Abstract
An estimated 65% of infective diseases are associated with the presence of bacterial biofilms. Biofilm-issued planktonic cells promote blood-borne, secondary sites of infection by the inoculation of the infected sites with bacteria from the intravascular space. To investigate the potential role of early detachment events in initiating secondary infections, we studied the phenotypic attributes of Staphylococcus aureus planktonic cells eroding from biofilms with respect to expression of the collagen adhesin, CNA. The collagen-binding abilities of S. aureus have been correlated to the development of osteomyelitis and septic arthritis. In this study, we focused on the impact of CNA expression on S. aureus adhesion to immobilized collagen in vitro under physiologically relevant shear forces. In contrast to the growth phase-dependent adhesion properties characteristic of S. aureus cells grown in suspension, eroding planktonic cells expressed invariant and lower effective adhesion rates regardless of the age of the biofilm from which they originated. These results correlated directly with the surface expression level of CNA. However, subsequent analysis revealed no qualitative differences between biofilms initiated with suspension cells and secondary biofilms initiated with biofilm-shed planktonic cells. Taken together, our findings suggest that, despite their low levels of CNA expression, S. aureus planktonic cells shed from biofilms retain the capacity for metastatic spread and the initiation of secondary infection. These findings demonstrate the need for a better understanding of the phenotypic properties of eroding planktonic cells, which could lead to new therapeutic strategies to target secondary infections.
Staphylococcus aureus is a bacterial pathogen that has been associated with diverse cases of community- and hospital-acquired infections. With higher morbidity and mortality numbers than other staphylococcal species, S. aureus is also one of numerous bacterial species that have been shown to colonize surfaces in organized biofilm communities (17). The increase in invasive medical procedures and prosthetic device implantations in hospital settings over the last decade has coincided with increased cases of S. aureus infections in the United States. In a recent clinical study, S. aureus bacteremia and device-related infections resulted in postimplantation surgery for 58% of all hospitalized patients with a prosthetic device and led to the death of over a quarter of them within 3 months (8). S. aureus infections may be complicated by the occurrence of secondary metastatic infections, which include infective endocarditis, osteomyelitis, or septic arthritis (20). It has been suggested that the detachment of bacterial cells from in vivo biofilms may justify the high rate of symptomatic metastatic infections seen with S. aureus (14).
Indeed, the prevalence of S. aureus as a human pathogen has been attributed both to its ability to form specific bonds with a wide variety of extracellular matrix proteins and to its capacity to colonize surfaces in organized biofilm communities (17). Therefore, indwelling surgical devices, catheters, and vascular prostheses may host sessile bacterial colonies that promote recurrent, chronic infections (31). Within the bloodstream, the evolution of bacterial biofilms involves three critical steps: (i) initial adhesion of planktonic cells to a substratum, (ii) proliferation and accumulation of sessile cells into biofilm layers, and (iii) detachment and dissemination of clusters or isolated cells under shear forces (12, 14, 34). Detachment of biofilm aggregates or individual cells consistently occurs under hydrodynamic conditions (5), and it has been suggested that the mode of dispersal may affect an organism's phenotypical characteristics and antimicrobial resistance properties (12). Extensive research has focused on the epidemiology, pathogenesis, and eradication of staphylococcal infections, but most of these studies have been based on the analysis of cells from shake flask cultures. Few studies have addressed the development of staphylococcal biofilms under physiologically relevant flow conditions. Similarly, few reports characterize the molecular and phenotypic differences between both planktonic and sessile S. aureus cells isolated under such growth conditions (6, 14, 31). In all likelihood, however, natural staphylococcal cultures develop as biofilms (9, 10, 33).
Metastatic infections such as septic arthritis and osteomyelitis undoubtedly result from inoculation of the infected site with bacteria from the intravascular space (29). Therefore, both ailments may result from biofilm-related dissemination events. Experimentally, the S. aureus collagen adhesin (CNA) was reported as a principal virulence factor in both septic arthritis (28) and osteomyelitis (13). A recent study investigating detachment dynamics of S. aureus biofilms in a 7-day in vitro catheter infection model suggested that detached biomasses consisted primarily of macroscopical clumps ranging from 11 to 1,000 packed cells (14). However, biofilm formation and the ability of shed planktonic cells to promote secondary infection through specific interactions with collagen have not been investigated previously. In light of the lack of information available concerning the process of cell detachment early in biofilm growth, we restricted our study to the first 24 h of sessile culture under shear forces. We analyzed the phenotypic properties of biofilm-shed, planktonic cells pertaining to their expression of CNA. Particular emphasis was given to the effect of the sessile mode of growth on the adhesion properties of planktonic cells onto an immobilized collagen. Thus, this analysis may give insight into the mechanisms governing proliferation of infectious disease via the development of secondary sites of infection.
MATERIALS AND METHODS
Media and components.
Tryptic soy broth (TSB; Difco, Detroit, MI) was used as the growth medium for both shake flask and biofilm cultures. Tryptic soy agar (TSA; Sigma-Aldrich, St. Louis, MO) was used as the growth medium on culture plates. Congo red agar (CRA) plates contained medium consisting of 36 g of sucrose (Sigma-Aldrich), 0.8 g of Congo red stain (Sigma-Aldrich), and 1 liter of brain heart infusion agar (Difco) (1). Where applicable, cells were harvested into solutions of phosphate-buffered saline (PBS; 138 mM NaCl, 2.7 mM KCl [pH 7.4]), with or without 0.2% sodium azide (Sigma-Aldrich). A solution of PBS plus 0.1% bovine serum albumin (BSA) was also used to block nonspecific bacterial adhesion when appropriate.
Bacterial strains and suspension cultures.
S. aureus strain Phillips is a clinical strain that was first isolated from a patient with osteomyelitis (28). This bacterial strain was selected due to the extensive description of its adhesion properties to collagen under hydrodynamic conditions (21-23) and for its biofilm-forming abilities, revealed by CRA plate assays (data not shown). S. aureus PH100 is a mutant variant of the Phillips strain (28). Although it retains biofilm-forming properties similar to those of the wild-type strain, PH100 lacks the collagen receptor (CNA) and was utilized in this study as a negative control for all adhesion assays. S. aureus Newman (ATCC 25904) was used as a negative control in CRA plate assays due to its poor biofilm-forming properties (3).
To generate primary shake flask cultures, 10 μl of S. aureus cells, collected from glycerol stocks stored at −80°C, was grown in 50 ml of TSB at 37°C with constant rotation. To generate secondary shake flask cultures, 100 μl of primary culture was inoculated in TSB in lieu of the frozen glycerol stock. Secondary and biofilm cultures were initiated from primary cultures harvested at mid-growth phase, at an optical density of approximately 6 (measured at 600 nm). At this growth phase, S. aureus cells are rapidly dividing and S. aureus Phillips cells express high levels of the collagen adhesin (23, 24).
Shed planktonic cells were defined as bacterial cells detached from the sessile culture under hydrodynamic shear forces. These cells were collected into sample vials directly downstream of the flow chamber and maintained at 0 to 4°C to prevent further growth. In contrast, sessile cells were defined as bacterial cells that remained attached by intercellular adhesion under hydrodynamic shear conditions and constituted the biofilm structure. These cells were recovered by flushing the flow chamber at the end of a 24-h biofilm run and resuspended in PBS for subsequent analysis.
Congo red agar assay.
CRA plates are commonly used for the detection of slime-producing bacterial strains (1). Bacterial cells from primary shake flask cultures were harvested in late growth phase into PBS at a 1:20 dilution and streaked onto CRA plates. Since the enriched medium enhances exopolysaccharide production, slime producers turn black while nonproducers appear clear or pink after 24 h or longer of culture at 37°C (1). Dark bacterial colonies were consistently achieved with S. aureus Phillips and PH100 cells, while smooth and clear Newman colonies confirmed the poor biofilm-forming properties of this strain.
Biofilm cultures.
Immobilized protein substrates were prepared as previously described (21, 25, 26). Briefly, a glass coverslip was coated with 100 μl of acid-insoluble fibrillar collagen type I (Sigma) or 100 μl fibrinogen (Sigma) and allowed to settle in a humid atmosphere for 1 h. Excess protein was rinsed off with 1 ml deionized water, and the slide was coated with 100 μl of PBS plus BSA.
A parallel plate flow chamber was used to study the effect of physiologically relevant fluid shear stress on biofilm development. Primary cells were diluted to a ratio of 1:40 in PBS plus BSA and perfused over the protein-coated slide at a wall shear rate of 300 s−1 for approximately 6 min. This resulted in an average attached cell density of approximately 2.0 × 104 cells mm−2. Adhesion times were adjusted slightly to achieve equivalent initial surface concentrations of bacteria from experiment to experiment. Subsequently, unattached cells were rinsed out with PBS plus BSA for 10 min at the same shear rate. Flow was then switched to TSB for 24 h at a wall shear rate of either 40 or 100 s−1. Typical shear rates in the human aorta range from 45 to 305 s−1 (16), and reported hemodynamic models for fluid flow through bones predict shear stresses on osteocytes up to 263 s−1, assuming Poiseuille flow and a whole-blood viscosity of 3.8 cP (19).
The flow apparatus was monitored via a videomicroscopy system consisting of a phase-contrast microscope with a computer-driven stage and a charge-coupled device camera connected to a Macintosh computer with public-domain NIH-Image capturing software (http://rsb.info.nih.gov/nih-image/download.html). The flow chamber and the microscope were held in a Plexiglas chamber maintained at 37°C as previously described (23, 24).
Adhesion assay under hydrodynamic conditions.
The adhesion properties of S. aureus on collagen have been extensively studied in our laboratory, and the experimental setup in this study remains as previously described (21, 25). Planktonic cells eroding from biofilm were collected at different time intervals and then resuspended at a concentration of 1 × 106 cells ml−1 in PBS plus BSA. All adhesion assays were performed at a wall shear rate of 300 s−1, which had been previously determined to be an optimal hydrodynamic condition for S. aureus adhesion to collagen in vitro (21).
Cell enumeration.
Cell counts and particle size estimation were performed with a Multisizer 3 Coulter Counter (Beckman Coulter, Inc., Fullerton, CA). These assays revealed that erosion of biofilm cells occurred within our flow system, disseminating single, free-floating cells rather than cellular clusters (data not shown). Viable cell counts were estimated in CFU/ml by plating diluted samples on solid TSA followed by incubation for 30 h at 37°C under aerobic conditions.
Determination of relative adhesin density by flow cytometry.
The expression of collagen adhesins present on cell surfaces was determined using a FACSCalibur flow cytometer (Becton Dickinson) and indirect immunofluorescence as previously described (23, 24). To label bacterial cells, F(ab′)2 fragments of mouse monoclonal antibody (MAb) 3B12 served as the primary antibody (kindly provided by Joseph Patti, Inhibitex, Inc., Alpharetta, GA). The MAb was raised against the minimal ligand binding domain, CNA-(151-318) (38). F(ab′)2 fragments of MAb 3B12 were cleaved using an Immunopure F(ab′)2 preparation kit (Pierce).
A standard calibration curve relating the amount of fluorescence detected to absolute number of surface adhesins was generated using Quantum Simply Cellular anti-mouse immunoglobulin G beads (Bangs Laboratory, Inc., Fishers, IN) (2). Negative controls performed in flow cytometry assays included (i) detection of unlabeled Phillips cells; (ii) detection of Phillips cells incubated with isotype-matched, nonbinding immunoglobulin as the primary antibody, followed by incubation with the secondary antibody; (iii) PH100 cells incubated with MAb 3B12 and the secondary antibody; and (iv) Phillips cells incubated with the secondary antibody, without prior exposure to a primary antibody.
Statistical analysis.
Unless otherwise specified, the data reported are the averages of the mean value of three or more experimental runs and error bars represent standard deviations of the means. Statistical significance, where applicable, was determined using the single-factor analysis of variance technique at a 95% confidence interval (equivalent to P < 0.05).
RESULTS
Biofilm formation on protein substrates.
To verify the ability of our strains to produce a viable biofilm within the flow system, initial adhesion of planktonic bacterial cells and subsequent development of a sessile community under TSB flow were routinely monitored by videomicroscopy. Qualitative analysis revealed the proliferation of bacterial biofilm within the flow chamber volume. Equivalent initial seed concentrations resulted in a complete surface coverage by the eighth hour of medium exposure for both wild-type and CNA mutant strains of either immobilized collagen (illustrated in Fig. 1) or fibrinogen (data not shown).
FIG. 1.
Top views of a representative S. aureus Phillips biofilm grown in vitro at 37°C under hydrodynamic shear conditions. TSB flows from left to right, maintaining a wall shear rate of 100 s−1. (A) An initial cell density of approximately 2 × 104 cells mm−2 was seeded onto collagen and subjected to a continuous flow of TSB medium. This led to a biofilm community, viewed here at 8 (B), 16 (C), and 24 (D) h via phase-contrast microscopy. The white bar in each panel represents 50 μm.
To demonstrate the ability of shed planktonic cells issued from primary S. aureus Phillips biofilms to form secondary biofilms, they were exposed to collagen-coated substrates under the same flow conditions as and with equivalent seed concentrations to the original shake flask population. Images revealed no qualitative differences between biofilms initiated with primary shake flask cultures and biofilms initiated with shed planktonic cells harvested from primary sessile cultures at different intervals (data not shown).
Quantification of eroding planktonic cells.
Biofilm growth in the flow chamber was accompanied by a continuous, quantifiable process of cell erosion, characteristic of biofilm development under flow conditions. Planktonic cell erosion is an important developmental phase and a clinically relevant feature of bacterial biofilms. We hypothesized that this process would be substrate dependent for the early biofilms cultured within our flow system. For each biofilm, planktonic cells were collected from the effluent media over successive 4-h intervals, resulting in six bacterial samples over 24 h of the experiment. Our results reveal that the erosion process commences within the first 4 h of sessile accumulation, yielding (1.99 ± 0.34) × 108 cells within the first 4 h of growth. The number of cells eroding from the biofilm then gradually increases under constant shear force to values as high as (2.80 ± 0.41) × 1010 cells for samples collected between 20 and 24 h of sessile growth (Fig. 2). A similar erosion trend was observed for S. aureus Phillips biofilms grown on collagen or fibrinogen. In addition, there was no statistically significant difference in erosion prior to 24 h of biofilm growth. Taken together, these results suggest that the rate of growth of the sessile culture was not affected by shear forces within our experimental range of shear rates. Rather, these results support the hypothesis that cell detachment was a shear-induced process within that shear range.
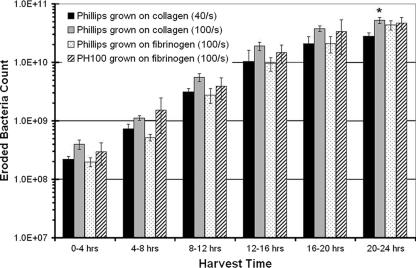
FIG. 2.
Total number of planktonic cells eroding from biofilm cultures over 4-h time intervals of cultures of Phillips cells grown on collagen at a wall shear rate of 40 s−1 (black bars), Phillips cells grown on collagen at 100 s−1 (gray bars), Phillips cells grown on fibrinogen at 100 s−1 (stippled bars), and PH100 cells grown on fibrinogen at 100 s−1 (diagonally striped bars). An asterisk indicates statistical significance with respect to the culture grown at 40 s−1 (P < 0.05). Data represent means ± standard deviations of three or more experiments.
PH100 biofilms grown on fibrinogen to serve as controls in adhesion assays reproduced similar erosion patterns. The number of PH100 cells collected in effluent media increased over 100-fold from as low as (2.98 ± 0.13) × 108 cells collected within the first 4 h of growth to concentrations on the order of (4.72 ± 0.11) × 1010 cells for the samples collected between 20 and 24 h (Fig. 2). For all experimental conditions, this trend correlated well with a visible increase of cell density within the biofilm community.
Collagen-binding properties of S. aureus planktonic cells.
To characterize the collagen-binding properties of planktonic cells issued from biofilm cultures, adhesion assays were performed at a wall shear rate of 300 s−1. This shear rate has been shown to be the optimal condition for CNA-mediated adhesion of S. aureus to collagen. As mentioned above, PH100 is a cna mutant of Phillips. It does not express the collagen receptor, CNA, and served as a negative control for all adhesion assays. Thus, the lack of binding events between PH100 cells issued from biofilm or suspension cultures with collagen (data not shown) demonstrated the specific nature of the collagen-binding events seen with Phillips cells.
The adherence properties of Phillips cells demonstrated a strong dependence on the mode of growth (biofilm or suspension culture). Although biofilm cultures and secondary shake flask cultures were initiated from the same seed population, cells collected from these respective cultures at identical time intervals demonstrated significantly different patterns of adhesion to collagen under shear forces (Fig. 3). Suspension cells followed a previously described, growth-curve-dependent adhesion profile. Shed planktonic cells revealed an initial increase in effective adhesion rate leading to a plateau value of approximately 6 cells mm−2 min−1 for all biofilm cultures. A similar trend was observed from cultures grown on collagen or fibrinogen at either shear rate. Data analysis revealed no statistical difference between results from cultures grown at 40 s−1 and those from cultures grown at 100 s−1.
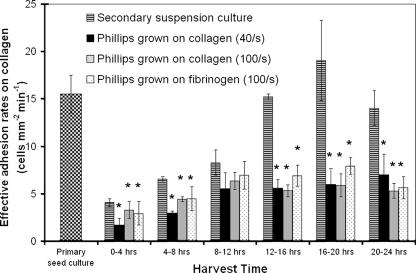
FIG. 3.
Effective adhesion rates of biofilm-shed and suspension culture S. aureus Phillips cells to a collagen substrate at a rate of 300 s−1. Shown is adhesion of bacterial cells from a primary suspension culture (checkered bars), a secondary suspension culture (horizontally striped bars) and planktonic cells shed from S. aureus Phillips biofilms grown on collagen at a wall shear rate of 40 s−1 (black bars) or 100 s−1 (gray bars) and grown on fibrinogen at 100 s−1 (stippled bars). Cells were collected from the effluent over successive 4-h intervals of biofilm culture as indicated. Asterisks represent statistical significance with respect to secondary suspension culture cells harvested at concurrent time intervals (P < 0.05). All values represent means ± standard deviations of three or more experiments. No binding events were observed with PH100 cells from suspension or biofilm cultures (data not shown).
Eroding cells collected within the first 4 h of biofilm growth were the cells least capable of adhesion to collagen. Cells from that population sample, grown over collagen at 40 s−1, had an average effective adhesion rate of 1.7 ± 0.7 cells mm−2 min−1 to immobilized collagen. This value was comparable in magnitude to the properties of cells harvested from either biofilm or suspension cultures within the same time interval. This suggests that the early planktonic population demonstrated a similar phenotype to early suspension cells with respect to cell adhesion. That in turn suggests that a “true” biofilm had yet to be established by 4 h of growth within our system. This was confirmed by low levels of polysaccharide staining observed on the coverslip surface after 4 h of biofilm growth (data not shown). In contrast, a cohesive sessile community was clearly formed by 8 h of growth (Fig. 1). Accordingly, the adhesion rates of planktonic cells were significantly different from that of suspension cells past 12 h of culture. The largest discrepancy between planktonic and suspension cells was observed within the 16- to 20-h time interval when suspension cells adhered approximately three times faster than eroding cells.
Determination of MCD of S. aureus cells.
Previous studies in our laboratory have shown that the adhesion of suspension cells to collagen under hydrodynamic shear forces is dependent on mean collagen adhesin density (MCD) on the cell surface (21, 25). In this work, we assessed the validity of that statement with biofilm-shed planktonic cells. Histogram plots from flow cytometry demonstrate that nearly all cell populations (primary seed, secondary suspension cultures, and planktonic cells shed at times greater than 4 h) featured a unique CNA-expressing population, as illustrated with representative samples in Fig. 4. Planktonic cells that had eroded between 0 and 4 h of growth were an exception and demonstrated a CNA-negative subpopulation that may be responsible for the lower adhesion rates observed for these cells (Fig. 3). Sessile cells also featured a small CNA-negative subpopulation (Fig. 4), which is absent from the seed population and thus likely occurs as a result of the sessile mode of growth.
FIG. 4.
Flow cytometry analysis of CNA expression. (A) Representative histograms of planktonic cells shed within the first 4 h of biofilm growth (light solid contour), planktonic cells shed between 4 and 8 h of sessile growth (dashed contour), and the primary seed culture (bold solid contour). M1 designates unlabeled S. aureus Phillips cells (gray-shaded area) that serve as a negative control. (B) Representative histogram of 24-h sessile cells (black-shaded area).
Using cells harvested from secondary shake flask cultures, we conclusively demonstrate that shed planktonic cells feature a different temporal expression pattern from that of suspension cells (Fig. 5). Overall, they carried lower CNA counts on their surfaces than cells from the seed population. After 24 h of sessile growth, shed planktonic cells expressed a threshold MCD value of 5,018 ± 558 and a 33.0% ± 7.4% reduction in MCD compared to cells from the primary shake flask culture (Fig. 5). In comparison, sessile cells carried an average MCD of 5,820 ± 115, which was equivalent to a 22.3% ± 1.5% reduction in MCD compared to cells from the primary shake flask culture (Fig. 5). Taken together, these results substantiate the lower adhesion rates observed for biofilm-shed planktonic cells and single out lower surface MCDs as the primary determining factor.
FIG. 5.
MCD on the surface of biofilm and suspension culture of S. aureus Phillips cells. Secondary suspension culture cells (horizontally striped bars) were harvested at characteristic growth phases: early growth (∼3 h), mid-growth (∼9 h), late growth (∼17 h), and stationary (∼22 h). Planktonic cells (gray bars) were collected at 4-h time intervals, and sessile cells (diagonally striped bar) were collected after 24 h of biofilm growth. Asterisks represent statistical significance (P < 0.05) with respect to primary culture cells (checkered bar). Reported values are means ± standard deviations of duplicate experiments.
DISCUSSION
There is an increasing body of research focused on the analysis of proliferation and resistance mechanisms underlying clinical biofilms. However, limited documentation is available regarding biofilm detachment and dissemination processes (10, 12, 17). Recent work by Purevdorj-Gage et al. (30) distinguished two characteristic phenomena exhibited in Pseudomonas aeruginosa biofilms: “seeding dispersal” and “erosion.” P. aeruginosa PAO1 was capable of “seeding dispersal” by a functional coordinated multicellular behavior through the formation of specialized microcolonies and hollow structures. In contrast, “erosion” or “sloughing” was defined by passive shedding of single, planktonic cells from a sessile culture by fluid shear (30). Furthermore, their results suggested that shear-dependent processes dominated during the first 2 days of biofilm growth (30). Such a distinction remains to be determined for S. aureus.
Our results reveal that for 24-h-old S. aureus biofilms, bacterial detachment mimics descriptions of shear-induced erosion. The continuous shedding of single planktonic cells from early S. aureus Phillips and PH100 biofilms is observed for cultures grown on extracellular matrix proteins at physiological wall shear rates. Results demonstrate that planktonic cells detach from sessile S. aureus microcolonies within the first 4 h of substrate colonization. The number of eroding cells increases exponentially for the first 20 h of biofilm proliferation. Similar erosion patterns were recorded for all cultures, regardless of the underlying substrate of initial adhesion. This suggests that with similar initial seed concentration and identical growth conditions, the shear-induced detachment process is independent of the protein substrate on which staphylococcal biofilms are grown. In turn, the detachment of planktonic cells from the sessile cultures under shear forces was independent of initial adhesion events. That is, initial adhesion rates and attachment substrates do not matter once cells are firmly affixed onto the surface and exposed to favorable growth conditions. Rather, parameters affecting the rate of cell detachment most likely include the rate of growth of the biofilm, its spatial organization, and the strength of intercellular bonds within the sessile culture. A thorough investigation of their respective effects remains to be completed for S. aureus, but the role played by physical properties of the biofilm structures in the detachment process has previously been demonstrated (32).
Cell dissemination from biofilm cultures is associated with the plague of persistent bacterial infections and the initiation of secondary sites of infections (10, 20, 27). Biofilm formation, exposure to fluid shear, and high rates of dissemination constitute a chain of events that have been linked to the pathology of S. aureus catheter-related bloodstream infections and endocarditis (32). Our kinetic data demonstrate for the first time the ability of S. aureus planktonic cells released from sessile cultures to bind to collagen via CNA-mediated interactions under physiologically relevant hydrodynamic shear forces. Additionally, we prove that when they adhere on collagen-coated substrate, shed planktonic cells retain the ability to form secondary biofilms in vitro under the same flow conditions as the original bacterial seed. The adhesion properties of planktonic cells eroding from S. aureus Phillips biofilms are time dependent during the early hours of biofilm accumulation, but the effective adhesion rates of detached Phillips cells reach a threshold limit by 12 h of sessile culture. Our data suggest that, under continuous shear forces, a potential “equilibrium” state is reached between the rate of passive detachment of planktonic cells and the rate of growth of the sessile structure. The existence of a “dynamic steady state” in biofilm activity and structure due to applied detachment forces has previously been predicted with multidimensional biofilm models (39). These results have relevant clinical implications, since the presence of CNA on staphylococcal species has been directly correlated to the development of osteomyelitis (13) and septic arthritis (28, 35).
Early work on the collagen binding capacity of S. aureus strains had suggested that mucoid strains featured reduced collagen binding capacity due to the presence of an extensive capsule which concealed the collagen adhesin (15). Thus, a particular concern was the characteristic production of polysaccharides by S. aureus biofilm communities and their effects on cellular interactions with collagen. Using PH100 cells as a negative control allowed us to conclusively determine that polysaccharides did not promote nonspecific adhesion to collagen. Additionally, immunofluorescence analysis revealed that the effective rates of adhesion to collagen featured by planktonic cells within the first 24 h of biofilm development were determined by surface MCD counts.
While CNA is not required for biofilm formation or intercellular aggregation, other required factors may be coordinately regulated with CNA, in which case, the indirect result of growing in a sessile community may be a decrease in the production of the S. aureus collagen adhesin. For example, the staphylococcal accessory regulator (sarA) is a global regulator that regulates transcription of over 100 genes and plays a major role in modulating S. aureus virulence (7, 18, 31, 37). SarA represses transcription of cna (4) and is required for biofilm development (3, 36, 37). A comparative proteome analysis of S. aureus biofilm and suspension cells recently revealed that SarA was expressed in larger amounts in biofilm cells than in suspension cells (31). Whether these two regulatory pathways are mutually exclusive or codependent is currently unknown.
The goal of the present work was to assess the potential of shed planktonic cells, issued from S. aureus Phillips biofilms, to promote secondary sites of infection through specific interactions with collagen under flow conditions. Using a physiologically relevant in vitro model, we have demonstrated that a significant number of cells detach from early staphylococcal biofilms under hydrodynamic shear forces. Eroding planktonic cells have adhesion properties that differ from those of classical shake flask cultures. These planktonic cells feature reduced but significant adhesion properties with respect to collagen, compared to rapidly growing cells in suspension. However, the reduced level of CNA expression associated with the biofilm phenotype did not impede the ability of shed planktonic cells to initiate secondary biofilms once attached to a collagen substrate under hydrodynamic conditions. Taken together, these results suggest that eroded S. aureus planktonic cells are likely to promote secondary sites of infections in vivo via CNA-mediated binding events. These findings clearly demonstrate the need for a better understanding of the phenotypic properties of eroding planktonic cells, and they offer further evidence of the potential roles of biofilms as “niduses” of acute infection as suggested by previous authors (10, 11).
Acknowledgments
This work was funded by the National Institutes of Health (grant RO1 AI059369 to Julia M. Ross) and in part by Grosvenor International through fellowship support to Patrick Ymele-Leki.
We thank Konstantinos Konstantopoulos for the use of his FACSCalibur flow cytometer. We also thank Joseph Patti and Inhibitex, Inc., for providing anti-CNA monoclonal antibodies.
Footnotes
Published ahead of print on 2 February 2007.
REFERENCES
- 1.Arciola, C. R., D. Campoccia, A. M. Borrelli, M. E. Donati, and L. Montanaro. 2001. Congo red agar plate method: improved accuracy and new extended application to Staphylococcus aureus. New Microbiol. 24:355-363. [PubMed] [Google Scholar]
- 2.Barnett, D., I. Storie, G. A. Wilson, V. Granger, and J. T. Reilly. 1998. Determination of leucocyte antibody binding capacity (ABC): the need for standardization. Clin. Lab. Haematol. 20:155-164. [DOI] [PubMed] [Google Scholar]
- 3.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins, J., A. Gillaspy, T. Rechtin, B. Hurlburt, and M. Smeltzer. 1999. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol. Microbiol. 33:317-326. [DOI] [PubMed] [Google Scholar]
- 5.Brading, M. G., J. Jass, and H. M. Lappin-Scott. 1995. Dynamics of bacterial biofilm formation, p. 46-63. In H. M. Lappin-Scott and J. W. Costerton (ed.), Microbial biofilms. Cambridge University Press, Cambridge, United Kingdom.
- 6.Brady, R. A., J. G. Leid, A. K. Camper, J. W. Costerton, and M. E. Shirtliff. 2006. Identification of Staphylococcus aureus proteins recognized by the antibody-mediated immune response to a biofilm infection. Infect. Immun. 74:3415-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassat, J., P. M. Dunman, E. Murphy, S. J. Projan, K. E. Beenken, K. J. Palm, S.-J. Yang, K. C. Rice, K. W. Bayles, and M. S. Smeltzer. 2006. Transcriptional profiling of a Staphylococcus aureus clinical isolate and its isogenic agr and sarA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology 152:3075-3090. [DOI] [PubMed] [Google Scholar]
- 8.Chu, V., D. Crosslin, J. Friedman, S. Reed, C. Cabell, R. Griffiths, L. Masselink, K. Kaye, G. Corey, L. Reller, M. Stryjewski, K. Schulman, and V. J. Fowler. 2005. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am. J. Med. 118:1416. [DOI] [PubMed] [Google Scholar]
- 9.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta, M. K. 2002. Biofilms and infection in dialysis patients. Semin. Dial. 15:338-346. [DOI] [PubMed] [Google Scholar]
- 12.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elasri, M. O., J. R. Thomas, R. A. Skinner, J. S. Blevins, K. E. Beenken, C. L. Nelson, and M. S. Smeltzer. 2002. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 30:275-280. [DOI] [PubMed] [Google Scholar]
- 14.Fux, C. A., S. Wilson, and P. Stoodley. 2004. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 186:4486-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillaspy, A., C. Lee, S. Sau, A. L. Cheung, and M. Smeltzer. 1998. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect. Immun. 66:3170-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsmith, H. L., and V. T. Turitto. 1986. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report-Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb. Haemostasis 55:415-435. [PubMed] [Google Scholar]
- 17.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 18.Heyer, G., S. Saba, R. Adamo, W. Rush, G. Soong, A. Cheung, and A. Prince. 2002. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect. Immun. 70:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillsley, M. V., and J. A. Frangos. 1994. Bone tissue engineering: the role of interstitial fluid flow. Biotechnol. Bioeng. 43:573-581. [DOI] [PubMed] [Google Scholar]
- 20.Lesens, O., Y. Hansmann, E. Brannigan, V. Remy, S. Hopkins, M. Martinot, P. Meyer, B. O. Connel, H. Monteil, D. Christmann, and C. Bergin. 2004. Positive surveillance blood culture is a predictive factor for secondary metastatic infection in patients with Staphylococcus aureus bacteraemia. J. Infect. 48:245. [DOI] [PubMed] [Google Scholar]
- 21.Mascari, L., and J. M. Ross. 2001. Hydrodynamic shear and collagen receptor density determine the adhesion capacity of S. aureus to collagen. Ann. Biomed. Eng. 29:956-962. [DOI] [PubMed] [Google Scholar]
- 22.Mascari, L., and J. M. Ross. 2003. Quantification of staphylococcal-collagen binding interactions in whole blood by use of a confocal microscopy shear-adhesion assay. J. Infect. Dis. 188:98-107. [DOI] [PubMed] [Google Scholar]
- 23.Mascari, L., and J. M. Ross. 2002. Quantifying the temporal expression of the Staphylococcus aureus collagen adhesin. Microb. Pathog. 32:99-103. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed, N., V. Livia, P. Speziale, and J. M. Ross. 2000. Quantification of Staphylococcus aureus cell surface adhesins using flow cytometry. Microb. Pathog. 29:357-361. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed, N., T. R. J. Rainier, and J. M. Ross. 2000. Novel experimental study of receptor-mediated bacterial adhesion under the influence of fluid shear. Biotechnol. Bioeng. 68:628-636. [PubMed] [Google Scholar]
- 26.Mohamed, N., A. M. Teeters, J. M. Patti, M. Höök, and J. M. Ross. 1999. Inhibition of Staphylococcus aureus adherence to collagen under dynamic conditions. Infect. Immun. 67:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 28.Patti, J. M., T. Bremell, D. Kajewski-Pietrasik, A. Abdelnour, A. Tarkowski, C. Rydén, and M. Höök. 1994. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 62:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry, C. 1999. Septic arthritis. Am. J. Orthop. 28:168-178. [PubMed] [Google Scholar]
- 30.Purevdorj-Gage, B., W. J. Costerton, and P. Stoodley. 2005. Phenotypic differentiation and seeding dispersal in non-mucoid and mucoid Pseudomonas aeruginosa biofilms. Microbiology 151:1569-1576. [DOI] [PubMed] [Google Scholar]
- 31.Resch, A., S. Leicht, M. Saric, L. Pásztor, A. Jakob, F. Götz, and A. Nordheim. 2006. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6:1867-1877. [DOI] [PubMed] [Google Scholar]
- 32.Rupp, C. J., C. A. Fux, and P. Stoodley. 2005. Viscoelasticity of Staphylococcus aureus biofilms in response to fluid shear allows resistance to detachment and facilitates rolling migration. Appl. Environ. Microbiol. 71:2175-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 34.Stoodley, P., S. Wilson, L. Hall-Stoodley, J. D. Boyle, H. M. Lappin-Scott, and J. W. Costerton. 2001. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 67:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Switalski, L., J. Patti, W. Butcher, A. Gristina, P. Speziale, and M. Hook. 1993. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol. Microbiol. 7:99-107. [DOI] [PubMed] [Google Scholar]
- 36.Trotonda, M. P., A. C. Manna, A. L. Cheung, I. Lasa, and J. R. Penadés. 2005. SarA positively controls Bap-dependent biofilm formation in Staphylococcus aureus. J. Bacteriol. 187:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle, J., A. Toledo-Arana, C. Berasain, J.-M. Ghigo, B. Amorena, J. R. Penadés, and I. Lasa. 2003. SarA and not sB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 38.Visai, L., Y. Xu, F. Casolini, S. Rindi, M. Hook, and P. Speziale. 2000. Monoclonal antibodies to CNA, a collagen-binding microbial surface component recognizing adhesive matrix molecules, detach Staphylococcus aureus from a collagen substrate. J. Biol. Chem. 275:39837-39845. [DOI] [PubMed] [Google Scholar]
- 39.Xavier, J. B., C. Picioreanu, and M. C. van Loosdrecht. 2005. A general description of detachment for multidimensional modelling of biofilms. Biotechnol. Bioeng. 91:651-669. [DOI] [PubMed] [Google Scholar]