Abstract
We have characterized Plasmodium vivax heat shock protein 70 (PvHSP70) and evaluated serodiagnostic applicability of recombinant PvHSP70 (rPvHSP70). In enzyme-linked immunosorbent assays and immunoblot analyses, rPvHSP70 showed high sensitivity (88.8%; 203/228 cases). P. falciparum-infected sera revealed positive reactions (78.8%). The predominant immunoglobulin G (IgG) subclasses were segregated with IgG1 and IgG3.
Plasmodium falciparum heat shock proteins (PfHSPs) might contribute to immunopathological alterations in infected hosts (1, 3). PfHSP70s may have a significant function during parasite adaptation in its environments (4, 7). They have also been a focus of considerable attention due to their immunodominant antigenic nature and their properties as mediators of protective immunity (1, 11). However, information regarding P. vivax HSP70 (PvHSP70) is not available. We herein characterize the molecular properties of PvHSP70 and provide evidence that PvHSP70 is highly antigenic against P. vivax infection sera.
Fifteen P. vivax isolates were obtained from Korea (6 isolates), Myanmar (7 isolates), Thailand (1 isolate), and Indonesia (1 isolate). Genomic DNA was extracted from patients' blood (8). The gene coding for PvHSP70 was amplified with forward (5′-ATGGCCGACGGAAAGGCGTCCAAGCCAA-3′) and reverse (5′-TCAATCGACTTCCTCGACGGTGGGTCCA-3′) primers. The sequences analyzed by the SeqEd.V1.0.3 and CLUSTAL programs were deposited in the GenBank database (see below). Full-length PvHSP70 was amplified using 5′-GGATCCATGGCCAGCGGAAAGGCGTCCAAG-3′ and 5′-CTGCAGTCAATCGACTTCCTCGACGGTGGG-3′ primers. The recombinant protein (rPvHSP70) was bacterially expressed using pQE30 vector (QIAGEN, Valencia, CA) and purified by nickel-nitrilotriacetic acid chromatography.
P. vivax (120 isolates from Korea and 108 isolates from Myanmar) and P. falciparum (33 isolates from Myanmar) infection sera were tested (8). Fifty healthy sera were employed. Informed consent was obtained. The study protocols were approved by the Ethical Committee of the National Institute of Health, Korea, and the Ethical Committee of the Department of Health, Upper Myanmar.
For Western blotting, rPvHSP70 was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and cut into strips. The strips were incubated with 1:200-diluted sera and subsequently with 1:1,000-diluted peroxidase-conjugated anti-human immunoglobulin G (IgG) (Cappel) and visualized using 4-chloro-1-naphthol.
For enzyme-linked immunosorbent assay (ELISA), 96-well microplates were coated with 200 μl of rPvHSP70 (0.2 and 0.5 μg/well for IgG and IgG subclass assays). Sera were diluted at 1:200, and conjugate was diluted at 1:1,000. Color reactions were developed using 0.05% ο-phenylenediamine. Absorbance (abs) was measured at 492 nm. Checkerboard titration was conducted using pooled sera from positive-reference individuals (n = 15) whose blood smears showed typical blood-stage P. vivax and from healthy individuals (n = 10). IgG subclass ELISAs were done using 1:1,000-diluted monoclonal antibodies (IgG1, clone 8c/6-39; IgG2, clone HP-6002; IgG3, clone HP-6050; and IgG4, clone HP-6025) (Sigma).
A 2,073-bp-long PvHSP70 gene encoded a 690-amino-acid polypeptide and showed high-level sequence identity with other HSP70s (95.8% to 99.1%; see Fig. S1 in the supplemental material). PvHSP70 harbored all of the characteristic domains, including a 45-kDa N-terminal ATPase domain, a 15-kDa substrate-binding motif, and a 10-kDa C terminus. Asp-21, Glu-187, Ala-190, and Thr-216, which might be involved in ATPase activity and putative calmodulin binding domain, were conserved. A cytosolic EEVD motif was recognized at the C terminus. The most relevant differences, including different numbers and locations of Gly-Gly-Met-Pro repeats, were also detected in the C-terminal region. These motifs contained highly antigenic epitopes and allowed for differentiation from other members (2, 5, 9). Analyses of PvHSP70 polymorphisms by use of P. vivax wild-type isolates revealed that PvHSP70 is highly conserved regardless of the geographical and genotypic origin of the isolate (data not shown).
The rPvHSP70 was expressed in soluble form with a molecular mass of approximately 72 kDa (Fig. 1A). The Vmax, Km, and kcat values for ATPase activity, as determined by Michaelis-Menten and Lineweaver-Burk plot analyses, were 12.3 nmol/min/mg, 478.7 μM, and 1.02 min−1. These parameters were higher than those of human HSP70 but were lower than those of PfHSP70 (6, 9, 10).
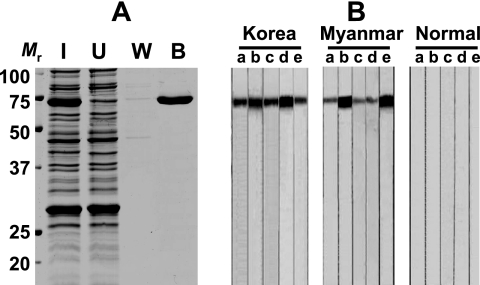
FIG. 1.
Expression and purification of rPvHSP70 and antibody reactivity of rPvHSP70. (A) Purified rPvHSP70 showed a single band with an approximate molecular mass of 72 kDa. Lanes: I, isopropyl-β-d-thiogalactopyranoside-induced Escherichia coli lysates; U, unbound fractions of nickel-nitrilotriacetic acid affinity chromatography; W, wash fractions; B, bound fractions. Mr, molecular mass (in kilodaltons). (B) Western blotting of rPvHSP70 tested against patient sera from P. vivax infections and healthy controls. rPvHSP70 exhibited immunoreactivity to P. vivax infection sera but not to those from healthy controls.
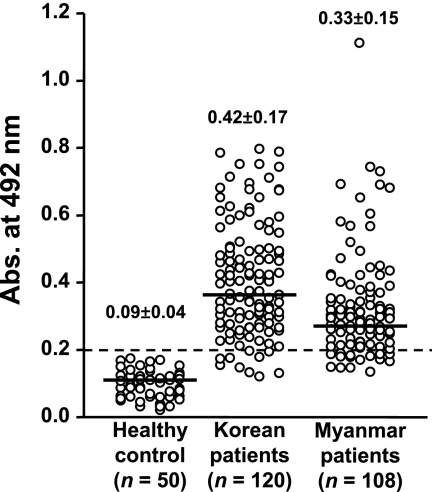
rPvHSP70 exhibited specific antibody responses to malarial infection sera but not to those from healthy controls (Fig. 1B). Sera (n = 5 each) from patients with cysticercosis, paragonimiasis, fascioliasis, and clonorchiasis showed no positive reaction (data not shown). We observed specific antibody levels against rPvHSP70 in P. vivax infection sera by ELISA (Fig. 2) . The means ± standard deviations (SD) of the values determined for the patients from areas where malaria is sporadic and areas where it is endemic were 0.42 ± 0.17, and 0.33 ± 0.15, while those of healthy controls were 0.09 ± 0.04. We set positive criteria as an abs of 0.20, which is between +2 and +3 SD of mean abs of healthy controls. The positive rates were determined to be 91.8% (110/120 cases) and 86.0% (93/108 cases) for patients from Korea and Myanmar (overall sensitivity, 88.8%). This observation was counter to our expectations, albeit there was no significant difference (P > 0.05). We expected that individuals inhabiting areas in which malaria is endemic might evidence higher antibody levels due to repeated infections and the presence of drug-resistant malaria than would patients residing in areas in which the disease is sporadic. We are currently unable to explain properly this apparent incongruity, which needs further study.
FIG. 2.
Result of micro-ELISA of rPvHSP70 testing sera from patients with P. vivax infection. The horizontal bar in the data for each group corresponds to the mean abs values. The cut-off value of 0.20 is indicated by a long broken horizontal line.
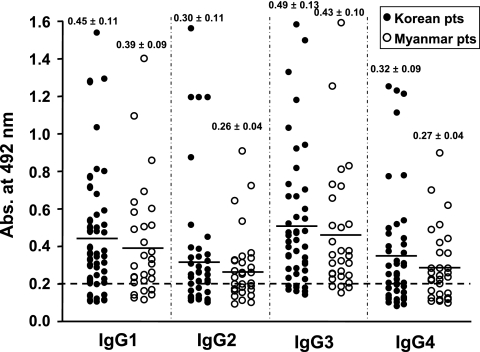
The patient sera reacted predominantly to the IgG1 and IgG3 subclasses (Fig. 3), which suggested that a cell-mediated protective mechanism might be operating during the course of P. vivax infection. A previous study demonstrated that IgG1 and IgG3 antibodies predominate in protected adults whereas IgG2 and IgG4 segregate in nonprotected children and adults who suffered from primary attack (3).
FIG. 3.
Scattergram showing IgG subclass responses to rPvHSP70 in sera from tertian malaria patients. The horizontal bar in the data for each group represents the mean abs value.
To analyze the relationships between the antibody levels specific to PvHSP70 and clinical manifestations, we classified patients into four groups: group I, with body temperature > 40°C and with parasitemia > 10,000 (n = 23); group II, with temperature > 40°C and with parasitemia < 1,000 (n = 13); group III, with temperature < 38.5°C and with parasitemia > 10,000 (n = 12); and group IV, with temperature < 38.5°C and with parasitemia < 1,000 (n = 22). The mean abs ± SD in each group was as follows: for group I, 0.48 ± 0.04; for group II, 0.42 ± 0.06; for group III, 0.60 ± 0.10; and for group IV, 0.46 ± 0.05. There was no significant difference among groups (P > 0.05). We also observed that rPvHSP70 revealed substantial antibody reactivity to P. falciparum-infected sera (0.35 ± 0.04) (sensitivity, 78.8%; 26/33 cases). These collective data strongly suggest that PvHSP70 is antigenic regardless of clinical magnification and parasitemia status of the patients. The antigenicity of PvHSP70 in naturally infected individuals, coupled with its high degree of conservation among wild-type isolates and different plasmodial species, makes the characterization of the immune responses elicited by malaria HSP70 of particular interest. The biological significance of immune responses to PvHSP70 would deserve further studies.
Nucleotide sequence accession number.
The nucleotide sequence of the gene coding for PvHSP70 was deposited in the GenBank database (DQ156547).
Supplementary Material
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (KOSEF R01-2003-000-10305-0).
Footnotes
Supplemental material for this article may be found at http://cvi.asm.org/.
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Behr, C., J. L. Sarthou, C. Rogier, J. F. Trape, M. H. Quan Dat, J. C. Michael, G. Aribot, A. Dieye, J. M. Claverie, P. Druihle, and P. Dubois. 1992. Antibodies and reactive T cells against the malaria heat-shock protein Pf72/Hsp70-1 and derived peptides in individuals continuously exposed to Plasmodium falciparum. J. Immunol. 149:3321-3330. [PubMed] [Google Scholar]
- 2.Fan, J. Y., and E. A. Davidson. 1996. Molecular cloning and antigenic mapping of heat-shock protein 70 from the malaria species Plasmodium berghei. Am. J. Trop. Med. Hyg. 55:570-576. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira, M. U., E. A. Kimura, A. M. Katzin, L. L. Santos-Neto, J. O. Ferrari, J. M. Villalobos, and M. E. de Carvalho. 1998. The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann. Trop. Med. Parasitol. 92:245-256. [DOI] [PubMed] [Google Scholar]
- 4.Kumar, N., and H. Zheng. 1992. Nucleotide sequence of a Plasmodium falciparum stress protein with similarity to mammalian 78 kDa glucose-regulated protein. Mol. Biochem. Parasitol. 56:353-356. [DOI] [PubMed] [Google Scholar]
- 5.Kumar, N., and H. Zheng. 1998. Evidence for epitope-specific thymus-independent response against a repeat sequence in a protein antigen. Immunology 94:28-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matambo, T. S., O. O. Odunuga, A. Boshoff, and G. L. Blatch. 2004. Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Protein Expr. Purif. 33:214-222. [DOI] [PubMed] [Google Scholar]
- 7.Mayer, M. P., and B. Bukau. 2005. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 62:670-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na, B. K., H. W. Lee, S. U. Moon, T. S. In, K. Lin, M. Maung, G. T. Chung, J. K. Lee, T. S. Kim, and Y. Kong. 2005. Genetic variations of the dihydrofolate reductase gene of Plasmodium vivax in Mandalay Division, Myanmar. Parasitol. Res. 96:321-325. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien, M. C., and D. B. McKay. 1993. Threonine 204 of the chaperone protein Hsc70 influences the structure of the active sites, but is not essential for ATP hydrolysis. J. Biol. Chem. 268:24323-24329. [PubMed] [Google Scholar]
- 10.Olson, C. L., K. C. Nadeau, M. A. Sullivan, A. G. Winquist, J. E. Donelson, C. T. Walsh, and D. M. Engman. 1994. Molecular and biochemical comparison of the 70-kDa heat shock proteins of Trypanosoma cruzi. J. Biol. Chem. 269:3868-3874. [PubMed] [Google Scholar]
- 11.Pavithra, S. R., G. Banumathy, O. Joy, V. Sigh, and U. Tatu. 2004. Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J. Biol. Chem. 279:46692-46699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.