Abstract
Biofilm formation (BF) in the setting of candiduria has not been well studied. We determined BF and MIC to antifungals in Candida spp. isolates grown from urine samples of patients and performed a retrospective chart review to examine the correlation with risk factors. A total of 67 Candida spp. isolates were grown from urine samples from 55 patients. The species distribution was C. albicans (54%), C. glabrata (36%), and C. tropicalis (10%). BF varied greatly among individual Candida isolates but was stable in sequential isolates during chronic infection. BF also depended on the growth medium and especially in C. albicans was significantly enhanced in artificial urine (AU) compared to RPMI medium. In nine of the C. albicans strains BF was 4- to 10-fold higher in AU, whereas in three of the C. albicans strains and two of the C. glabrata strains higher BF was measured in RPMI medium than in AU. Determination of the MICs showed that planktonic cells of all strains were susceptible to amphotericin B (AMB) and caspofungin (CASPO) and that three of the C. glabrata strains and two of the C. albicans strains were resistant to fluconazole (FLU). In contrast, all biofilm-associated adherent cells were resistant to CASPO and FLU. The biofilms of 14 strains (28%) were sensitive to AMB (MIC50 of <1 μg/ml). Correlation between degree of BF and MIC of AMB was not seen in RPMI grown biofilms but was present when grown in AU. A retrospective chart review demonstrated no correlation of known risk factors of candiduria with BF in AU or RPMI. We conclude that BF is a stable characteristic of Candida strains that varies greatly among clinical strains and is dependent on the growth medium. Resistance to AMB is associated with higher BF in AU, which may represent the more physiologic medium to test BF. Future studies should address whether in vitro BF can predict treatment failure in vivo.
Candiduria is rare in otherwise healthy people (16) but relatively frequent in hospitalized patients. The clinical significance of candiduria is unclear (33, 34). Urinary tract infection (UTI) is the most common type of nosocomial infection (37), and 10 to 15% of UTIs are caused by Candida species (2). Candiduria is even more common in the setting of indwelling catheters. Although the majority of infections are caused by Candida albicans, C. glabrata is emerging as a nosocomial pathogen with a predilection for the urinary tract (17). Of concern is that candiduria is associated with higher mortality (20), especially in patients with comorbidities. Most studies report a relatively low percentage for concomitant candidemia in patients with candiduria. Hence, it has been suggested that candiduria represents a surrogate marker that is associated with, but not causative, of increased mortality (19). Alternatively, candiduria may reflect a relevant infection and appropriate aggressive treatment could improve the outcome. Treatment recommendations are largely based on expert opinion and anecdotal reports. A large prospective study (41-43) demonstrated that fluconazole (FLU) was effective for short-term eradication of candiduria. The precise reason for the inefficacy of therapy in long-term eradication has not been thoroughly investigated. Higher eradication rates were achieved with urinary catheter removal. Another study in renal transplant patients also did not demonstrate an effect of treatment on survival (35). Candida readily forms biofilms that attach to solid surfaces (23, 24). Biofilm formation (BF) can be affected by growth conditions and coinfection with other pathogens (11). In addition, several studies have demonstrated that BF can interfere with antifungal therapy (30, 32).
Although candiduria is associated with indwelling devices and low eradication rates with antifungal therapy, systematic studies that measure BF in urine Candida isolates and compare it to clinical data are lacking. To date, biofilm studies have been carried out primarily with laboratory strains, and some variability in BF among Candida isolates and species has been reported (22, 26). The goal of the present study was to broaden our understanding of the epidemiology and the natural history of candiduria. The BF of Candida isolates isolated from candiduric patients was compared in different media. In addition, the response of biofilms to antifungal agents and the effect of known risk factors on BF were assessed.
MATERIALS AND METHODS
Study population and study design.
The study was conducted from September 2004 to April 2005 at a teaching hospital of the Albert-Einstein College of Medicine in the Bronx, NY. During that time all urine samples positive for yeast on microscopy were sent that same day to a research laboratory for determination of the fungal burden (i.e., the CFU count) and species identification. No attempts were made to influence the physicians' responses to the report of urine culture or analysis, yielding yeast. The data on patient demographics, underlying diseases, therapy, and hospital outcome were collected by retrospective chart review, which was done according to Albert-Einstein College of Medicine internal review board regulations. A total of 137 urinalysis samples were obtained from 117 patients during this time. The distribution of isolates in the overall cohort was 46% C. albicans, 40% C. glabrata, 10% C. tropicalis, and 4% other species, including C. parapsilosis and C. dubliensis. BF was measured in a subgroup of these isolates. To avoid artifact from variation in storage conditions, we determined the BF for the latter half of this cohort because these strains were all frozen under identical conditions.
Candida strains and molecular strain characterization.
Urine samples were reexamined for the presence of bacteria and hyphae by microscopy at ×400 magnification in the research laboratory. Aliquots of urine were plated on Sabouraud agar plates to determine the CFU per milliliter of urine. Candida speciation was determined by plating the isolates on BBL CHROM-agar. In some unclear cases where this technique produced ambiguous results, the Candida isolates were speciated by VITEK using biochemical methods. To investigate the strain relatedness of sequential isolates, molecular typing was performed by sequencing of multiple PCR-amplified loci (multilocus sequence typing) as previously described (8, 44). For C. albicans the loci AAT1a, AAT1b, MP1, ZWF1, CaVPS13, CaADP1, CaSYA1, and CaRPN2 were amplified with published primers. For C. glabrata the loci FKS, LEU2, NMT1, TRP1, UGP1, and URA3 were amplified.
Measurement of BF.
BF was examined by adherence to polystyrene in pH-balanced RPMI and in artificial urine (AU). Streaked colonies were grown up overnight in SD broth and washed three times in phosphate-buffered saline (PBS). Cells were prepared at a concentration of 106/ml in RPMI and AU. AU has a pH of 5.8 and was slightly modified that described previously (7, 15); it was composed of CaCl2 (0.65 g/liter), MgCl2 (0.65 g/liter), NaCl (4.6 g/liter), Na2SO4 (2.3 g/liter), sodium citrate (0.65 g/liter), sodium oxalate (0.02 g/liter), KH2PO4 (2.8 g/liter), KCl (1.6 g/liter); NH4Cl (1.0 g/liter), urea (25.0 g/liter), creatinine (1.1 g/liter), 5% (vol/vol) YNB broth, and 8% dextrose (38). In selected experiments 3.25 and 32.5 μM nicotinic acid was added. A 100-μl volume of suspension was plated on 96-well plates (polystyrene, nontreated) and incubated for 48 h at 37°C for adherence and BF. The plates were washed three times manually with PBS to remove nonadherent cells. A semiqualitative measure of BF and viability was detected by an XTT [2,3-bis(2-methoxy-4nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide] reduction assay as described previously (31). Briefly, XTT at 0.5 g/liter was added with menadione (10 mM in acetone) to a final concentration of 1 μM in PBS. A 100-μl portion of the XTT-menadione suspension was added to each well, followed by incubation in the dark at 37°C for 2 h. Biofilm was observed by calorimetric changes in each well by determining the optical density at 490 nm. SC5314, a standard C. albicans strain, was used as a control strain in each experiment that allowed comparison of the BF between plates. We performed the measurement of BF in three to six wells per sample depending on the test.
Determination of the MIC50 in biofilm-associated and planktonic cells.
The MICs for amphotericin B (AMB), caspofungin (CASPO), and FLU were determined by microdilution method as described by Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) M27-A methodology (28) with RPMI 1640 buffered to pH 7 by using MOPS. As a quality control measure, the results were considered valid only when the MICs of the quality control isolates fell within the prespecified ranges: C. parapsilosis ATCC 22019 (FLU, 2 to 8 mg/liter) and C. krusei ATCC 6258 (FLU, 16 to 64 mg/liter). The MIC50 was determined as the lowest concentration that produced at least 50% inhibition compared to the growth of the control well for FLU and the absence of visible growth in the case of AMB. The turbidity was then compared both visually and spectrophotometrically.
For the measurement of in vitro antifungal susceptibility on biofilm, the biofilm was prepared for each cell population as described above. After a washing step, the biofilm was exposed to a range of antifungal concentration for FLU (1 to 1,024 μg/ml), CASPO (1 to 1,024 μg/ml), and AMB (0.125 to 32 μg/ml) for 48 h. After 48 h, the cells were washed with PBS three times, and an XTT reduction assay was performed as described above. The calorimetric reading at 490 nm was used to compare the reduction of growth relative to the control as a result of coincubation with antifungal drugs.
Statistical methods.
Chi-square, t tests, and linear regression were performed with SSPS version 11.0.3 (SSPS, Inc., Chicago, IL).
RESULTS
Characteristics of patients with candiduria.
BF was determined in 67 Candida isolates recovered from 55 patients, including 2 Candida isolates from blood and 10 sequential isolates from urine. The species distribution was as follows: C. albicans (54%), C. glabrata (36%), and C. tropicalis (10%). A retrospective chart review determined that the patients exhibited the expected standard risk factors associated with candiduria, including female sex, old age, diabetes, prior antibiotic use, and indwelling urinary tract catheters (Table 1).
TABLE 1.
Patient characteristicsa
| Variable | No. of patients (%) |
|---|---|
| Female patients | 40 (77.4) |
| Race | |
| White | 13 (36.1) |
| Black | 19 (43.6) |
| Hispanic | 16 (21.3) |
| Nursing home resident | 20 (36.3) |
| Dialysis | 6 (10.3) |
| Hospitalized within the last 6 mo | 26 (47.2) |
| Diabetes | 30 (54.5) |
| Foley catheter | 34 (61.8) |
| Antibiotic use in the prior mo | 30 (54.4) |
| Hospitalized in ICU | 14 (26.4) |
| Central line | 17 (32.6) |
| UA, >20 WBC | 20 (36.6) |
The median age in years for the patients was 76.5. WBC, white blood cell count.
BF in Candida isolates from urine varies greatly and depends on the medium.
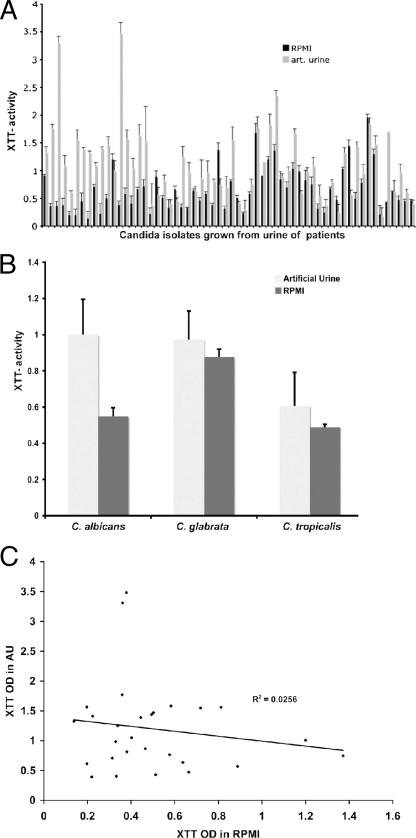
The metabolic activity in preformed biofilms was measured by the XTT reduction assay after 48 h of growth in standard morpholinepropanesulfonic acid (MOPS)-buffered RPMI and in AU, a medium that better mimics the in vivo growth environment of urine. BF varied considerably among clinical isolates (Fig. 1A) under both growth conditions. Under standard growth conditions in MOPS-buffered RPMI, BF was significantly enhanced in C. glabrata isolates compared to C. albicans and C. tropicalis isolates (Fig. 1B). This difference, however, was not found when BF was measured after growth in AU, mainly because C. albicans formed more biofilm. Of note is, however, that there was no correlation between XTT values obtained in RPMI and AU in the individual C. albicans isolate (R2 = 0.026, P = 0.4) (Fig. 1C). In contrast, BF in AU and RPMI of C. glabrata were correlated (R2 = 0.738, P < 0.001). Nine C. albicans isolates produced more biofilm in RPMI than in AU, whereas three C. albicans and two C. glabrata isolates produced more biofilm in AU than in RPMI. More detailed growth studies performed with eight clinical strains established that BF in AU or RPMI reflected growth and correlated with the colony count of scraped biofilms (R2 = 0.9). Also, the addition of glucose to RPMI (8%) resulted in increased BF predominantly in C. albicans strains but not in C. glabrata or C. tropicalis (data not shown). Previous studies reported that nicotinic acid limitation regulates the silencing of adherence genes in C. glabrata (9), which we hypothesized could also affect BF. We examined BF in the presence or absence of nicotinic acid in 12 C. glabrata strains and found no difference (0.42 ± 0.052 versus 0.44 ± 0.09 and 0.46 ± 0.08 [P > 0.5] for AU alone, AU with 3.25 μM nicotinic acid, and 32.5 μM nicotinic acid, respectively). In addition, BF was comparable on standard polystyrene and latex 96-well plates (data not shown) both if grown in AU and if grown in RPMI.
FIG. 1.
(A) BF is highly variable among clinical isolates and is dependent on the growth medium. “High” and “low” biofilm formers were defined as clinical isolates that exhibited XTT activity greater than or less than the geometrical mean (optical density of 0.55). (B) Species-dependent differences in BF were only observed if biofilms were grown in RPMI. Here C. glabrata isolates produced significantly (P = 0.003) more biofilm than did C. albicans and C. tropicalis (P = 0.01) isolates. (C) No correlation was found between BF in AU and RPMI.
Sequential Candida isolates from patients.
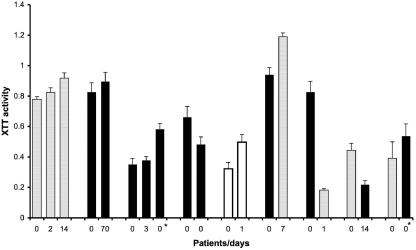
Sequential urinary Candida spp. isolates were available from nine patients (Fig. 2). In two cases blood isolates were also available. Molecular typing determined that the sequential isolate in five patients represented the same strain, whereas in four patients both C. albicans and C. glabrata were recovered from urine. The time between the first and last urine samples ranged from 0 to 70 days. The fungal burden in urine (log CFU) between sequential urine samples did not differ significantly (<10% of total log change) in patients with identical strains but varies in infections with more than one strain. In one patient two different Candida species were grown from blood and urine. The degree of BF among sequential isolates remained stable even in isolates that were recovered 70 days apart. In contrast, in patients infected with more than one strain the capacity for BF among the recovered strains was more variable. In one patient the C. glabrata blood isolate produced more biofilm than did the urine isolate. We conclude that BF is a stable characteristic of a Candida strain that is affected by the chronicity of infection.
FIG. 2.
BF for sequential isolates is stable unless there were more than one species in the sample. Gray bars indicate C. albicans isolates, black bars indicate C. glabrata, and white bars indicate C. krusei. *, Candida isolated from blood.
MIC in biofilm-associated and planktonic Candida.
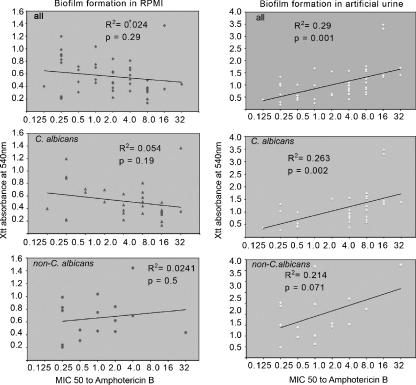
The MICs of CASPO, AMB, and FLU were measured in 50 urine Candida isolates that were grown as planktonic or biofilm-associated cell phenotypes (Table 2). As expected, the majority of the planktonic phenotypes of Candida isolates were sensitive to standard antifungal drugs. Two C. albicans and three C. glabrata strains were found to be resistant to FLU. In contrast, biofilm-associated yeast cells were not inhibited by FLU, and only 30% of them were inhibited by AMB. Most of them also exhibited inhibition by CASPO but the MICs were greater than the drug concentrations that are expected in serum. Because BF was dependent on medium, we grew the isolates in both AU and RPMI and then compared the MICs to biofilm-associated cells under standard conditions (Fig. 3). These experiments determined that the degree of BF in AU correlated (R2 = 0.29, P = 00.1) with inhibition by AMB, whereas there was no correlation in RPMI (R2 = 0.0024, P = 0.29) in the Candida isolates. We conclude from these data that BF in AU may be a better growth medium to predict the response to antifungal treatment.
TABLE 2.
MICs of planktonic and biofilm-associated Candida
| Species | MIC (μg/ml) in biofilms grown in RPMI or AU
|
MIC50 (μg/ml) for planktonic Candida
|
|||||
|---|---|---|---|---|---|---|---|
| AMB AU | AMB RPMI | CASPO AU | CASPO RPMI | AMB | FLU | CASPO | |
| C. albicans | 16 | 8 | 512 | 256 | 0.125 | 0.25 | 0.125 |
| 8 | 8 | 512 | 512 | 0.25 | 0.25 | 0.125 | |
| 8 | 8 | 512 | 512 | 0.06 | 0.25 | 0.125 | |
| 0.125 | 0.25 | 16 | 8 | 0.06 | 32 | 0.125 | |
| 32 | 2 | 256 | 256 | 0.06 | 0.25 | 0.125 | |
| 8 | 8 | 512 | 512 | 0.06 | 0.5 | 0.125 | |
| 4 | 4 | 512 | 512 | 0.25 | 1 | 0.125 | |
| 8 | 8 | 512 | 512 | 0.125 | 1 | 0.125 | |
| 16 | 8 | 512 | 512 | 0.25 | >64 | 0.125 | |
| 4 | 2 | 512 | 512 | 0.06 | 1 | 0.125 | |
| 0.25 | 0.125 | 32 | 32 | 0.25 | 0.25 | 0.125 | |
| 16 | 4 | 256 | 512 | 0.06 | 4 | 0.125 | |
| 4 | 2 | 512 | 512 | 0.125 | 8 | 0.125 | |
| 16 | 8 | 512 | 512 | 0.06 | 0.5 | 0.125 | |
| 0.5 | 1 | 16 | 16 | 0.125 | 0.125 | 0.125 | |
| 8 | 4 | 512 | 256 | 0.06 | 2 | 0.125 | |
| 4 | 4 | 32 | 16 | 0.125 | 64 | 0.125 | |
| 4 | 2 | 512 | 512 | 0.06 | 0.25 | 0.125 | |
| 8 | 4 | 32 | 16 | 0.5 | 8 | 0.125 | |
| 0.5 | 0.5 | 16 | 8 | 0.25 | 16 | 0.125 | |
| 8 | 1 | 128 | 512 | 0.125 | 16 | 0.125 | |
| 2 | 0.5 | 32 | 32 | 0.06 | 2 | 0.125 | |
| 8 | 4 | 1024 | 1024 | 0.25 | 4 | 0.5 | |
| 0.5 | 0.25 | 512 | 512 | 0.125 | 4 | 0.125 | |
| 0.25 | 0.25 | 4 | 4 | 0.25 | 2 | 0.125 | |
| C. glabrata | 0.5 | 0.25 | 16 | 8 | 0.25 | 8 | 0.125 |
| 4 | 16 | 512 | 512 | 0.5 | 1 | 0.5 | |
| 0.25 | 0.25 | 32 | 32 | 0.25 | >64 | 0.125 | |
| 0.5 | 0.25 | 32 | 32 | 0.25 | >64 | 0.125 | |
| 0.5 | 0.5 | 32 | 16 | 0.25 | >64 | 0.125 | |
| 1 | 0.5 | 16 | 8 | 0.25 | 4 | 0.125 | |
| 2 | 4 | 512 | 512 | 0.125 | 2 | 0.125 | |
| 4 | 2 | 16 | 16 | 0.125 | 4 | 0.125 | |
| 2 | 0.25 | 4 | 2 | 0.125 | 4 | 0.125 | |
| 8 | 2 | 512 | 256 | 0.25 | 32 | 0.25 | |
| 0.25 | 0.25 | 16 | 8 | 0.25 | 2 | 0.125 | |
| 1 | 1 | 32 | 32 | 0.06 | 0.5 | 0.125 | |
| C. tropicalis | 2 | 4 | 32 | 32 | 0.125 | 4 | 0.125 |
| 32 | 32 | 512 | 512 | 0.125 | 2 | 0.125 | |
| 2 | 2 | 16 | 32 | 0.5 | 0.125 | 0.125 | |
FIG. 3.
The degree of inhibition by AMB correlated with the degree of BF only if the biofilms were grown in AU and not in RPMI (R as determined by linear regression).
Association of patient characteristics and BF.
Because of the extensive variability of BF observed among strains and because environmental factors such as glucose, coinfection, and adherence can affect BF, it is reasonable to hypothesize that certain patient characteristics may predispose a patient to be infected or colonized with a high or low biofilm former. To examine this hypothesis, a retrospective chart review was performed. We determined that BF in Candida isolates of patients with potential risk factors, including urinary catheter, diabetes, or antibiotic treatment, did not differ from the BF of Candida isolates of patients that did not exhibit these risk factors (data not shown). Furthermore, the degree of BF was not associated with a higher CFU in urine, leucocyturia (white blood cell count of >20) or concomitant bacterial infection. We conclude from these data that high BF in Candida isolates appears to be a stable but highly variable characteristic of an individual Candida strain that does not appear to be associated with specific conditions or characteristics in the host. This was irrespective of the medium (AU or RPMI) the BF was measured in.
DISCUSSION
BF is associated with adherence to surfaces and presumed to promote persistence of infection (10, 27). In the present study we investigated BF in Candida isolates of patients with candiduria. We can draw several conclusions from our data. (i) BF varies greatly among clinical Candida isolates from urine and is highly dependent on growth conditions. (ii) Biofilm production by C. albicans is enhanced if it is grown in AU. (iii) The degree of BF and the inhibition by AMB correlate better if the Candida isolate is grown in AU than if grown in standard RPMI. (iv) BF is stable during chronic infection. (v) No association between individual host factors and the capacity for BF in the Candida spp. was observed.
Studies of BF often use Candida strains passaged in laboratories (3, 31) that have been adapted to culture media, and thus they may not be representative of BF in clinical strains. Microevolution of fungal pathogens occurs in vitro and in vivo and can affect major virulence factors (5, 6, 13, 14, 36). In the present study we determined that BF among clinical Candida strains varied significantly and ranged from high to low. These findings differed from previous studies in which 26 Candida strains isolated from patients with oropharyngeal candidiasis, where most isolates manifested comparable BF (18). Overall, we found that a larger percentage of Candida isolates produce biofilm in the conditions described here than has previously been described (38), although more recent studies have also found BF to be common especially in non-albicans strains (25). It is not known whether this finding could be specific to urine isolates, although some studies suggest that there is no difference between the BF of Candida isolated from blood versus that isolated from other sites (39). Several studies have documented that BF correlates with XTT absorbance (26, 31), although one study also reported limitations of the XTT assay in studies comparing different Candida species (21). We found that more CFU is recovered from biofilms that exhibit higher XTT values. However, most studies determine the BF after growth in RPMI or in YNB growth medium (30, 31), whereas we also examined BF in AU, which presumably represents a more physiologic growth environment. Interestingly, we found that BF is highly dependent on the growth medium. In particular, C. albicans strains produced significantly more biofilm in AU than in RPMI. To some extent this difference may be the result of increased glucose levels in artificial urine. More importantly, however, a change in growth conditions did not simply increase BF in all Candida isolates but could have a variable effect on the individual Candida strain. As such, no correlation of BF in RPMI and AU was documented. For example, some C. albicans strains that were identified as high biofilm producers in one medium could be classified as a low biofilm producer when grown in another medium. This finding suggests that BF is not a stable characteristic in a Candida strain. However, this conclusion is unlikely given that BF was consistent in repeated assays and was stable in serial isolates from individual patients. Our data raise the question as to which assay would best predict BF in vivo. The decision regarding which assay to use should take into consideration that BF in Candida isolates from blood and urine may differ. In one patient from which the same Candida strain was recovered from blood and urine, BF in the blood isolate was increased relative to the urine isolate. Especially for non-albicans species other studies have also reported more BF in the blood-derived Candida strains compared to strains from other sites (25, 38). Factors such as pH may affect BF and may in part explain these observations.
Cells in biofilms display phenotypic traits, such as increased resistance to antimicrobial agents and protection from host defenses, that are dramatically different from their planktonic counterparts. Our assays confirm previous findings that Candida biofilms display resistance to FLU, whereas the planktonic cells are susceptible (1, 12, 45). This is of particular importance since FLU is the drug of choice in patients with candiduria (41, 42). At the same time this may explain why FLU treatment often does not achieve long-term eradication even if indwelling urinary catheters are removed (35, 41, 42). CASPO is a commonly used antifungal drug, although it is usually not the drug of choice for UTI because less than 1% of the drug is excreted in urine. In contrast to previous studies, we did not document in vitro activity of CASPO against Candida biofilms (4). Our study examined MIC in a large number of clinical isolates, and this may be in part explained by differences in CASPO activity between Candida strains and species (4, 40). In addition, the length of treatment of biofilms differs significantly between in vitro and in vivo models of antifungal susceptibility in the setting of biofilms because in the in vivo model rabbits are treated for 7 days with antifungal medication (40). About one-third of biofilms were successfully inhibited by AMB. The MIC of AMB was comparable in biofilms grown in AU and in RPMI. However, for some strains, and especially for C. albicans, the degree of AMB-induced inhibition correlated only with the metabolic activity of biofilms grown in AU and not biofilms grown in RPMI. In summary, our data demonstrate that even in Candida isolates classified as low biofilm producers FLU is not an effective drug. The growth of Candida as an adherent biofilm-associated cell phenotype is not suppressed by FLU. These data further support the view that FLU resistance in Candida biofilms is multifactorial (29). In contrast, resistance to AMB is correlated with the degree of BF in AU and may be effective against Candida biofilms. Hence, our data support revisiting strategies to treat candiduric patients with brief courses of intravenous AMB rather than azoles or CASPO (12).
In vitro BF can be affected by external factors such as coinfection with other pathogens, glucose concentration, antibiotic treatment, and pH. In addition, a more adherent cell phenotype may be selected in the presence of a surface such as a urinary catheter. Hence, we examined the hypothesis that host factors and specific conditions in the local microenvironment of the bladder may select for strains with high BF and constitute a risk factor for persistent candiduria. Our retrospective analysis did not document a correlation with host characteristics, nor did our results support evidence that an indwelling urinary catheter, diabetes, or concomitant bacterial infection select for a Candida population that produces more BF. In contrast, we conclude from our data that BF is an inherent and stable characteristic of a Candida strain. Supportive of our conclusion is molecular typing data that also could not associate multilocus genotypes of Candida with extensive variation in BF (26).
The extent to which strain differences in BF affect the pathogenesis and outcome of candiduria is not clear. A limitation of our study was that treatment of UTI was not controlled for because it was a retrospective analysis. However, it is conceivable that the failure to eradicate candiduria in these individuals is a result of BF. We propose that controlled studies should be undertaken to optimize antifungal treatment based on strain characteristics of the Candida strain because candiduria is associated with decreased survival and treatment is not successful. These studies will help to shed light on the question of whether candiduria is a condition that should be treated aggressively or if it is only a surrogate marker for poor outcome.
Acknowledgments
This work was supported by the NIH (grant RO-1 AI59681 to B.C.F.), by the AIDS International Training Research Program (AITRP; grant D34TW001403), and by the Center of AIDS Research (CFAR; grant A1-051519) at Albert Einstein College of Medicine.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Al-Fattani, M. A., and L. J. Douglas. 2004. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 48:3291-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amer, F. A., H. A. Mohtady, I. M. el-Behedy, S. Khalil, Y. A. el-Hendy, E. A. el-Gindy, and H. E. Salem. 2004. Bacteria of nosocomial urinary tract infections at a university hospital in Egypt: identification and associated risk factors. Infect. Control Hosp. Epidemiol. 25:895-897. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., J. Nett, P. Oschel, R. Albrecht, K. Marchillo, and A. Pitula. 2004. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72:6023-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. Lopez-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasi, E., A. Brozzetti, D. Francisci, R. Neglia, G. Cardinali, F. Bistoni, V. Vidotto, and F. Baldelli. 2001. Evidence of microevolution in a clinical case of recurrent Cryptococcus neoformans meningoencephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 20:535-543. [DOI] [PubMed] [Google Scholar]
- 6.D'Antonio, D., F. Romano, E. Pontieri, G. Fioritoni, C. Caracciolo, S. Bianchini, P. Olioso, T. Staniscia, R. Sferra, S. Boccia, A. Vetuschi, G. Federico, E. Gaudio, and G. Carruba. 2002. Catheter-related candidemia caused by Candida lipolytica in a patient receiving allogeneic bone marrow transplantation. J. Clin. Microbiol. 40:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Martino, P., A. Merieau, R. Phillips, N. Orange, and C. Hulen. 2002. Isolation of an Escherichia coil strain mutant unable to form biofilm on polystyrene and to adhere to human pneumocyte cells: involvement of tryptophanase. Can. J. Microbiol. 48:132-137. [DOI] [PubMed] [Google Scholar]
- 8.Dodgson, A. R., C. Pujol, D. W. Denning, D. R. Soll, and A. J. Fox. 2003. Multilocus sequence typing of Candida glabrata reveals geographically enriched clades. J. Clin. Microbiol. 41:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domergue, R., I. Castano, A. De Las Penas, M. Zupancic, V. Lockatell, J. R. Hebel, D. Johnson, and B. P. Cormack. 2005. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 308:866-870. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 11.El-Azizi, M. A., S. E. Starks, and N. Khardori. 2004. Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J. Appl. Microbiol. 96:1067-1073. [DOI] [PubMed] [Google Scholar]
- 12.Fisher, J. F., K. Woeltje, A. Espinel-Ingroff, J. Stanfield, and J. T. DiPiro. 2003. Efficacy of a single intravenous dose of amphotericin B for Candida urinary tract infections: further favorable experience. Clin. Microbiol. Infect. 9:1024-1027. [DOI] [PubMed] [Google Scholar]
- 13.Franzot, S. P., J. Mukherjee, R. Cherniak, L. C. Chen, J. S. Hamdan, and A. Casadevall. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect. Immun. 66:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fries, B. C., and A. Casadevall. 1998. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J. Infect. Dis. 178:1761-1766. [DOI] [PubMed] [Google Scholar]
- 15.Griffith, D. P., D. M. Musher, and C. Itin. 1976. Urease: the primary cause of infection-induced urinary stones. Investig. Urol. 13:346-350. [PubMed] [Google Scholar]
- 16.Guze, L., and L. Harley. 1957. Fungus infections of the urinary tract. Yale J. Biol. Med. 1957:292-305. [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, A. D., J. Castro, D. C. Sheppard, Y. Carmeli, and M. H. Samore. 1999. Risk factors for nosocomial candiduria due to Candida glabrata and Candida albicans. Clin. Infect. Dis. 29:926-928. [DOI] [PubMed] [Google Scholar]
- 18.Jin, Y., L. P. Samaranayake, Y. Samaranayake, and H. K. Yip. 2004. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral Biol. 49:789-798. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman, C. A. 2005. Candiduria. Clin. Infect. Dis. 41(Suppl. 6):S371-S376. [DOI] [PubMed] [Google Scholar]
- 20.Kauffman, C. A., J. A. Vazquez, J. D. Sobel, H. A. Gallis, D. S. McKinsey, A. W. Karchmer, A. M. Sugar, P. K. Sharkey, G. J. Wise, R. Mangi, A. Mosher, J. Y. Lee, W. E. Dismukes, et al. 2000. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin. Infect. Dis. 30:14-18. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn, D. M., M. Balkis, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn, D. M., J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect. Immun. 70:878-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumamoto, C. A. 2002. Candida biofilms. Curr. Opin. Microbiol. 5:608-611. [DOI] [PubMed] [Google Scholar]
- 24.Kumamoto, C. A., and M. D. Vinces. 2005. Alternative Candida albicans lifestyles: growth on surfaces. Annu. Rev. Microbiol. 59:113-133. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, C. P., and T. Menon. 2006. Biofilm production by clinical isolates of Candida species. Med. Mycol. 44:99-101. [DOI] [PubMed] [Google Scholar]
- 26.Li, X., Z. Yan, and J. Xu. 2003. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology 149:353-362. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee, P. K., G. Zhou, R. Munyon, and M. A. Ghannoum. 2005. Candida biofilm: a well-designed protected environment. Med. Mycol. 43:191-208. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., J. Rhine-Chalberg, A. L. Barry, and J. H. Rex. 1995. Strain variation and antifungal susceptibility among bloodstream isolates of Candida species from 21 different medical institutions. Clin. Infect. Dis. 21:1507-1509. [DOI] [PubMed] [Google Scholar]
- 29.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 30.Ramage, G., and J. L. Lopez-Ribot. 2005. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol. Med. 118:71-79. [DOI] [PubMed] [Google Scholar]
- 31.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramage, G., K. Vandewalle, B. L. Wickes, and J. L. Lopez-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 33.Richards, M. J., J. R. Edwards, D. H. Culver, R. P. Gaynes, et al. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 34.Richards, M. J., J. R. Edwards, D. H. Culver, R. P. Gaynes, et al. 1999. Nosocomial infections in pediatric intensive care units in the United States. Pediatrics 103:e39. [DOI] [PubMed] [Google Scholar]
- 35.Safdar, N., W. R. Slattery, V. Knasinski, R. E. Gangnon, Z. Li, J. D. Pirsch, and D. Andes. 2005. Predictors and outcomes of candiduria in renal transplant recipients. Clin. Infect. Dis. 40:1413-1421. [DOI] [PubMed] [Google Scholar]
- 36.Samaranayake, Y. H., L. P. Samaranayake, J. Y. Yau, R. S. Dassanayake, T. K. Li, and S. Anil. 2003. Phenotypic diversity of oral C. albicans isolated on single and sequential visits in an HIV-infected Chinese cohort. APMIS 111:329-337. [DOI] [PubMed] [Google Scholar]
- 37.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 91:72S-75S. [DOI] [PubMed] [Google Scholar]
- 38.Shin, J. H., S. J. Kee, M. G. Shin, S. H. Kim, D. H. Shin, S. K. Lee, S. P. Suh, and D. W. Ryang. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuford, J. A., K. E. Piper, J. M. Steckelberg, and R. Patel. 30November2006, posting date. In vitro biofilm characterization and activity of antifungal agents alone and in combination against sessile and planktonic clinical Candida albicans isolates. Diagn. Microbiol. Infect. Dis. doi: 10.1016/j .diagmicrobio.2006.09.004. [DOI] [PubMed]
- 40.Shuford, J. A., M. S. Rouse, K. E. Piper, J. M. Steckelberg, and R. Patel. 2006. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J. Infect. Dis. 194:710-713. [DOI] [PubMed] [Google Scholar]
- 41.Sobel, J. D. 1999. Management of asymptomatic candiduria. Int. J. Antimicrob. Agents 11:285-288. [DOI] [PubMed] [Google Scholar]
- 42.Sobel, J. D., C. A. Kauffman, D. McKinsey, M. Zervos, J. A. Vazquez, A. W. Karchmer, J. Lee, C. Thomas, H. Panzer, W. E. Dismukes, et al. 2000. Candiduria: a randomized, double-blind study of treatment with fluconazole and placebo. Clin. Infect. Dis. 30:19-24. [DOI] [PubMed] [Google Scholar]
- 43.Sobel, J. D., and T. Lundstrom. 2001. Management of candiduria. Curr. Urol. Rep. 2:321-325. [DOI] [PubMed] [Google Scholar]
- 44.Tavanti, A., N. A. Gow, S. Senesi, M. C. Maiden, and F. C. Odds. 2003. Optimization and validation of multilocus sequence typing for Candida albicans. J. Clin. Microbiol. 41:3765-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tortorano, A. M., A. Prigitano, E. Biraghi, and M. A. Viviani. 2005. The European Confederation of Medical Mycology (ECMM) survey of candidaemia in Italy: in vitro susceptibility of 375 Candida albicans isolates and biofilm production. J. Antimicrob. Chemother. 56:777-779. [DOI] [PubMed] [Google Scholar]