Abstract
Cell-mediated immune (CMI) responses to an acellular pertussis vaccine administered to 49 subjects, a subset of participants in the National Institutes of Health-funded adult acellular pertussis vaccine efficacy trial, were evaluated and compared with antibody responses to vaccine antigens. Levels of proliferation of and cytokine secretion from lymphocytes cultured in the presence of pertussis toxin, filamentous hemagglutinin, or pertactin were measured before vaccination and 1 month and 1 year after vaccination. Statistically significant increases in lymphocyte stimulation indices and cytokine secretion were noted at both 1 month and 1 year after vaccination. Brisk pertussis antigen-specific immunoglobulin G responses were also noted at 1 month after vaccination, but these responses had declined by nearly 50% at 1 year after vaccination. These studies clearly demonstrate that both cellular and humoral immune responses occur after the administration of acellular pertussis vaccines to adolescents and adults but that the CMI responses are of greater magnitude and longer duration. CMI responses may be a better correlate of long-term protection.
The precise immunologic mechanisms by which pertussis vaccines confer protection against infection and disease are not defined. By using murine models, it has been demonstrated that either the adoptive transfer of CD4+ cells from immune mice in the absence of antibody (15) or the presence of passive antibody alone (11) confers protection against challenge with Bordetella pertussis. This finding suggests that either humoral or cell-mediated immunity (CMI) is protective in the murine model. In humans, the lack of a clear correlation between the levels of antibody to pertussis antigens and protection against disease, the persistence of protective immunity long after the disappearance of pertussis antigen-specific antibody, and the longer duration of cell-mediated responses to pertussis antigens lend credence to the possibility that CMI provides primary protection against disease. In an attempt to better understand both the CMI and humoral responses to acellular pertussis vaccines in adolescents and adults and to see if these immune responses could be correlated with each other, we studied a subset of participants in the National Institutes of Health (NIH)-funded adult acellular pertussis vaccine efficacy trial (APERT). Few data for adolescents and adults are presently available to address these important issues (8, 22).
MATERIALS AND METHODS
Study design.
Details of the study design and study results from APERT have been published previously (24). In brief, this prospective trial enrolled 2,781 healthy adolescents and adults between 15 and 65 years of age with no history of pertussis disease or vaccination within the previous 5 years. Patients were randomly selected to receive either an acellular pertussis vaccine (aP) or a control vaccine (a hepatitis A vaccine [HAV]) and were carefully monitored for signs of pertussis. Studies measuring CMI and humoral immune responses in vaccine recipients were conducted with a subset of the participants enrolled at Vanderbilt University Medical Center (one of eight study sites). Written informed consent was obtained before enrollment. The study protocol was approved by the local ethical review board and was performed in compliance with the World Health Organization Declaration of Hong Kong/Helsinki.
Vaccines.
Subjects were randomly assigned in a double-blind manner to receive either aP containing 8 μg of pertussis toxin (PT), 8 μg of filamentous hemagglutinin (FHA), and 2.5 μg of pertactin (PRN) or HAV containing 720 enzyme-linked immunosorbent assay (ELISA) units (EU) of hepatitis A antigen (HAVRIX) by deep intramuscular injection into the left deltoid. Both vaccines were adsorbed to aluminum hydroxide and were kindly provided by GlaxoSmithKline Biologicals, Rixensart, Belgium.
Blood samples.
Both sera and heparinized whole blood were collected from the subset of subjects enrolled in the CMI studies before, 1 month after, and 1 year after vaccination. Sera were stored at −80°C until analysis. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll (Serva, Heidelberg, Germany) density gradient centrifugation and resuspended in inactivated fetal calf serum (Cambrex, NJ)-10% dimethyl sulfoxide for long-term storage in liquid nitrogen. Fetal calf sera were screened and were not associated with nonspecific-lymphocyte proliferation. Assays were not performed according to consecutive sample collection during the study period, but all samples were studied within a short time period to ensure consistent results. Furthermore, we compared the performances of fresh versus frozen cells in our assay. Although we demonstrated a 23% decrease in overall lymphoproliferation rates with freezing, we obtained highly consistent responses regarding differences in culture conditions (data not shown).
Serology.
Immunoglobulin G (IgG) antibodies to PT, FHA, and PRN were assayed using standardized ELISAs modified from methods described previously (6, 7, 9, 17). ELISA units for IgG were determined by using U.S. reference pertussis antigen antisera (human) lots 3 and 4. The minimum level of detection for IgG antibody to each antigen was 2 EU/ml. The limit of quantitation was defined as the boundary below which the precision of assay quantitation declined; it was determined to be 6 EU/ml for PT-specific IgG and 8 EU/ml for FHA- and PRN-specific IgG.
Lymphocyte proliferation assay.
PBMC were thawed according to a standardized protocol including quick warming to 4°C and immediate removal of freezing medium by washing with complete culture medium (RPMI 1640 medium with 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, 1× amino acids from minimal essential medium, 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% human AB serum [PAN Biotech, Aidenbach, Germany]) at room temperature. Lymphocytes were resuspended in complete culture medium and cultured at a cell density of 150,000 cells/200 μl on 96-well round-bottom culture plates (Greiner, Germany) for 5.5 days at 37°C and 5% CO2 in the presence of 10 μg/ml of PT, FHA, or PRN. Positive controls to show the general capacity for proliferation included PBMC cultured with 5 μg/ml of the mitogen phytohemagglutinin (Boehringer Mannheim, Germany) for 2.5 days. Negative controls included PBMC incubated for 2.5 days and 5.5 days in the absence of mitogens or antigens. Proliferative rates were determined by measuring [3H]thymidine (0.5 μCi of [3H]thymidine/well added during the last 16 h of culture) uptake by cultured PBMC by using a liquid scintillation counter (Betaplater 1205; WALLAC, Finland). All cultures were performed in triplicate.
To ensure the viability and suitability of the cells subjected to culture, only samples complying with each of the following criteria were used for final evaluation: (i) >80% of PBMC excluded propidium iodide at the start of the culture period, (ii) [3H]thymidine uptake exceeded 50,000 cpm after 2.5 days in the presence of the mitogen phytohemagglutinin in the positive control wells, and (iii) [3H]thymidine uptake was clearly below 9,000 cpm in the negative control wells.
The proliferative rates were expressed as geometric means of values for triplicate cultures. In accordance with previously published procedures (21), a positive CMI response was defined as a rate of antigen-stimulated proliferation at least fourfold higher than the rate of spontaneous proliferation (stimulation index, ≥4).
Detection of IFN-γ and IL-5.
The cytokines gamma interferon (IFN-γ) and interleukin 5 (IL-5) were measured in the culture supernatant after 5 days of antigenic stimulation by using commercial ELISA systems (Biosource, CA). IFN-γ activity reflects Th1 responses, while IL-5 activity reflects Th2 responses.
Statistics.
Antibody distributions were expressed as geometric mean titers (GMTs) by using logarithm-transformed data. Levels below the minimum level of detection were assigned a value of 1. Rates of antigen-specific-lymphocyte proliferation were compared with rates of proliferation in negative controls containing only media by using log-transformed geometric means of values for triplicate cell cultures (t test). Statistical analyses were performed using SigmaStat software (SPSS, Munich, Germany). Analyses of the correlation between antibody levels and quantitative cell proliferation data were performed by using linear regression of log ratios of values from 1 month and 1 year postvaccination to the prevaccination values.
RESULTS
Study population and study specimens.
Eighty-five subjects consented to participate in the evaluation of pertussis antigen-specific CMI. To reduce the costs of performing assays, a smaller control group that received HAV (10 subjects) was included. These control subjects were randomly selected by the NIH to ensure blinding of both the investigators and the laboratory personnel in Germany. Cryopreserved lymphocytes were recovered with an overall yield of 85%, showing a mean viability of 93% with low spontaneous proliferation (mean, 1,595 cpm; σ, 4,669 cpm). Samples from 55 subjects complied with all inclusion criteria and had all corresponding CMI and serologic data. Forty-nine of these subjects received aP, and six of these subjects received HAV. Demographically, excluded subjects did not differ from included ones (data not shown). The subset undergoing CMI studies had a mean age of 31.5 years (range, 16.1 to 59.4 years), 69.1% were female, and 41.8% were health care workers, and these characteristics were similar to those of the overall APERT participant group.
Pertussis antigen-specific antibodies in serum.
One month and 1 year after vaccination, concentrations of IgG antibodies to PT, FHA, and PRN in the aP group were significantly higher than before vaccination (Table 1) Pertussis antigen-specific-antibody increases were not observed in controls, and their postimmunization titers were comparable to prevaccination levels (Table 1). More detailed immunogenicity results from the combined study have been published elsewhere (10).
TABLE 1.
IgG antibody concentrations before and after vaccinationa
| Group (n) and time point | Concn of antibody to:
|
% of subjects with indicated concn of antibody to:
|
||||
|---|---|---|---|---|---|---|
| PT | FHA | PRN | PT, >6 EU/ml | FHA, >8 EU/ml | PRN, >8 EU/ml | |
| Vaccine (aP) group (49) | ||||||
| Prevaccination | 8 | 24 | 16 | 73 | 100 | 100 |
| Postvaccination (1 mo) | 40 | 419 | 438 | 100 | 100 | 100 |
| Postvaccination (1 yr) | 17 | 173 | 129 | 98 | 100 | 100 |
| Control (HAV) group (6) | ||||||
| Prevaccination | 6 | 16 | 16 | 67 | 100 | 83 |
| Postvaccination (1 mo) | 6 | 16 | 18 | 67 | 100 | 83 |
| Postvaccination (1 yr) | 14 | 15 | 18 | 100 | 100 | 83 |
Concentrations are given as geometric mean titers. Adults (n = 49) from the APERT subset for CMI analysis were vaccinated with a dose of an acellular pertussis vaccine.
Pertussis antigen-specific-lymphocyte proliferation.
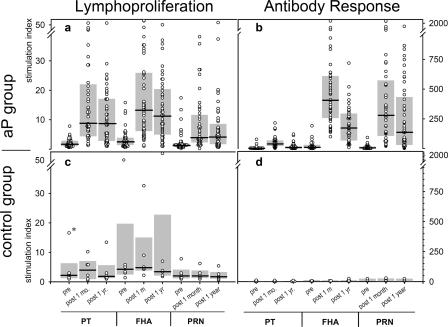
Pertussis antigen-specific-lymphocyte proliferation responses to each of the three pertussis antigens are represented in Fig. 1. Minimal proliferation responses were seen in the prevaccination blood samples from aP recipients and in all samples from HAV controls.
FIG. 1.
Responses of subjects to an acellular pertussis vaccine. The proliferation of pertussis antigen-specific lymphocytes (a and c) and antibody concentrations (b and d) were measured. Lymphocytes from 49 vaccinated subjects (aP group) (a) and those from the 6 members of the control group (c) were cultured in the presence of antigens (10 μg/ml PT, 10 μg/ml FHA, or 10 μg/ml PRN). [3H]thymidine (0.5 μCi) was added during the last 16 h of culture, and the level of incorporation was measured by scintillation counting (counts per minute). Stimulation indices were calculated as follows: (counts per minute for the culture with the antigen)/(counts per minute for the medium control). Serum samples from the vaccinated subjects (b) and the control group (d) were analyzed for pertussis antigen-specific IgG with ELISA. IgG concentrations are expressed as EU/ml. Lymphoproliferation measurements and serum antibody concentrations increased significantly at 1 month (post 1 m) and 1 year (post 1 yr) postvaccination compared to corresponding prevaccination (pre) values. Shaded bars indicate interquartile ranges, and black horizontal lines indicate median values. The outlier value (*) is attributed to a single subject; although the value for pertussis antigen-specific lymphoproliferation is high, prevaccination versus postvaccination data for the outlier did not reflect an impact of the vaccination.
Compared to prevaccination levels, significantly increased rates of proliferation of lymphocytes specific for all three antigens in samples from the aP subjects were observed at both the 1-month- and the 1-year-postvaccination time points (P < 0.05) (Fig. 1). The responses at 1 year were lower than those noted at 1 month postimmunization. Subjects with pertussis antigen-specific-lymphocyte responses following aP administration did not differ from nonresponders in gender, age, or occupation (data not shown). None of the participants in the CMI subset of the APERT group had culture-confirmed pertussis or serologic conversions during the pertussis surveillance period, suggesting that there was no natural exposure to pertussis among these subjects after vaccination.
The secretion of pertussis antigen-specific IFN-γ by lymphocytes was measured prevaccination and at both 1 month and 1 year after vaccination (Table 2). One month after vaccination, the secretion of IFN-γ specific for PT, FHA, and PRN had increased 53-fold, 80-fold, and 66-fold, respectively. One year after vaccination, IFN-γ secretion remained elevated compared with that in controls but was diminished by 60 to 70% relative to the 1-month-postvaccination levels. Little or no secretion of pertussis antigen-specific gamma interferon in HAV recipients was noted at any time point (Table 2).
TABLE 2.
Increases and decreases in IFN-γ and IL-5 pertussis antigen-specific-lymphocyte proliferation responsesa
| Group (n) and time point postimmunizationb | Change (n-fold) in IFN-γ response to:
|
Change (n-fold) in IL-5 response to:
|
||||
|---|---|---|---|---|---|---|
| PT | FHA | PRN | PT | FHA | PRN | |
| aP group (49) | ||||||
| 1 mo | 52.7 | 80.01 | 65.57 | 12.22 | 12.61 | 1.0 |
| 1 yr | 37.87 | 50.05 | 46.09 | 9.6 | 3.2 | 1.0 |
| Control group (6) | ||||||
| 1 mo | 0.57 | 0.76 | 0.08 | 0.09 | 1.00 | 0.1 |
| 1 yr | 0.00 | 0.00 | 7.90* | 0.02 | 1.00 | 6.54* |
Asterisks indicate cases in which one outlier was responsible for the high value.
Values at the indicated time points were compared to preimmunization values to determine the amount of change.
As for the secretion of antigen-specific IL-5, a slight increase in the aP group both at 1 month and at 1 year postvaccination was noted (Table 2), but it was less than that observed for IFN-γ. HAV recipients had very little IL-5 secretion at any assessment point.
Comparison of antibody and lymphoproliferative responses.
Titers of individual antibodies to each pertussis antigen in the acellular pertussis vaccine recipients were compared to lymphoproliferative responses to the same antigens by linear regression. Statistically significant correlations between antibody titers and lymphoproliferative responses in the aP group were noted at 1 month postvaccination (Table 3) but not at 1 year after vaccination. The decay in antibody levels was greater than the decay in the interferon stimulation response.
TABLE 3.
Correlation between the lymphoproliferative (CMI) response and the IgG antibody responsea
| Time point postimmunization | Correlation between CMI and IgG antibody responses to:
|
|||||
|---|---|---|---|---|---|---|
| PT
|
FHA
|
PRN
|
||||
| r | P | r | P | r | P | |
| 1 mo | 0.31 | 0.03 | 0.27 | 0.046 | 0.29 | 0.046 |
| 1 yr | 0.12 | 0.38 | 0.18 | 0.20 | 0.30 | 0.03 |
Responses were assessed at 1 month and 1 year after the immunization of adults (n = 49) with an acellular pertussis vaccine. r, correlation coefficient; P, probability.
DISCUSSION
A trivalent acellular pertussis vaccine without diphtheria or tetanus toxoid was administered to healthy adolescents and adults enrolled in APERT, a prospective NIH-sponsored multicenter efficacy trial conducted in the United States. As part of this study, cell-mediated and humoral immune responses were measured before, 1 month after, and 1 year after vaccination. One month after the administration of the acellular pertussis vaccine, antibodies to all three vaccine antigens were detected, but levels declined nearly 50% by 1 year after vaccination, especially those of PT antibodies (5, 23). In contrast, significantly elevated cell-mediated immune responses were observed at 1 month after vaccination and these responses remained elevated at 1 year. The decay in cell-mediated immune responses to the acellular pertussis vaccine was less than the decay in antibody levels.
The role of humoral and cellular immunity in the prevention of pertussis infection and disease remains unclear. Studies of immune responses to acellular pertussis vaccines suggest that both B- and T-cell responses are elicited in mice (11, 18, 20) and humans (4, 16). In the present study, a significant increase in the proliferation of PBMC specific to all three pertussis vaccine antigens was demonstrated at 1 month after vaccination. This finding suggests the induction of an immunological memory response, presumably consisting of restimulated effector memory T cells as well as B-helper T cells. The observed level of proliferation is in accordance with data from other studies with human adults (1, 19) and also reflects data from pediatric vaccine trials (2, 3, 25). Additionally, our data suggest that pertussis antigen-specific humoral and cell-mediated immune responses correlate with each other at 1 month after acellular pertussis immunization.
In contrast to the appreciable decay in PT, FHA, and PRN antibody levels by 1 year after immunization, pertussis antigen-specific T-cell responses persisted at high levels. The prolonged presence of detectable T-cell-mediated immunity after the administration of the acelluar pertussis vaccine has been demonstrated previously with both infants (25, 26) and adults (4, 22). Mahon et al. (12) hypothesized that T-cell-mediated immune memory might be a major determinant of more prolonged protection whereas the immediate induction of antibodies may serve to combat the acute infection. The physiological basis of this difference has not been described. It has been recognized that following the cognate contact of helper T cells with B cells in the germinal center of secondary lymph nodes, affinity-maturated B-cell subsets develop. Antibody-secreting memory B cells are available for secondary antigen-specific responses (14). Thus, the decrease in antibody levels with time is likely attributable to a loss of plasma cells over time but the retention of memory B cells, which likely offer “boostability” when receiving instruction from memory T cells like those detected in our study. A correlation between the antibody levels and memory-T-cell responses during the early (prevaccination-to-1-month-postvaccination) phase exists, and the memory-B-cell pool could be activated upon a secondary antigen contact at a later time (1 year postvaccination).
We further measured specific cytokine secretion by pertussis antigen-specific stimulated T cells. Significant increases in IFN-γ secretion by PBMC were seen 1 month after vaccination. Since increases in IL-5 were lower than increases in IFN-γ, we speculate that the CMI responses to pertussis antigens were more Th1-like. Similar Th1 cytokine secretion profiles for adults (1, 19) and children (13) receiving acellular vaccines have been observed previously. The results of these studies indicate that healthy adolescents and adults immunized with a three-component acellular pertussis vaccine generate both humoral and sustained cell-mediated immune responses.
Acknowledgments
We thank GlaxoSmithKline Biological, Rixensart, Belgium, for contributing vaccines and antigenic material for the CMI response assays.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Ausiello, C. M., R. Lande, A. la Sala, F. Urbani, and A. Cassone. 1998. Cell-mediated immune response of healthy adults to Bordetella pertussis vaccine antigens. J. Infect. Dis. 178:466-470. [DOI] [PubMed] [Google Scholar]
- 2.Ausiello, C. M., R. Lande, F. Urbani, B. Di Carlo, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 2000. Cell-mediated immunity and antibody responses to Bordetella pertussis antigens in children with a history of pertussis infection and in recipients of an acellular pertussis vaccine. J. Infect. Dis. 181:1989-1995. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., R. Lande, F. Urbani, A. la Sala, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassone, A., P. Mastrantonio, and C. M. Ausiello. 2000. Are only antibody levels involved in the protection against pertussis in acellular pertussis vaccine recipients? J. Infect. Dis. 182:1575-1577. [DOI] [PubMed] [Google Scholar]
- 5.Cherry, J. D., S. J. Chang, D. Klein, M. Lee, S. Barenkamp, D. Bernstein, R. Edelman, M. D. Decker, D. P. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Prevalence of antibody to Bordetella pertussis antigens in serum specimens obtained from 1793 adolescents and adults. Clin. Infect. Dis. 39:1715-1718. [DOI] [PubMed] [Google Scholar]
- 6.Cherry, J. D., U. Heininger, P. D. Christenson, T. Eckhardt, S. Laussucq, J. G. Hackell, J. R. Mezzatesta, and K. Stehr. 1995. Surrogate serologic tests for the prediction of pertussis vaccine efficacy. Ann. N. Y. Acad. Sci. 754:359-363. [DOI] [PubMed] [Google Scholar]
- 7.Deen, J. L., C. A. Mink, J. D. Cherry, P. D. Christenson, E. F. Pineda, K. Lewis, D. A. Blumberg, and L. A. Ross. 1995. Household contact study of Bordetella pertussis infections. Clin. Infect. Dis. 21:1211-1219. [DOI] [PubMed] [Google Scholar]
- 8.Edelman, K. J., Q. He, J. P. Makinen, M. S. Haanpera, N. N. Tran Minh, L. Schuerman, J. Wolter, and J. A. Mertsola. 2004. Pertussis-specific cell-mediated and humoral immunity in adolescents 3 years after booster immunization with acellular pertussis vaccine. Clin. Infect. Dis. 39:179-185. [DOI] [PubMed] [Google Scholar]
- 9.Hodder, S. L., J. D. Cherry, E. A. Mortimer, Jr., A. B. Ford, J. Gornbein, and K. Papp. 2000. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin. Infect. Dis. 31:7-14. [DOI] [PubMed] [Google Scholar]
- 10.Le, T., J. D. Cherry, S. J. Chang, M. D. Knoll, M. L. Lee, S. Barenkamp, D. Bernstein, R. Edelman, K. M. Edwards, D. Greenberg, W. Keitel, J. Treanor, and J. I. Ward. 2004. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J. Infect. Dis. 190:535-544. [DOI] [PubMed] [Google Scholar]
- 11.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahon, B. P., M. T. Brady, and K. H. Mills. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181:2087-2091. [DOI] [PubMed] [Google Scholar]
- 13.Mascart, F., V. Verscheure, A. Malfroot, M. Hainaut, D. Pierard, S. Temerman, A. Peltier, A. S. Debrie, J. Levy, G. Del Giudice, and C. Locht. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170:1504-1509. [DOI] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams, L. J., L. P. Malherbe, and M. G. McHeyzer-Williams. 2006. Checkpoints in memory B-cell evolution. Immunol. Rev. 211:255-268. [DOI] [PubMed] [Google Scholar]
- 15.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mink, C. A., N. M. Sirota, and S. Nugent. 1994. Outbreak of pertussis in a fully immunized adolescent and adult population. Arch. Pediatr. Adolesc. Med. 148:153-157. [DOI] [PubMed] [Google Scholar]
- 18.Petersen, J. W., P. H. Ibsen, K. Haslov, and I. Heron. 1992. Proliferative responses and gamma interferon and tumor necrosis factor production by lymphocytes isolated from tracheobroncheal lymph nodes and spleens of mice aerosol infected with Bordetella pertussis. Infect. Immun. 60:4563-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds, E., B. Walker, D. Xing, J. Southern, C. Asokanathan, B. Dagg, M. Corbel, and E. Miller. 2006. Laboratory investigation of immune responses to acellular pertussis vaccines when used for boosting adolescents after primary immunisation with whole cell pertussis vaccines: a comparison with data from clinical study. Vaccine 24:3248-3257. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 69:7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran Minh, N. N., K. Edelman, Q. He, M. K. Viljanen, H. Arvilommi, and J. Mertsola. 1998. Antibody and cell-mediated immune responses to booster immunization with a new acellular pertussis vaccine in school children. Vaccine 16:1604-1610. [DOI] [PubMed] [Google Scholar]
- 22.Tran Minh, N. N., Q. He, K. Edelman, A. Putto-Laurila, H. Arvilommi, M. K. Viljanen, and J. Mertsola. 2000. Immune responses to pertussis antigens eight years after booster immunization with acellular vaccines in adults. Vaccine 18:1971-1974. [DOI] [PubMed] [Google Scholar]
- 23.Ward, J. I., J. D. Cherry, S. J. Chang, S. Partridge, W. Keitel, K. Edwards, M. Lee, J. Treanor, D. P. Greenberg, S. Barenkamp, D. I. Bernstein, and R. Edelman. 2006. Bordetella pertussis infections in vaccinated and unvac-cinated adolescents and adults, as assessed in a national prospective randomized acellular pertussis vaccine trial (APERT). Clin. Infect. Dis. 43:151-157. [DOI] [PubMed] [Google Scholar]
- 24.Ward, J. I., J. D. Cherry, S. J. Chang, S. Partridge, H. Lee, J. Treanor, D. P. Greenberg, W. Keitel, S. Barenkamp, D. I. Bernstein, R. Edelman, and K. Edwards. 2005. Efficacy of an acellular pertussis vaccine among adolescents and adults. N. Engl. J. Med. 353:1555-1563. [DOI] [PubMed] [Google Scholar]
- 25.Zepp, F., M. Knuf, P. Habermehl, H. J. Schmitt, C. Meyer, R. Clemens, and M. Slaoui. 1997. Cell-mediated immunity after pertussis vaccination and after natural infection. Dev. Biol. Stand. 89:307-314. [PubMed] [Google Scholar]
- 26.Zepp, F., M. Knuf, P. Habermehl, J. H. Schmitt, C. Rebsch, P. Schmidtke, R. Clemens, and M. Slaoui. 1996. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect. Immun. 64:4078-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]