Abstract
The Th1/Th2 balance deregulation toward a Th2 immune response plays a central role in allergy. We previously demonstrated that administration of recombinant Lactococcus lactis strains expressing bovine β-lactoglobulin (BLG), a major cow's milk allergen, partially prevents mice from sensitization. In the present study, we aimed to improve this preventive effect by coadministration of L. lactis BLG and a second recombinant L. lactis strain producing biologically active interleukin-12 (IL-12). This L. lactis strain producing IL-12 was previously used to enhance the Th1 immune response in a tumoral murine model (L. G. Bermúdez-Humarán et al., J. Immunol. 175:7297-7302, 2005). A comparison of the administration of either BLG alone or BLG in the presence of IL-12 was conducted. A BLG-specific primary Th1 immune response was observed only after intranasal coadministration of both L. lactis BLG and IL-12-producing L. lactis, as demonstrated by the induction of serum-specific immunoglobulin G2a (IgG2a) concomitant with gamma interferon secretion by splenocytes, confirming the adjuvanticity of IL-12-producing L. lactis. Immunized mice were further sensitized by intraperitoneal administration of purified BLG, and the allergic reaction was elicited by intranasal challenge with purified BLG. Mice pretreated with BLG in either the presence or the absence of IL-12 were rendered completely tolerant to further allergic sensitization and elicitation. Pretreatment with either L. lactis BLG or L. lactis BLG and IL-12-producing L. lactis induces specific anti-BLG IgG2a production in serum and bronchoalveolar lavage (BAL) fluid. Although specific serum IgE was not affected by these pretreatments, the levels of eosinophilia and IL-5 secretion in BAL fluid were significantly reduced after BLG challenge in the groups pretreated with L. lactis BLG and L. lactis BLG-IL-12-producing L. lactis, demonstrating a decreased allergic reaction. Our data demonstrate for the first time (i) the induction of a protective Th1 response by the association of L. lactis BLG and IL-12-producing L. lactis which inhibits the elicitation of the allergic reaction to BLG in mice and (ii) the efficiency of intranasal administration of BLG for the induction of tolerance.
Food allergies, i.e., immediate-type, immunoglobulin E (IgE)-mediated immune responses, affect 2 to 2.5% of the general populations of Western countries (22, 24, 25). It results from the activation of the Th2-type helper lymphocytes. In mice, this response is mainly characterized by the production of interleukin-4 (IL-4) and IL-13, which induce the production of IgE and IgG1, and of IL-5, which attracts eosinophils. In contrast, the Th1 response is characterized by the induction of gamma interferon (IFN-γ) and IL-2 production and the stimulation of cellular immunity. Th1 and Th2 cells regulate their own development via the cytokines produced: IFN-γ suppresses Th2-cell proliferation and promotes Th1-cell proliferation, whereas IL-4 promotes additional Th2-cell proliferation and inhibits Th1-cell development (39, 43, 45).
A recent study suggests that an early allergen-specific induction of Th1 cells before allergy sensitization could not efficiently prevent the development of atopic disorders: Th1 priming was abolished in the presence of allergen-specific Th2 cells, whereas Th1 cells could not inhibit subsequent priming of Th2 cells (58). The use of adjuvants that increase the proliferation of Th1 cells and that render them more efficient in inhibiting Th2 cells would therefore be of great interest in the management of allergic diseases. These observations prompted us to explore the adjuvant effect of IL-12 in a mouse model of allergic reaction to bovine β-lactoglobulin (BLG), a major milk allergen (2). IL-12 is a heterodimeric cytokine produced by antigen-presenting cells that promotes the development of naïve Th cells into Th1 effector cells and that induces IFN-γ production (51, 52, 54). IL-12 also inhibits Th2 class switching by repression of the IL-4 cytokine (15, 17, 46, 54, 55). Moreover, treatment with recombinant IL-12 has been shown to have positive effects on bronchial hyperresponsiveness and the eosinophilic response (11). Unfortunately, despite the therapeutic potential of this molecule, the toxicity of systemic IL-12 treatment observed during clinical trials has limited its use (18, 31, 32, 33, 40). This toxicity correlates with increased IFN-γ levels, decreased glucose levels, and altered histological responses in the spleen and duodenum. This has motivated several recent investigations demonstrating that intranasal delivery of IL-12 is a less toxic route of inoculation compared to the commonly used systemic one (6, 7, 26).
The gram-positive and nonpathogenic lactic acid bacteria are used as delivery vehicles for therapeutic proteins (23). Their immunostimulatory effects in patients with allergies have demonstrated that they stimulate Th1 cells and inhibit Th2 cells (35, 37, 47). A recent study demonstrated the inhibition of Der p5-induced airway inflammation and hyperreactivity by recombinant Lactobacillus acidophilus or Streptococcus thermophilus (12). Lactococcus lactis is a lactic acid bacterium widely used in the food industry and extensively engineered for the production of therapeutic proteins (9). L. lactis is of particular interest for the mucosal delivery of functional proteins since it is a noninvasive and nonpathogenic food bacterium that does not survive when it is administered to animal and humans (19, 30), and it was recently shown that the use of genetically modified L. lactis for the mucosal delivery of proteins to humans is feasible. These properties could ensure transient and limited IL-12 expression, thereby limiting the risks of IL-12-related toxicity.
We previously engineered L. lactis strains to produce biologically active IL-12 (L. lactis IL-12) and demonstrated that intranasal administration of L. lactis IL-12 to mice resulted in high levels of stimulation of Th1 cells without the induction of any toxicity (8, 10, 57). Administration of recombinant L. lactis producing BLG allowed the induction of a BLG-specific immune response in mice (13, 14) and partial protection for further sensitization (1). In order to improve the immunomodulatory effect of L. lactis BLG on allergen sensitization and on the elicitation of the allergic reaction, we investigated the effect of the prophylactic coadministration of L. lactis BLG and L. lactis IL-12 in a mouse model of allergy (2). So far, as contradictory results have been reported with ovalbumin (OVA) and house dust mite allergens after intranasal administration (tolerance versus sensitization) (28, 29), we also investigated the effect of intranasal administration of BLG as a protein either alone or coadministered with the IL-12 protein.
MATERIALS AND METHODS
Apparatus and reagents.
All enzyme immunoassays were performed in 96-well microtiter plates (Immunoplate Maxisorb; Nunc, Roskilde, Denmark) by using specialized Titertek microtitration equipment from Labsystems (Helsinki, Finland). BLG was purified from cow's milk, as described previously (41). Unless otherwise stated, all reagents, including recombinant IL-12, were of analytical grade and were obtained from Sigma (St Louis, MO).
Recombinant lactococcal strains and preparation of live inocula for immunization.
The constructions of recombinant L. lactis NZ9000 strains expressing a secreted form of IL-12 (L. lactis IL-12) and BLG fused with the LEISSTCDA propeptide (L. lactis BLG) have been described previously (8, 42). The control strain, i.e., L. lactis NZ9000, containing an empty vector and all derived recombinant strains were grown at 30°C without shaking in M17 medium (Difco) supplemented with 0.5% glucose and 10 μg/ml chloramphenicol. To induce the nisin promoter, all strains (including the control strain) were grown to an optical density at 600 nm (OD600) of 0.4 and induced with 10 ng/ml nisin for 3 to 4 h to achieve an OD600 of ∼1.0. Cellular pellets were then harvested by centrifugation (5,000 × g, 10 min at 4°C) and washed two times with saline (sterile solution of 0.9% NaCl). The pellets were resuspended in saline to a final concentration of 5 × 109 CFU in 10 μl (inoculum) and were immediately administered to mice. The production and amounts of IL-12 and BLG in recombinant lactococci strains were assessed by immunoblotting and were quantified by enzyme-linked immunosorbent assay (ELISA).
Administration of proteins or lactococci and evaluation of primary immune response.
Specific-pathogen-free female BALB/c mice (age, 6 weeks; Centre d'Elevage René Janvier, Le Genest Saint-Isle, France) were maintained under normal husbandry conditions. All animal experiments were started after the animals were allowed 2 weeks of acclimation and were performed according to European Community rules of animal care and with authorization 91-122 of the French Veterinary Services. The immunization protocol is detailed in Fig. 1. Briefly, the mice were slightly anesthetized with isofluorane and were intranasally immunized twice for 3 consecutive days, with a 2-week interval used between the two sets of immunizations. Treatments consisted of the administration of 10 μl into one nostril with the use of a micropipette. Two groups of 12 mice received intranasally an inoculum of L. lactis BLG alone or mixed with L. lactis IL-12. In parallel, two other groups of 12 mice each received intranasally 5 μg of BLG either alone or in combination with 50 ng of recombinant active IL-12 (rIL-12); the concentrations corresponded to the BLG and IL-12 concentrations contained in the inoculum of recombinant lactococci. Control mice (n = 12) received either saline or 5 × 109 CFU of L. lactis. One last group of mice (n = 10) was untreated (naïve mice).
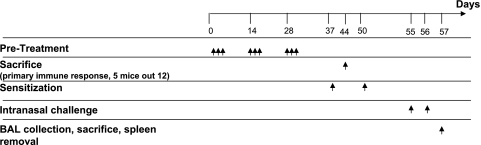
FIG. 1.
Experimental protocol. Groups of mice (n = 12) were intranasally immunized on days 1 to 3, 14 to 16, and 28 to 30 with a live recombinant L. lactis strain producing BLG, L. lactis BLG plus IL-12-producing recombinant L. lactis, nonrecombinant L. lactis, purified protein BLG alone, or BLG in combination with IL-12. Control animals were immunized with saline. On days 37 and 50 the mice were intraperitoneally sensitized with 5 μg of BLG emulsified in incomplete Freund's adjuvant. Allergen challenge was performed on days 55 and 56 by intranasal administration of 20 μg BLG. Naïve mice (n = 5) were left untreated.
The primary immune response was analyzed by using serum samples collected 5 days after the last administration (day 35), as described previously (1). On day 44, five mice from each group were humanely killed, and their spleens were removed under sterile conditions to evaluate cytokine production under specific ex vivo restimulation (see below).
Sensitization and intranasal challenge.
On days 37 and 50, treated mice (n = 7 per group) were sensitized by intraperitoneal injection of 5 μg of BLG emulsified in incomplete Freund's adjuvant (Difco Laboratories, Detroit, MI), as described previously (2, 3). Naïve mice were left untreated (n = 5). Allergen challenge was performed with all mice on days 55 and 56 by intranasal administration of 20 μg of BLG in 50 μl of saline, as described previously (2).
Serum samples were collected on days 48 and 54. On day 57, after the intranasal challenge, the mice were deeply anesthetized by intraperitoneal injection of urethane (15 mg/10 g body weight) and the trachea was cannulated to recover bronchoalveolar lavage (BAL) fluid by infusion with saline. The cells in the BAL fluid were counted on a Malassez slide after trypan blue exclusion, and differential cells counts were performed after cytocentrifugation and staining with May-Grunwald and Giemsa stains (LaboModerne). Morphological characteristics were used for the differentiation of at least 300 cells/sample and allowed quantification of eosinophils, lymphocytes, neutrophils, and macrophages. Aliquots of the remaining BAL fluids were centrifuged and stored at −80°C until the cytokines were assayed.
Quantification of anti-BLG IgE, IgG1, and IgG2a.
The anti-BLG IgE, IgG1, and IgG2a titers of individual serum samples were determined by immunoassays, as described previously (4). Quantitative determination of specific IgE after a booster injection (i.e., on day 54) was preceded by removal of serum IgG by using protein G (PROSEP; Bioprocessing, Consett, United Kingdom) to avoid the interference of IgG with IgE detection (2, 3).
Cytokine assays.
The spleens were harvested under sterile conditions. After lysis of red blood cells (180 mM NH4Cl, 17 mM disodium EDTA) and several washes, the splenocytes were resuspended in RPMI-10 (RPMI supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U penicillin, 100 μg/ml streptomycin). Cells were incubated for 60 h at 37°C (5% CO2) in 96-well culture plates (106 cells/well) in the presence of BLG (20 μg/ml) or concanavalin A (1 μg/ml), which was used as a positive control (1, 2, 3). Incubations with saline or OVA (20 μg/ml) were performed as negative controls. The supernatants were then removed and stored at −80°C until further assays. IL-4 and IFN-γ were assayed by using CytoSets kits (BioSource International Europe, Nivelles, Belgium). IL-5 was assayed by using an immunometric assay with monoclonal antibody TRFK4 for capture and acetylcholinesterase-labeled TRFK5 monoclonal antibody for development (21).
Cytokine levels in BAL fluid were analyzed by using a Bio-plex multiple cytokine assay system, according to the recommendations of the manufacturer (Bio-Rad).
Statistical analyses.
Data were analyzed by analysis of variance with JMP statistical software. Tukey-Kramer's test was used to compare the differences between groups. Comparisons with a control were performed by Dunnett's method. A P value of <0.05 was considered significant.
RESULTS
Effect of intranasal administration of proteins and live recombinant lactococci producing BLG and IL-12 on BLG-specific IFN-γ and IgG2a production.
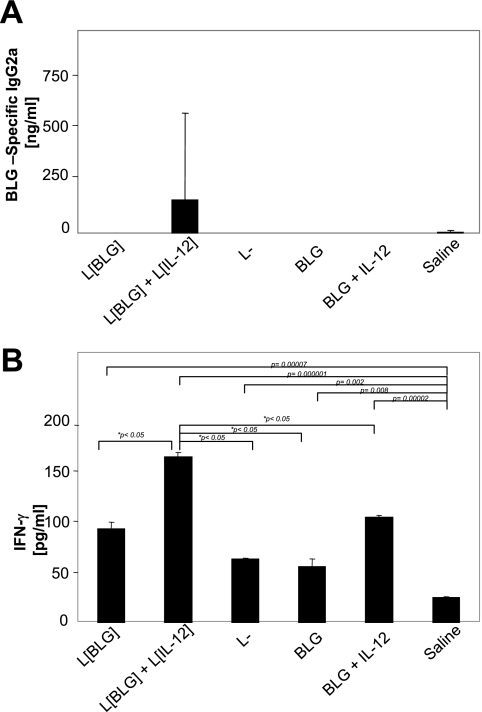
Mice treated with BLG, BLG plus IL-12, L. lactis BLG, or L. lactis did not produce significant levels of anti-BLG IgG2a, anti-BLG IgE, or anti-BLG IgG1 (Fig. 2A and data not shown). In contrast, anti-BLG IgG2a was significantly induced in mice that received a coadministration of L. lactis BLG and L. lactis IL-12 (Fig. 2A). Reactivated splenocytes from those mice also produced significantly higher levels of IFN-γ (P < 0.05) than the splenocytes from the other groups (Fig. 2B). It is noteworthy that the level of IFN-γ production was higher in all treated groups than in untreated mice, whereas specific antibodies were undetectable. This demonstrates the induction of a discrete cellular immune response in the treated mice.
FIG. 2.
Intranasal treatment of mice with L. lactis BLG (L[BLG]) plus L. lactis IL-12 (L[IL-12]) induces a BLG-specific Th1 immune response. Immunized mice (n = 5; Fig. 1) were killed on day 42. Serum samples and spleen cells restimulated in vitro with BLG were tested for IgG2a (A) and IFN-γ (B) production, respectively. Bars represent the means ± standard deviations. The P values obtained with JMP software after comparison of the treated group and the saline (control) group are shown, and a P value of <0.05 indicated statistical significance when the results for the different groups are compared. L−, L. lactis strain containing an empty vector.
These results indicate that the IL-12 delivered by recombinant lactococci favored the induction of a specific Th1 systemic response in L. lactis BLG-treated mice, whereas BLG administered as a protein in the presence or absence of IL-12 or BLG delivered by a recombinant L. lactis strain did not induce any detectable humoral response.
Immunomodulatory effects of BLG and IL-12 proteins and recombinant lactococci on further sensitization.
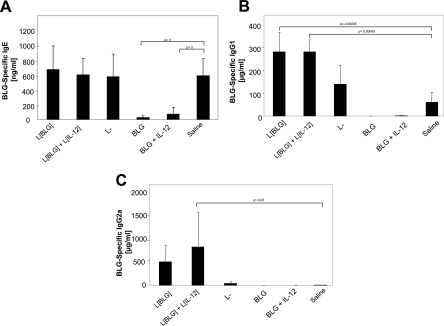
We then investigated the effects of the different treatments on a further sensitization (Fig. 1). After the booster injection (day 54), mice treated with saline (control) demonstrated a high Th2 response, as shown by the induction of specific IgE (Fig. 3A) and IgG1 (Fig. 3B) without the induction of specific IgG2a (Fig. 3C). Mice pretreated with L. lactis BLG alone or L. lactis BLG in combination with L. lactis IL-12 produced equivalent levels of IgE (Fig. 3A) and higher levels of IgG1 (Fig. 3B) than the control mice. Interestingly, these mice also developed a higher anti-BLG IgG2a response (Fig. 3C). However, the last difference was significant only for mice treated with L. lactis BLG plus L. lactis IL-12 due to high individual variability. L. lactis treatment led to nonsignificant changes in the response to the sensitization compared to that in the control mice.
FIG. 3.
Pretreatment of recombinant lactococci did not affect BLG-specific IgE antibody production. Five days after the second sensitization (day 55), the sera of mice (n = 7) were collected and tested for BLG-specific IgE (A), IgG1 (B), and IgG2a (C) antibodies. Bars represent the means ± standard deviations. The P values obtained with JMP software after comparison of treated group and the saline (control) are shown. L[BLG], L. lactis BLG; L[IL-12], L. lactis IL-12; L−, L. lactis strain containing an empty vector.
The absence of a specific antibody (i.e., IgE, IgG1, and IgG2a) response after sensitization was observed in mice treated with BLG via the intranasal route (Fig. 3), suggesting the induction of an efficient tolerance. Addition of IL-12 did not inhibit the induction of the tolerance. Thus, intranasal administration of BLG with or without IL-12 led to the induction of an efficient humoral tolerance in the periphery. In contrast, when BLG and IL-12 were codelivered by recombinant lactococci, such a tolerance was not induced and a Th1-dependent antibody response was observed, confirming the adjuvant role of this bacterium and its efficiency as a delivery vector.
Treatment with BLG or live recombinant lactococci protects sensitized mice against intranasal challenge with BLG allergen.
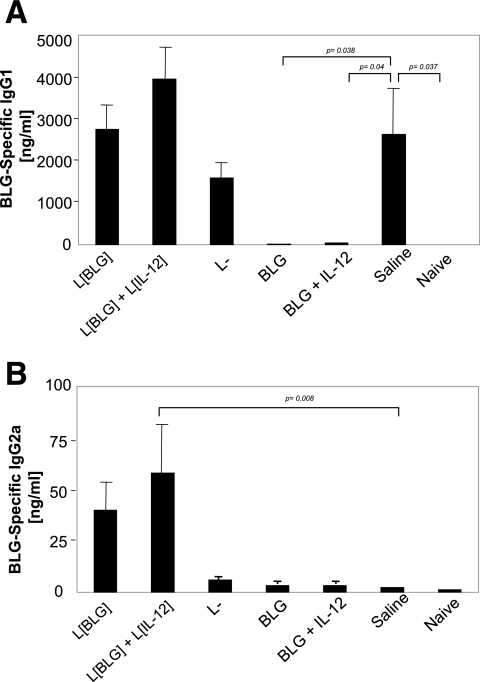
After two intranasal challenges of sensitized control mice, specific IgG1 (Fig. 4A) was evidenced in BAL fluid, whereas specific IgG2a were undetectable (Fig. 4B). Conversely, a significant increase in anti-BLG IgG2a was observed in mice pretreated with L. lactis BLG and L. lactis IL-12 (Fig. 4B), whereas the IgG1 levels were equivalent to those in the control mice (Fig. 4A). The same profile was observed for mice treated with L. lactis BLG alone, although the increase in the IgG2a level was not significant compared to the level in the control mice (Fig. 4B). No significant differences in anti-BLG specific IgG1 and IgG2a levels were observed between the L. lactis and control groups. Mice treated with BLG in the presence or in absence of rIL-12 became tolerant to subsequent sensitization and did not react to the challenge, as demonstrated by the absence of specific antibodies in BAL fluid.
FIG. 4.
Pretreatment with L. lactis BLG (L[BLG]) and L. lactis BLG plus L. lactis IL-12 (L[IL-12]) allows the production of IgG2a in BAL fluid. On day 57, sensitized and challenged mice (n = 7) were killed and BAL fluids were collected as described in Materials and Methods. BLG-specific IgG1 (A) and IgG2a (B) antibody concentrations were determined by quantitative immunoassays. Bars represent the means ± standard deviations. The P values obtained with JMP software after comparison of treated group and the saline (control) are shown. L−, L. lactis strain containing an empty vector.
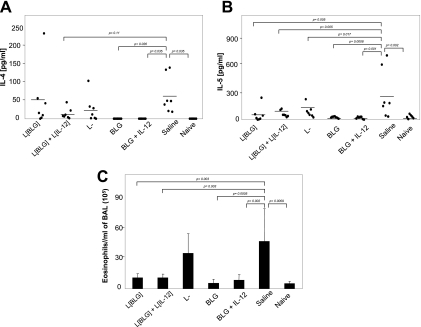
The cytokine levels in BAL fluid were then assayed. Local secretions of IL-4 (Fig. 5A) and IL-5 (Fig. 5B) were detected in control mice after challenge, demonstrating the local elicitation of an allergic reaction. On the other hand, a significant decrease in the level of IL-5 production was observed in the BAL fluid of all treated mice compared to that in the BAL fluid of the control mice (Fig. 5B), together with a significant decrease in the level of IL-4 secretion in the BAL fluid of mice treated with the proteins (Fig. 5A). Due to high individual variability in the group treated with L. lactis BLG, no significant decrease in the level of IL-4 secretion in BAL fluid was observed in any of the groups treated with live recombinant lactococci (Fig. 5A). Nevertheless, exclusion from the statistical analysis of the data for the mouse in the L. lactis BLG group that responded with high IL-4 levels provided evidence of a significant protective effect of coadministration of L. lactis IL-12 and L. lactis BLG on IL-4 secretion. No significant differences in the levels of production of the other cytokines were observed between the different groups.
FIG. 5.
Intranasal pretreatment with L. lactis BLG (L[BLG]) and L. lactis BLG plus L. lactis IL-12 (L[IL-12]) reduced IL-5 and IL-4 levels and decreased the level of airway eosinophilia. On day 57, sensitized and challenged mice were killed and BAL fluids were collected. IL-4 (A) and IL-5 (B) cytokines were measured by ELISA, and eosinophil influx (C) was quantified by differential cellular counts under May-Grunwald and Giemsa staining. Bars represent the means ± standard deviations. The P values obtained with JMP software after comparison of treated group and the saline (control) are shown. L−, L. lactis strain containing an empty vector.
Finally, eosinophil influx was also evaluated in the different groups of mice. Significant eosinophilia was detected in the BAL fluid of control mice sensitized and challenged with BLG (Fig. 5C), confirming the local elicitation of the allergic reaction in those mice. The same result was obtained for L. lactis-treated mice. Interestingly, a significantly decreased eosinophilia was observed in mice treated with L. lactis BLG and L. lactis BLG plus L. lactis IL-12. This decrease was equivalent to that observed in mice rendered tolerant by treatment with BLG in the presence or the absence of IL-12 (Fig. 5C).
DISCUSSION
Previously, we have demonstrated that oral administration of recombinant L. lactis strains producing BLG allows the partial prevention of sensitization to this major cow's milk allergen (1). The highest level of IgE inhibition was obtained with the L. lactis strain producing the largest amounts of BLG fused with the staphylococcal nuclease (Nuc). However, the potential immune response induced against Nuc limits its use for human application. Although IgE was not suppressed, significant increases in specific IgG2a and IFN-γ responses were obtained in a prophylaxis protocol with the recombinant strain producing BLG (L. lactis BLG) fused with the synthetic propeptide LEISSTCDA, whereas strains producing smaller amounts of BLG alone were ineffective. We also demonstrated that intranasal administration of a recombinant L. lactis strain secreting biologically active IL-12 induces a Th1 antigen-specific immune response in mice (8, 10). To enhance the efficiency of prophylaxis with the L. lactis BLG-producing strain, in the present study we coadministered L. lactis IL-12 and L. lactis BLG strains, and the immune response evoked and its effects on further sensitization and elicitation of the allergic response were evaluated.
Our results demonstrate that intranasal coadministration of live L. lactis BLG and L. lactis IL-12 allow the induction of a primary Th1 systemic immune response, as shown by the induction of BLG-specific IgG2a in serum and IFN-γ-producing Th1 cells in the spleen. In contrast, specific IgG2a was not detected either in mice treated with L. lactis BLG or in mice treated with BLG plus IL-12. These results confirmed the adjuvant effect of IL-12 delivered by L. lactis on the BLG-specific response and the efficiency of the intranasal route of administration in our model. Interestingly, mice treated with an empty L. lactis strain produced significantly higher levels of IFN-γ (P < 0.05) than control mice during the primary immune response. The intrinsic Th1 adjuvant effect of L. lactis or other lactic acid bacterial strains has already been observed in several studies (1, 34, 38, 48, 50).
Furthermore, we observed that administration of L. lactis BLG with or without coadministration of L. lactis IL-12 suppressed the further development of airway eosinophilia after intranasal challenge, whereas specific IgE was not reduced. These findings were correlated with the decreased levels of IL-5 in BAL fluid and the level of increased IgG2a production in serum and BAL fluid. These observations suggest that treatments with recombinant lactococci allow the induction of BLG-specific Th1 cells that downregulate Th2 and effector cells. This preventive effect was more efficient when L. lactis BLG was coadministered with L. lactis IL-12. Surprisingly, specific IgE levels were not decreased in those pretreated groups. However, similar data have already been reported by different laboratories (16, 20, 36, 58). For example, Erb et al. (20) observed that infection of mice with Mycobacterium bovis bacillus Calmette-Guérin (BCG) resulted in the inhibition of airway eosinophilia correlated with reduced levels of IL-5, while IgE production remained unaffected. The authors proposed that the induction of a Th1 immune response (e.g., IFN-γ production) during local M. bovis BCG infection was responsible for this protection and suggested that this protection did not extend to the systemic immune response against the allergen. However, our results revealed the induction of both systemic and local Th1 immune responses after intranasal pretreatment with recombinant L. lactis, as demonstrated by specific IgG2a in serum and BAL fluid. It appears, then, that although the systemic Th1 immune response induced by treatment with L. lactis BLG and L. lactis BLG plus L. lactis IL-12 cannot totally inhibit the systemic Th2 response induced by intraperitoneal sensitization, it allows the redirection of the Th subset toward a mixed systemic Th1/Th2 response. Moreover, the local Th1 immune response generated by recombinant lactococci induced the production of specific IgG2a in BAL fluid. Those IgG2a antibodies can efficiently capture BLG, which then allows decreased levels of IgE binding to the allergen and, finally, decreased levels of Th2 cytokine production and eosinophilia after allergen challenge. This is in accordance with the results obtained by Yasumi et al. (58), who showed that primed Th2 cells proliferate more vigorously than primed Th1 cells. Although the authors concluded that the induction of allergen-specific Th1 cells, even before sensitization, will not prevent the development of atopic disorder, these observations suggest that treatment with recombinant lactococci should be more efficient for therapy than for prophylaxis protocols.
An interesting finding of this study is that intranasal administration of low doses of BLG protein (5 μg) for 3 consecutive days, repeated twice at a 2-week interval, induced a profound state of tolerance in BALB/c mice, as demonstrated by the total absence of both systemic and local responses in sensitized and challenged mice. Indeed, previous studies have demonstrated that short-term or continuous intranasal exposure of mite allergen, but not of OVA, caused eosinophilia in BAL fluid and bronchoconstriction in BALB/c mice, along with elevated Th2-cell-associated production of antibodies in serum and Th2-cell-associated production of cytokines by splenocytes (28, 49), whereas other studies have demonstrated that repeated exposure of BALB/c mice to an inhaled low dose of OVA inhibited the potential to develop a specific Th2 response in the periphery due to a noncompartmentalized induced immune tolerance (5, 44). In a recent study, the prevention of a cellular and a humoral Th2-mediated allergic response was obtained by intranasal administration of three consecutive high doses of OVA (100 μg), whereas lower doses (10 μg) were found to be less effective and intranasal treatment was less effective than orally induced tolerance (29). Thus, our results suggest that BLG shares the same tolerogenic properties as OVA when it is administered intranasally before sensitization.
Studies by Winkler et al. (56) demonstrate an increase in transforming growth factor β (TGF-β) mRNA in spleen cells from mice made Bet v1 tolerant intranasally. In this study, we did not observe the production of cytokines such as IL-10 or TGF-β in BAL fluid or reactivated splenocytes in mice pretreated with BLG (data not shown). This suggests a cell-contact-dependent tolerance, which may involve CD4+ T cells expressing membrane-bound TGF-β, as previously demonstrated in a study of low-dose induced airway tolerance and in peptide intranasal immunotherapy (27, 44). IL-12 was inefficient in breaking tolerance induction in our model. This observation is surprising, as IL-12 has been demonstrated to have a Th1 adjuvant effect, inhibiting bronchial hyperresponsiveness and the eosinophilic response (11). This suggests either that the doses of IL-12 that we administered were too low to exert a Th1 adjuvant effect or that intranasal tolerance is preferred to the Th1 response in the nose in our model.
The effect of intranasal administration of BLG in an already sensitized animal should be evaluated to determine if it will abrogate or amplify the elicitation of the allergic reaction in the respiratory tract. As examples, intranasal application of recombinant birch allergen Bet v1 led to the suppression of the allergic immune response in sensitized mice (56), and continuous intranasal exposure to OVA in sensitized BALB/c animals led to the complete abrogation of airway inflammation (53). However, the effectiveness of mucosal OVA administration in suppressing T-cell immunity declined when mucosal antigen delivery started after immunization and became selective for the Th2-mediated pulmonary allergic response but not for T-cell-mediated antibody production (29). The Th1 adjuvant effect of L. lactis BLG and L. lactis IL-12 should then be of great interest in controlling the ongoing Th2 response in our model.
In conclusion, our study provides evidence of efficient tolerance induction by intranasal administration of low doses of BLG protein. It also demonstrates the efficient prevention of the elicitation of the allergic reaction to BLG by coadministration of recombinant L. lactis strains producing BLG and IL-12 by induction of a specific Th1 immune response regulating systemic and local Th2 and effectors cells. These promising results therefore represent a step toward the development of new strategies for the management of allergies.
Acknowledgments
Naima G. Cortes-Perez was the recipient of a grant from the Human Nutrition Department of INRA.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Adel-Patient, K., S. Ah-Leung, C. Creminon, S. Nouaille, J. M. Chatel, P. Langella, and J. M. Wal. 2005. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin. Exp. Allergy 35:539-546. [DOI] [PubMed] [Google Scholar]
- 2.Adel-Patient, K., M. A. Nahori, B. Proust, J. R. Lapa e Silva, C. Creminon, J. M. Wal, and B. B. Vargaftig. 2003. Elicitation of the allergic reaction in beta-lactoglobulin-sensitized Balb/c mice: biochemical and clinical manifestations differ according to the structure of the allergen used for challenge. Clin. Exp. Allergy 33:376-385. [DOI] [PubMed] [Google Scholar]
- 3.Adel-Patient, K., C. Creminon, D. Boquet, J. M. Wal, and J. M. Chatel. 2001. Genetic immunisation with bovine beta-lactoglobulin cDNA induces a preventive and persistent inhibition of specific anti-BLG IgE response in mice. Int. Arch. Allergy Immunol. 126:59-67. [DOI] [PubMed] [Google Scholar]
- 4.Adel-Patient, K., C. Creminon, H. Bernard, G. Clement, L. Negroni, Y. Frobert, J. Grassi, J. M. Wal, and J. M. Chatel. 2000. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J. Immunol. Methods 235:21-32. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez, D., F. K. Swirski, T. C. Yang, R. Fattouh, K. Croitoru, J. L. Bramson, M. R. Stämpfli, and M. Jordana. 2006. Inhalation tolerance is induced selectively in thoracic lymph nodes but executed pervasively at distant mucosal and non mucosal tissues. J. Immunol. 176:2568-2580. [DOI] [PubMed] [Google Scholar]
- 6.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 7.Arulanandam, B. P., and D. W. Metzger. 1999. Modulation of mucosal and systemic immunity by intranasal interleukin 12 delivery. Vaccine 17:252-260. [DOI] [PubMed] [Google Scholar]
- 8.Bermúdez-Humarán, L. G., N. G. Cortes-Perez, F. Lefevre, V. Guimaraes, S. Rabot, J. M. Alcocer-Gonzalez, J. J. Gratadoux, C. Rodriguez-Padilla, R. S. Tamez-Guerra, G. Corthier, A. Gruss, and P. Langella. 2005. A novel mucosal vaccine based on live lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 175:7297-7302. [DOI] [PubMed] [Google Scholar]
- 9.Bermúdez-Humarán, L. G., G. Corthier, and P. Langella. 2004. Recent advances in the use of Lactococcus lactis as live recombinant vector for the development of new safe mucosal vaccines. Recent Res. Dev. Microbiol. 8:147-160. [Google Scholar]
- 10.Bermúdez-Humarán, L. G., P. Langella, N. G. Cortes-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Saucedo-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjermer, L., and Z. Diamant. 2004. Current and emerging nonsteroidal anti-inflammatory therapies targeting specific mechanisms in asthma and allergy. Treat. Respir. Med. 3:235-246. [DOI] [PubMed] [Google Scholar]
- 12.Charng, Y. C., C. C. Lin, and C. H. Hsu. 2005. Inhibition of allergen-induced airway inflammation and hyperreactivity by recombinant lactic-acid bacteria. Vaccine 24:5931-5936. [DOI] [PubMed] [Google Scholar]
- 13.Chatel, J. M., S. Nouaille, K. Adel-Patient, Y. Le Loir, H. Boe, A. Gruss, J. M. Wal, and P. Langella. 2003. Characterization of a Lactococcus lactis strain that secretes a major epitope of bovine beta-lactoglobulin and evaluation of its immunogenicity in mice. Appl. Environ. Microbiol. 69:6620-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chretien, I., J. Pene, F. Briere, R. de Waal Malefyt, F. Rousset, and J. E. de Vries. 1990. Regulation of human IgE synthesis. I. Human IgE synthesis in vitro is determined by the reciprocal antagonistic effects of IL-4 and IFN-gamma. Eur. J. Immunol. 20:243-251. [DOI] [PubMed] [Google Scholar]
- 16.Chun-Keung, Y., L. Yi-Hsia, and C. Chih-Long. 2002. Dehydroepiandrosterone attenuates allergic airway inflammation in Dermatophagoides farinae-sensitized mice. J. Microbiol. Immunol. Infect. 35:199-202. [PubMed] [Google Scholar]
- 17.Coffman, R. L., and J. Carty. 1986. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J. Immunol. 136:949. [PubMed] [Google Scholar]
- 18.Cohen, J. 1995. IL-12 deaths: explanation and a puzzle. Science 270:908. [DOI] [PubMed] [Google Scholar]
- 19.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erb, K. J., J. W. Holloway, A. Sobeck, H. Moll, and G. Le Gros. 1998. Infection of mice with Mycobacterium bovis-bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 16:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eum, S. Y., S. Haile, J. Lefort, M. Huerre, and B. B. Vargaftig. 1995. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc. Natl. Acad. Sci. USA 92:12290-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford, R. P., D. J. Hill, and C. S. Hosking. 1983. Cows' milk hypersensitivity: immediate and delayed onset clinical patterns. Arch. Dis. Child. 58:856-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanniffy, S., U. Wiedermann, A. Repa, A. Mercenier, C. Daniel, J. Fioramonti, H. Tlaskolova, H. Kozakova, H. Israelsen, S. Madsen, A. Vrang, P. Hols, J. Delcour, P. Bron, M. Kleerebezem, and J. Wells. 2004. Potential and opportunities for use of recombinant lactic acid bacteria in human health. Adv. Appl. Microbiol. 56:1-64. [DOI] [PubMed] [Google Scholar]
- 24.Hefle, S. L., and S. L. Taylor. 2004. Food allergy and the food industry. Curr. Allergy Asthma Rep. 4:55-59. [DOI] [PubMed] [Google Scholar]
- 25.Hill, D. J., M. A. Firer, G. Ball, and C. S. Hosking. 1989. Recovery from milk allergy in early childhood: antibody studies. J. Pediatr. 114:761-766. [DOI] [PubMed] [Google Scholar]
- 26.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3:801-809. [DOI] [PubMed] [Google Scholar]
- 27.Hufnagl, K., B. Winkler, M. Focke, R. Valenta, O. Scheiner, H. Renz, and U. Wiedermann. 2005. Intranasal tolerance induction with polypeptides derived from 3 noncross-reactive major aeroallergens prevents allergic polysensitization in mice. J. Allergy Clin. Immunol. 116:370-376. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, J. R., R. E. Wiley, R. Fattouh, F. K. Swirski, B. U. Gajewska, A. J. Coyle, J. C. Gutierrez-Ramos, R. Ellis, M. D. Inman, and M. Jordana. 2004. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 169:378-385. [DOI] [PubMed] [Google Scholar]
- 29.Keller, A. C., D. Mucida, E. Gomes, E. Faquim-Mauro, A. M. Faria, D. Rodriguez, and M. Russo. 2006. Hierarchical suppression of asthma-like responses by mucosal tolerance. J. Allergy Clin. Immunol. 117:283-290. [DOI] [PubMed] [Google Scholar]
- 30.Kimoto, H., M. Nomura, M. Kobayashi, K. Mizumachi, and T. Okamoto. 2003. Survival of lactococci during passage through mouse digestive tract. Can. J. Microbiol. 49:707-711. [DOI] [PubMed] [Google Scholar]
- 31.Leonard, J. P., M. L. Sherman, G. L. Fisher, L. J. Buchanan, G. Larsen, M. B. Atkins, J. A. Sosman, J. P. Dutcher, N. J. Vogelzang, and J. L. Ryan. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90:2541-2548. [PubMed] [Google Scholar]
- 32.Lotze, M. T., L. Zitvogel, R. Campbell, P. D. Robbins, E. Elder, C. Haluszczak, D. Martin, T. L. Whiteside, W. J. Storkus, and H. Tahara. 1996. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann. N. Y. Acad. Sci. 795:440-454. [DOI] [PubMed] [Google Scholar]
- 33.Lui, V. W., L. D. Falo, and L. Huang. 2001. Systemic production of IL-12 by naked DNA mediated gene transfer: toxicity and attenuation of transgene expression in vivo. J. Gene Med. 3:384-393. [DOI] [PubMed] [Google Scholar]
- 34.Maassen, C. B., C. van Holten-Neelen, F. Balk, M. J. den Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 35.Majamaa, H., and E. Isolauri. 1997. Probiotics: a novel approach in the management of food allergy. J. Allergy Clin. Immunol. 99:179-185. [DOI] [PubMed] [Google Scholar]
- 36.Matheu, V., O. Back, E. Mondoc, and S. Issazadeh-Navikas. 2003. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 112:585-592. [DOI] [PubMed] [Google Scholar]
- 37.Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 64:5403-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamadzadeh, M., S. Olson, W. V. Kalina, G. Ruthel, G. L. Demmin, K. L. Warfield, S. Bavari, and T. R. Klaenhammer. 2005. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 102:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 40.Motzer, R. J., A. Rakhit, L. H. Schwartz, T. Olencki, T. M. Malone, K. Sandstrom, R. Nadeau, H. Parmar, and R. Bukowski. 1998. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin. Cancer Res. 4:1183-1191. [PubMed] [Google Scholar]
- 41.Negroni, L., H. Bernard, G. Clement, J. M. Chatel, P. Brune, Y. Frobert, J. M. Wal, and J. Grassi. 1998. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J. Immunol. Methods 220:25-37. [DOI] [PubMed] [Google Scholar]
- 42.Nouaille, S., L. G. Bermúdez-Humarán, K. Adel-Patient, J. Commissaire, A. Gruss, J. M. Wal, V. Azevedo, P. Langella, and J. M. Chatel. 2005. Improvement of bovine ss-lactoglobulin production and secretion by Lactococcus lactis. Braz. J. Med. Biol. Res. 38:353-359. [DOI] [PubMed] [Google Scholar]
- 43.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 44.Ostroukhova, M., C. Seguin-Devaux, T. B. Oriss, B. Dixon-McCarthy, L. Yang, B. T. Ameredes, T. E. Corcoran, and A. Ray. 2004. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Investig. 114:28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paludan, S. R. 1998. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 48:459-468. [DOI] [PubMed] [Google Scholar]
- 46.Pene, J., F. Rousset, F. Briere, I. Chretien, J. Y. Bonnefoy, H. Spits, T. Yokota, N. Arai, K. I. Arai, J. Banchereau, and J. E. de Vries. 1988. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc. Natl. Acad. Sci. USA 85:6880-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pochard, P., P. Gosset, C. Grangette, C. Andre, A. B. Tonnel, J. Pestel, and A. Mercenier. 2002. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J. Allergy Clin. Immunol. 110:617-623. [DOI] [PubMed] [Google Scholar]
- 48.Repa, A., C. Grangette, C. Daniel, R. Hochreiter, K. Hoffmann-Sommergruber, J. Thalhamer, D. Kraft, H. Breiteneder, A. Mercenier, and U. Wiedermann. 2003. Mucosal application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine 22:87-95. [DOI] [PubMed] [Google Scholar]
- 49.Shibamori, M., K. Ogino, Y. Kambayashi, and H. Ishiyama. 2006. Intranasal mite allergen induces allergic asthma-like responses in NC/Nga mice. Life Sci. 78:987-994. [DOI] [PubMed] [Google Scholar]
- 50.Shida, K., R. Takahashi, E. Iwadate, K. Takamizawa, H. Yasui, T. Sato, S. Habu, S. Hachimura, and S. Kaminogawa. 2002. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin. Exp. Allergy 32:563-570. [DOI] [PubMed] [Google Scholar]
- 51.Skeen, M. J., M. A. Miller, T. M. Shinnick, and H. K. Ziegler. 1996. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J. Immunol. 156:1196-1206. [PubMed] [Google Scholar]
- 52.Song, F., G. Matsuzaki, M. Mitsuyama, and K. Nomoto. 1996. Differential effects of viable and killed bacteria on IL-12 expression of macrophages. J. Immunol. 156:2979-2984. [PubMed] [Google Scholar]
- 53.Swirski, F. K., D. Sajic, C. S. Robbins, B. U. Gajewska, M. Jordana, and M. R. Stampfli. 2002. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J. Immunol. 169:3499-3506. [DOI] [PubMed] [Google Scholar]
- 54.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 55.Venkataraman, C., S. Leung, A. Salvekar, H. Mano, and U. Schindler. 1999. Repression of IL-4-induced gene expression by IFN-gamma requires Stat1 activation. J. Immunol. 162:4053-4061. [PubMed] [Google Scholar]
- 56.Winkler, B., K. Baier, S. Wagner, A. Repa, H. G. Eichler, O. Scheiner, D. Kraft, and U. Wiedermann. 2002. Mucosal tolerance as therapy of type I allergy: intranasal application of recombinant Bet v 1, the major birch pollen allergen, leads to the suppression of allergic immune responses and airway inflammation in sensitized mice. Clin. Exp. Allergy 32:30-36. [DOI] [PubMed] [Google Scholar]
- 57.Wu, C., G. Yang, L. G. Bermúdez-Humarán, P. Qingfeng, Z. Yinming, J. Wang, and X. Gao. 2006. Immunomodulatory effects of IL-12 secreted by Lactococcus lactis on Th1/Th2 balance in ovalbumin (OVA)-induced asthma model mice. Int. Immunopharmacol. 6:610-615. [DOI] [PubMed] [Google Scholar]
- 58.Yasumi, T., K. Katamura, I. Okafuji, T. Yoshioka, T. A. Meguro, R. Nishikomori, T. Kusunoki, T. Heike, and T. Nakahata. 2005. Limited ability of antigen-specific Th1 responses to inhibit Th2 cell development in vivo. J. Immunol. 174:1325-1331. [DOI] [PubMed] [Google Scholar]