 month also could not grow on 0.1% DTAC medium. The strain was therefore adapted to high concentrations of DTAC as follows: after almost completely degrading DTAC during growth, the culture was transferred to fresh medium containing a higher concentration of the surfactant. The DTAC concentration of the fresh medium was increased by 0.05%, whenever the culture was transferred. This stabilization on higher-concentration DTAC medium was repeated at a concentration range between 0.1 and 0.4%. Subsequently, the strain grew well on basal medium containing 0.4% (wt/vol) DTAC, even if it had been maintained for 1 month on basal medium containing 0.1% (wt/vol) DTAC.
Assimilation of DTAC and total carbon and nitrogen in the culture.
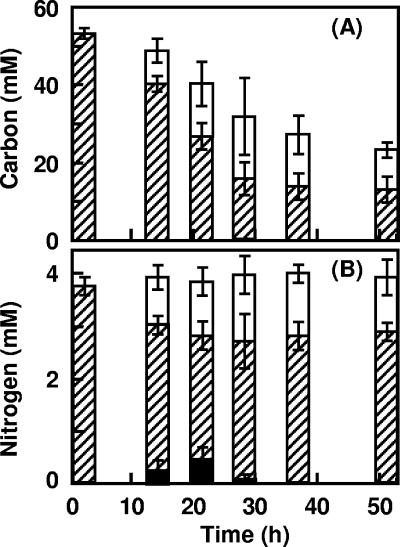
Strain 7-6 grew well on basal medium containing 0.1% (wt/vol) DTAC without NH4NO3 (Fig. 1A). Figure 2 shows the changes in total carbon and nitrogen in the supernatant of the culture and in the cells with an increasing number of bacterial cells reflecting the growth of strain 7-6 DTAC was almost completely utilized in 40 h. After 52 h of cultivation, 23% of the total carbon originally provided was detected in the culture supernatant and 18% of that was in the cells of the strain (Fig. 2A). Ammonium ion accumulated (Fig. 2B) but then disappeared. Approximately 30% of the total nitrogen originally provided was observed in the cells at 52 h of cultivation.
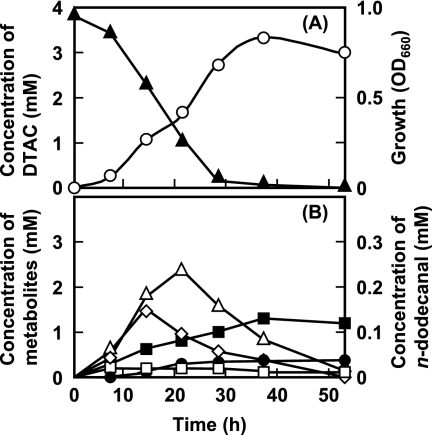
FIG. 1.
Growth of Pseudomonas sp. strain 7-6 on DTAC medium and metabolism of DTAC. (A) Strain 7-6 was cultivated on medium containing 0.1% (wt/vol) DTAC (3.8 mM) as the sole carbon, nitrogen, and energy source at 30°C with shaking. Growth was determined by measuring the optical density at 660 nm (OD600) (○); the residual DTAC (▴) was measured spectrophotometrically. (B) Intermediates derived from DTAC, dodecanal (⧫), n-dodecyldimethylamine (□), formaldehyde (•), lauric acid (▵), and trimethylamine (▪) were measured by GC. The analytical procedures are described in the text.
FIG. 2.
Analysis of total carbon and nitrogen in culture supernatants and cells of Pseudomonas sp. strain 7-6. Strain 7-6 was cultivated on DTAC medium containing 0.1% (wt/vol) DTAC (3.8 mM) as the sole carbon, nitrogen, and energy source (7 ml/test tube) at 30°C with shaking. After centrifugation, 14 ml of culture supernatant and the cells were divided and used for further analysis. (A) Total carbon in the supernatant (▨) and in the cells (□) was converted to d-glucose. (B) Total nitrogen in the supernatant (▨) and in the cells (□) was measured by using the Kjeldahl method. Ammonium (▪) released into the culture was measured by the indophenol blue method. Analytical procedures are described in the text.
Effects of various factors on the growth and degradation of DTAC.
Since DTAC is a surfactant, culture flasks incubated with shaking filled up with bubbles, especially at the beginning of cultivation and in cultures containing more than 0.1% (wt/vol) DTAC. The bubbles could have produced a semiaerobic condition in the culture, thereby inhibiting the growth of strain 7-6. To rule out this possibility, the effect of shaking on growth was examined by culturing strain 7-6 either in 7 ml of 0.1% (wt/vol) DTAC basal medium in a test tube (18 by 180 mm) or 70 ml of test medium in a 500-ml flask or in 400 ml of test medium in a 3-liter flask, with shaking. Culturing the bacterium in the test tube yielded reproducible results with respect to the growth curve and degradation of DTAC; thus, test tubes were used in all subsequent experiments.
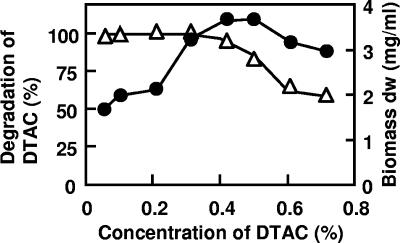
Strain 7-6 grew well in 0.4% (wt/vol) DTAC medium containing 0.02% (wt/vol) yeast extract and completely degraded the substrate in 48 h. Although the strain grew on 0.7% (wt/vol) DTAC medium, only 60% of the DTAC in the medium was degraded after 48 h (Fig. 3). The strain grew well and degraded DTAC completely at pH 7.0 to 7.5. At pH 6.5, 36% (wt/vol) of the DTAC remained after 72 h of cultivation. However, the strain was unable to grow at a pH of <6.0 and >8.5.
FIG. 3.
Effect of DTAC concentration on the growth of Pseudomonas sp. strain 7-6 and the degradation of DTAC. The test medium (7 ml in a test tube) contained DTAC at a concentration ranging between 0.01% (wt/vol) (0.38 mM) and 1.0% (wt/vol) (38 mM). Each culture was incubated until the growth of strain 7-6 reached stationary phase. Growth was determined by measuring the dry weight of the biomass (•); residual DTAC (▵) in the culture was measured spectrophotometrically.
Substrate specificity for growth.
Table 1 shows the QAC substrate specificity of strain 7-6 with respect to growth and the growth limit for each QAC. DTAC was the best growth substrate among QACs with a C6 to C18 alkyl group that were tested. The strain grew slightly on medium containing a QAC with a C6 alkyl group but could not degrade a QAC with a C8 alkyl group in a test medium containing a 1 mM concentration of the compound. In contrast, it grew well and degraded QACs with a C12, C16, or C14 alkyl group.
Identification of the metabolites from DTAC.
Metabolites derived from DTAC were isolated from the culture supernatant and analyzed by GC and GC-MS (Table 2). Sample 1 was analyzed by GC and GC-MS, which revealed major peaks at 5.2, 6.5, and 7.1 min. The mass spectra and the GC retention times of metabolites I, II, and III agreed with those of n-dodecanal, n-dodecyldimethylammonium, and lauric acid, respectively. Analysis of pentafluorophenylhydrazone-derivatized sample 2 showed one major peak at 3.8 min. The mass spectrum and retention time agreed with those of authentic pentafluorophenylhydrazone-derivatized formaldehyde. The mass spectrum and GC retention time of sample 3, containing metabolite V, agreed with those of trimethylamine. Benzensulfonyl derivatization of sample 4 and subsequent analysis showed that the mass spectrum and GC retention time of metabolite VI agreed with those of benzensulfonyl-derivatized dimethylamine.
TABLE 2.
Mass spectra of the metabolites from DTAC
| Metabolite
|
No. of fragments of the trimethylsilylated product (m/z assignment, percent relative intensity) | |
|---|---|---|
| No. | Name | |
| I | n-Dodecanal | 166 (M+-H2O, 4.1), 156 (M+-(CH2)×2, 5.4), 140 (M+-(CH2 = CH-OH), 13.2) |
| II | n-Dodecyldimethylamine | 213 (M+, 54.4), 198 (M+-CH3, 2.7), 128 (M+-)(CH3-(CH2)5, 4.1), 114 (M+-(CH3-(CH2)6, 3.5) |
| III | Lauric acid | 201 (M+, 5.4), 73 (M+-CH3-(CH2)5, 40.9), 45 (M+-(CH3-(CH2)11, 54.5) |
| IV | Formaldehydea | 210 (M+, 100), 182 (M+-N = CH2, 50) |
| V | Trimethylamine | 58 (M+-H, 19.1), 43 (M+-H-CH3, 47.7) |
| VI | Dimethylamineb | 185 (M+, 100), 141 (M+-N(CH3)2, 61.0), 125 (M+-N(CH3)2-O, 72.3) |
Fragments of pentafluorophenylhydrazone derivative.
Fragments of benzenesulfonylated derivative.
Strain 7-6 almost completely degraded DTAC during 28 h of growth (Fig. 1A). The metabolites lauric acid and n-dodecanal accumulated and were metabolized during stationary phase (Fig. 1B). In contrast, trimethylamine and formaldehyde accumulated with the degradation of DTAC. Although dimethylamine was detected until the exponential-growth phase, residual dimethylamine could not be accurately measured and the data were therefore omitted from Fig. 1B.
Assimilation of the identified metabolites.
Strain 7-6 was cultivated on basal medium containing the identified metabolites as sole carbon or nitrogen sources instead of DTAC (Table 3). The strain could utilize n-dodecanal and lauric acid as carbon and energy sources and trimethylamine and dimethylamine as nitrogen sources. However, it grew poorly on test medium containing n-dodecyldimethylamine, degrading only 23% of the substrate.
TABLE 3.
Growth of strain 7-6 and degradation of identified metabolites
| Mediuma | Growth (OD660 at 1 day) | Degradation (%)b |
|---|---|---|
| Control 1 (DTAC) | 1.4 | 95.1 |
| Control 2 (yeast extract) | 0.25 | - |
| n-Dodecanal | 1.6 | 90.2 |
| Dodecyldimethylamine | 0.41 | 23.0 |
| Lauric acid | 1.4 | 97.6 |
| Trimethylamine | 1.2 | 11.0 |
| Dimethylamine | 1.4 | -c |
DTAC medium was used as a positive control (control 1). Another control consisting of the same medium but containing 0.02% (wt/vol) yeast extract without DTAC and NH4NO3 was also used. The identified intermediates n-dodecanal, dodecyldimethylamine, and lauric acid were added to DTAC medium containing 0.05% (wt/vol) NH4NO3 instead of DTAC as the sole carbon source. Trimethylamine or dimethylamine was added to DTAC medium containing 1% (wt/vol) d-glucose instead of DTAC and NH4NO3 as the sole nitrogen source. After 1 day of cultivation, the concentration of each substrate was measured by using GC as described in the text. Growth was measured as the OD660.
Initial concentration, 5 mM.
Residual dimethylamine was omitted because it could not be accurately measured.
DISCUSSION
Pseudomonas sp. strain 7-6 was isolated from an enrichment culture inoculated with activated sludge and DTAC. Although its ability to tolerate the surfactant was easily lost, tolerance was restored by recurrent exposure of the bacterium to DTAC. Consequently, the bacterium grew well on 50 mM DTAC medium and was able to completely degrade the compound. This resulted in successful preparation of the metabolic intermediates of DTAC in strain 7-6. Efflux pump proteins that transport a range of structurally dissimilar compounds, including several antibiotics, are present in antibiotic-susceptible and antibiotic-resistant bacteria (15). Bacteria therefore have an innately low susceptibility to most biocides, and this property can be enhanced by continuous exposure to these compounds.
Adaptation to QACs can be enhanced, for example, by continuous exposure. Regular exposure to QACs of gram-positive bacteria, such as Staphylococcus aureus, brings about their adaptation and resistance to these chemicals (16). Gram-negative bacteria, such as Pseudomonas spp., can adapt to sanitizers used in food-processing industries (9, 10). Langsrud et al. suggested that Pseudomonas spp. can adapt to survival in the presence of high concentrations of QACs. Pseudomonas aeruginosa strain PFRB, isolated from a stock solution of benzalkonium chloride, was exposed to QACs and gradually showed high-level resistance to hexadecyltrimethylammonium bromide (11 mM) (the C16 alkyl QAC in this report) (12). A strain used for disinfectants testing, Pseudomonas aeruginosa ATCC 15422, and Pseudomonas spp. isolated from cold-stored chicken developed tolerance to high concentrations of QACs after several rounds of reinoculations in Mueller-Hinton broth containing gradually higher amounts of QACs (10). The tested strains, Pseudomonas fluorescens and Pseudomonas lundensis, grew on broth containing didecyldimethylammonium chloride (DDAC) (0.11 mM).
Strain 7-6 can degrade QACs containing C6-C18 of alkyl groups, except octyltrimethylammonium chloride (C8). The toxicity of QACs increases with increasing chain length of the alkyl moiety (6). The DDAC-degrading bacterium Pseudomonas fluorescens TN4 showed a very similar pattern regarding specificity of the growth substrate (14). Strain TN4 grew on basal medium containing C10-C16 QACs but not on basal medium containing C18 QACs. Strain 7-6 did not grow well on test medium containing short-chain QACs, such as hexyltrimethylammonium chloride (C6) and octyltrimethylammonium chloride (C8). Thus, the enzyme in the initial degradation step in which the C-N bond of QAC is cleaved may not be able to attack toward short-chain substrates.
Analyses of total carbon and nitrogen in the culture and identification of the metabolites from DTAC indicated that strain 7-6 readily utilizes DTAC as the sole carbon and nitrogen source. GC-MS analysis (Table 2) and bacterial utilization of the identified metabolites (Table 3) support our hypothesis of two pathways for DTAC metabolism in strain 7-6 (Fig. 4). Pathways 1 and 2 differ in the order in which groups are removed from DTAC. In pathway 1, DTAC (compound I, Fig. 4) was converted to lauric acid (compound III, Fig. 4) via n-dodecanal (compound II, Fig. 4), with the release of trimethylamine (compound IV, Fig. 4). In pathway 2, DTAC was converted to n-dodecyldimethylamine (compound VI, Fig. 4) with the release of formaldehyde. The metabolite was converted into n-dodecanal, with the release of dimethylamine (compound V, Fig. 4). Strain 7-6 can utilize n-dodecanal and lauric acid as a sole carbon sources and trimethylamine and dimethylamine as sole nitrogen sources (Table 3). Trimethylamine would be converted to ammonium via dimethylamine and methylamine. Strain NT4 also utilizes DDAC as the sole carbon source. However, Nishihara et al. suggested that the metabolite dimethylamine, from DDAC, is further metabolized by strain TN4 and/or evaporated from the medium due to its volatility (14). Xanthomonas sp. and Pseudomonas sp. strain B1 utilize QAC as a carbon and energy source but not as a nitrogen source (2, 17). To our knowledge, our report provides the first evidence of a pseudomonad that can utilize QAC as the sole carbon, nitrogen, and energy source. Strain 7-6, precultured on DTAC medium, was also able to utilize intermediates from DTAC as the sole carbon or nitrogen source. However, among the six substrates tested, including DTAC, the bacterium did not readily metabolize n-dodecyldimethylamine, suggesting that proposed pathway 1 is the main route for DTAC assimilation.
We attempted to obtain additional evidence for the conversion of DTAC. Unfortunately, cell extracts from strain 7-6 grown on DTAC medium did not always show activity toward the compound. GC analysis indicated that the extracts converted DTAC to n-dodecanal and trimethylamine in the presence of NAD(P)H, but the extract lost activity shortly after it had been prepared. Thus, it may be that DTAC denatures the enzymes involved in its metabolism. The enzyme in the initial step of DTAC metabolism is probably a monooxygenase, as reported by van Ginkel et al. (17). We are currently attempting to establish enzyme assay for DTAC metabolism.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Ahlstroem, B., M. Chelminska-Bertilsson, R. A. Thompson, and L. Edebo. 1997. Submicellar complexes may initiate the fungicidal effects of cationic amphiphilic compounds on Candida albicans. Antimicrob. Agents Chemother. 41:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean-Raymond, D., and M. Alexander. 1977. Bacterial metabolism of quaternary ammonium compounds. Appl. Environ. Microbiol. 33:1037-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edward, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S rRNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiom, S. J., J. R. Furr, A. D. Russell, and J. R. Dickinson. 1993. Effects of chlorhexidine diacetate and cetylpyridinium chloride on whole cells and protoplasts of Saccharomyces cerevisiae. Microbios 74:111-120. [PubMed] [Google Scholar]
- 5.Komagata, K. 1985. Aerobic bacteria, p. 99-160. In T. Hasegawa (ed.), Classification and identification of microorganism. Japan Scientific Societies Press, Tokyo, Japan.
- 6.Korai, H., and K. Takeichi. 1970. Antimicrobial activity of quaternary ammonium bromide. Hakko Kogaku Zasshi 48:635-640. [Google Scholar]
- 7.Kouma, H., S. Shibata, and T. Nagata. 2003. Preparation of quaternary ammonium salts having antibacterial and antifungal activity. Jpn. Kokai Tokkyo Koho patent JP 2003137706.
- 8.Kunisako, S. (ed.). 2006. Quaternary ammonium salt, p. 716-720. In 14906 manufactured chemical products. Chemical Daily, Tokyo, Japan.
- 9.Langsrud, S., and G. Sundheim. 1997. Factors contributing to the survival of poultry associated Pseudomonas spp. exposed to a quaternary ammonium compound. J. Appl. Microbiol. 82:705-712. [DOI] [PubMed] [Google Scholar]
- 10.Langsrud, S., G. Sundheiml, and R. B. Strahsen. 2003. Intrinsic and acquired resistance to quaternary ammonium compounds in food-related Pseudomonas spp. J. Appl. Microbiol. 95:874-882. [DOI] [PubMed] [Google Scholar]
- 11.McBain, A. J., R. G. Ledder, L. E. Moore, C. E. Catrenich, and P. Gilbert. 2004. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 70:3449-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai, K., T. Murata, S. Ohta, H. Zenda, M. Ohnishi, and T. Hyashi. 2003. Two different mechanisms are involved in the extremely high-level benzalkonium chloride resistance of a Pseudomonas fluorescens strain. Microbiol. Immunol. 47:709-715. [DOI] [PubMed] [Google Scholar]
- 13.Nelson, D. W., and L. E. Sommers. 1982. Total carbon, organic carbon, and organic matter, p. 539-579. In A. L. Page, R. H. Miller, and D. R. Keeney (ed.), Methods of soil analysis, part 2, 2nd ed. Agron. Monogr. 9. ASA, CSSA, and SSSA, Madison, WI.
- 14.Nishihara, T., T. Okamoto, and N. Nishiyama. 2000. Biodegradation of didecyldimethylammonium chloride by Pseudomonas fluorescens TN4 isolated from activated sludge. J. Appl. Microbiol. 88:641-647. [DOI] [PubMed] [Google Scholar]
- 15.Piddock, L. J. 2006. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 19:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundheim, G., T. Hagtvedt, and R. Dainty. 1992. Resistance of meat associated staphylococci to a quaternary ammonium compound. Food Microbiol. 9:161-167. [Google Scholar]
- 17.van Ginkel, C. G., J. B. van Dijk, and A. G. Kroon. 1992. Metabolism of hexadecyltrimethylammonium chloride in Pseudomonas strain B1. Appl. Environ. Microbiol. 58:3038-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventullo, R. M., and R. J. Larson. 1986. Adaptation of aquatic microbial communities to quaternary ammonium compounds. Appl. Environ. Microbiol. 51:356-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versteeg, D. J., and S. J. Shorter. 1992. Effect of organic carbon on the uptake and toxicity of quaternary ammonium compounds to the fathead minnow, Pimephales promelas. Environ. Toxicol. Chem. 11:571-580. [Google Scholar]
- 20.Vives-Rego, J., D. Vaqué, and J. Martinez. 1986. Effect of heavy metals and surfactants on glucose metabolism, thymidine incorporation, and exoproteolytic activity in sea water. Water Res. 20:1411-1415. [Google Scholar]
- 21.Weatherburn, M. W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39:971-974. [Google Scholar]
- 22.Yamamoto, K., and K. Kadono. 1999. Spectrophotometric determination of ionic surfactants on basis of a color change of fluorescein dyes with a quaternary ammonium ion. Bunseki Kagaku 48:87-93. [Google Scholar]