Abstract
An enzyme-linked immunosorbent assay (ELISA) was developed for specific antibody detection in serum specimens of patients with sporotrichosis. The assay was made with mycelial-phase Sporothrix schenckii exoantigens and was tested against 90 sera from patients with different clinical forms of sporotrichosis. Potential cross-reactions were analyzed with 72 heterologous sera from patients with paracoccidioidomycosis, cryptococcosis, aspergillosis, histoplasmosis, tuberculosis, and American tegumentary leishmaniasis, as well as 76 sera from healthy controls. We found a sensitivity of 97% and a specificity of 89% in this assay. Some cross-reactions were seen, as observed in other immunoassays for the diagnosis of sporotrichosis. The ELISA appears to be especially useful for cutaneous forms of disease, since these are not promptly diagnosed with available immunoprecipitation or agglutination techniques. These results suggest that the ELISA using mycelial-phase S. schenckii exoantigens is a very sensitive diagnostic tool for the serodiagnosis of sporotrichosis and can be used in conjunction with conventional methods of diagnosis, particularly in cases where cross-reactions or false-positive results are experienced with the serodiagnosis.
Sporotrichosis is a subcutaneous infection caused by the dimorphic fungus Sporothrix schenckii that occasionally results in disseminated disease. This disease has a worldwide distribution, and infection is usually acquired by traumatic inoculation of the fungus into the subcutaneous tissue (18, 23). S. schenckii has been isolated from several environmental sources (8, 11, 18, 29), and most sporotrichosis cases occur in professionals who work with thorny plants, such as farmers, gardeners, and forestry workers (8, 11). However, in a recent sporotrichosis outbreak in Rio de Janeiro, Brazil, transmission of the disease occurred broadly in the populace through the scratches or bites of S. schenckii-infected cats, along with a notable increased incidence in veterinarians (4).
Definitive diagnosis of sporotrichosis relies either on direct visualization of the organism in clinical specimens, which lacks sensitivity, and/or the isolation of the source of the organism in culture, which is time-consuming and, in some manifestations of the disease, such as S. schenckii-induced arthritis, the collection of material for culture is difficult (23). Although some cases of sporotrichosis are benign, severe disease or unusual presentations can occur in immunocompromised individuals such as human immunodeficiency virus-infected patients and patients with chronic granulomatous disease (3, 15). Therefore, sporotrichosis may be mistaken for other infections, such as tuberculosis, leishmaniasis, paracoccidioidomycosis, gummatous syphilis, and chromoblastomycosis (28, 31, 33). The delay in the diagnosis of sporotrichosis can lead to fatality (3).
Detection of patients' antibodies offers a more rapid alternative to the diagnosis than that afforded by histology or culture. At present, the detection of host antibodies to S. schenckii is accomplished by immunoelectrophoresis and tube agglutination (1, 16). Although several new immunoassays have been developed for the detection of antibodies in the serum samples of patients with histoplasmosis (10, 26), paracoccidioidomycosis (2, 21), chromoblastomycosis (9, 34), and candidiasis (17), there is a paucity of effective immunoassays for the serodiagnosis of sporotrichosis. In addition, these immunoassays involve laborious antigen production procedures (6, 30).
The mycelial phase of the fungus produces a number of exoantigens in culture, especially those of 90 and 50 kDa, which appear to be species specific. The optimum expression of these principal antigenic components of S. schenckii occurs in the stationary phase of cultures grown in Sabouraud dextrose broth (22). Accordingly, we describe here the application of mycelial S. schenckii exoantigens in an enzyme-linked immunosorbent assay (ELISA) for the detection of antibody responses in patients with sporotrichosis.
MATERIALS AND METHODS
S. schenckii strain and antigen production.
S. schenckii 23508 was used in the present study. It was isolated from the dwelling of a patient with sporotrichosis. This isolate was identified by biochemical testing, typical colony morphology, and microscopic appearance of growth on culture medium at 25 and 37°C (8, 18, 28), and this strain is available in the culture collection from the Mycology Branch of Instituto de Pesquisa Clínica Evandro Chagas, Fiocruz, Brazil. This strain was compared to other isolates and was demonstrated to be a prolific antigen producer, with an excellent response when probed with a rabbit hyperimmune antiserum by Western blot analysis (R. Almeida-Paes, unpublished results). The exoantigen used in the ELISA was prepared from the mycelial form of this strain according to the method of Mendoza et al. (22). In brief, Sabouraud dextrose broth (Difco Laboratories, Detroit, MI) was inoculated with S. schenckii mycelial phase, followed by incubation at 28°C in a rotatory shaker at 100 rpm for 14 days. Subsequently, culture supernatants were filtered through a 0.45-μm-pore-size mixed cellulose acetate membrane (Millipore Corp., Billerica, MA), concentrated 10-fold by pervaporation, and dialyzed against distilled water at 4°C for 3 days. Thimerosal (1:5,000) was added as a preservative. The protein concentration was measured by a dye-binding assay with respect to an albumin standard, and the protein profile was studied according to the method of Laemmli (19) in a silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4% stacking gel and 7.5% resolving gel). The antigen mixture was stored at 4°C until use.
Serum samples.
Ninety serum specimens obtained from different patients with sporotrichosis (21 male and 69 female, 40.9 ± 17.2 years [mean age ± the standard deviation]) were used in the present study. The sera were collected at Fiocruz between March 2000 and December 2004 and stored at −20°C until use. The diagnosis of sporotrichosis was based on the isolation of S. schenckii in culture, and the disease manifestations were fixed cutaneous (n = 22, 24.4%), lymphocutaneous (n = 49, 54.4%), disseminated cutaneous (n = 16, 17.8%), extracutaneous (n = 2, 2.2% [one osteoarticular, one central nervous system]), and disseminated (n = 1, 1.1%). All of the patients with sporotrichosis were evaluated for evidence of underlying immunosuppression in our clinic, and none of the patients were found to have immunological abnormalities. A total of 72 heterologous serum samples from patients with culture-proven diseases that can mimic sporotrichosis were also examined, including paracoccidioidomycosis (n = 12), cryptococcosis (n = 10), aspergillosis (n = 5), histoplasmosis (n = 15), tuberculosis (n = 12), and American tegumentary leishmaniasis (n = 18). Sera from homologous and heterologous groups had been collected before the treatment of their disease, and none of patients had received the sporotrichin skin test. All of the clinical samples were chosen randomly and were obtained from the Immunodiagnostic Section Serum Bank, Mycology Branch, IPEC, Fiocruz, Brazil. In addition, 76 serum samples from blood donors—healthy individuals previously tested for human immunodeficiency virus infection, syphilis, hepatitis B, and Chagas' disease and showing negative results—were included in the present study as negative controls. All serum specimens were collected from individuals living in the city of Rio de Janeiro, Brazil, a region where sporotrichosis is endemic (4).
ELISA.
Indirect ELISA was performed as described previously (17), with slight modifications to detect immunoglobulin G (IgG) class antibodies to S. schenckii. Antigen was added (40 ng of protein in 100 μl of carbonate buffer [63 mM; pH 9.6] per well) to 96-well microtiter plates (Nunc-Immuno Starwell, MaxiSorp Surface). This concentration of S. schenckii protein was determined by checkerboard titration of twofold dilutions of antigen and high-titer human serum. The plates were incubated for 90 min at 37°C and overnight at 4°C. Plates were washed three times with washing buffer (10 mM phosphate-buffered saline [PBS], 0.1% Tween 20 [pH 7.3]) and blocked with Superblock blocking buffer in PBS (Pierce Biotechnology, Inc., Rockford, IL) according to the manufacturer's instructions. The plates were then washed three times with washing buffer, and serum samples were added in duplicate to wells at a 1:4,000 dilution in incubation buffer (10 mM PBS, 0.1% Tween 20, 5% nonfat skimmed milk powder [pH 7.3]) and then incubated at 37°C for 1 h. After three washes, plates were incubated at 37°C for 1 h with goat anti-human IgG-peroxidase conjugate Fc fragment specific (Jackson Immunoresearch Laboratories, PA) diluted 1:32,000 in incubation buffer at a final volume of 100 μl per well. Plates were washed three times, and then the enzymatic reaction was developed with the addition of 100 μl per well of 0.4 mg of o-phenylenediamine dihydrochloride/ml and 0.04% hydrogen peroxide in 10 mM sodium citrate buffer (pH 5.5) at 37°C for 30 min. The reaction was stopped by the addition of 50 μl of 3 M HCl per well. The absorbances were measured on a microplate reader (Bio-Rad model 550) at 490 nm. This experiment was done twice under uniform laboratory conditions to avoid internal variations in order to ascertain the reproducibility of the assay. For each experiment two controls were made: a control to ensure that the secondary antibody was not interacting with the antigen in the plate and a blank control. These two controls consisted of duplicate wells in which no serum was applied but antigen was coated and secondary antibody was added, and additional duplicate wells in which neither antigen, serum, nor conjugate was applied, respectively. The absorbance value for each serum was the mean of the values for each well to which serum was applied. The cutoff point for serum reactivity to the S. schenckii mycelial exoantigen was set as the mean plus three standard deviations of values of the healthy control patients' sera. Serum samples with absorbance values above the cutoff were considered positive.
Statistical analyses.
Analyses were performed by using SPSS 11.0 for Windows software. Comparison between sporotrichosis patients, heterologous patients, and control individuals was made by using the chi-square test. Comparisons of means were made by using the Student unpaired t test. A P value of ≤0.01 was considered statistically significant. In addition, we performed a receiver-operating-characteristic (ROC) analysis in which sensitivity and specificity were calculated as a function of the cutoff value. For this, “1 − the specificity” was plotted against sensitivity, and the area under the curve was calculated.
RESULTS
Antigen analysis.
The S. schenckii 23508 exoantigen contained 280 ng of protein/μl as assayed by the Bradford method (7). The characteristics of the exoantigen are shown in Fig. 1. Silver-stained electrophoresis gel revealed the presence of five protein bands of 90, 70, 63, 51, and 42 kDa, with the 50- and 63-kDa bands being most prominent.
FIG. 1.
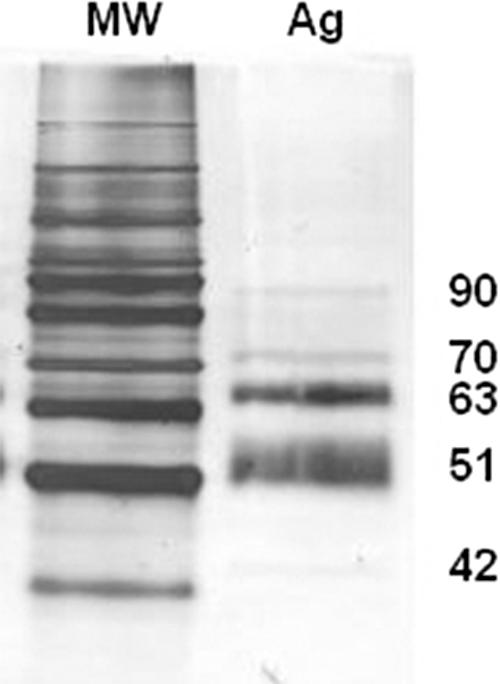
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis profile of the mycelial-phase S. schenckii 23508 exoantigens. MW, standard molecular mass; Ag, antigen. The molecular masses (in kDa) of the five protein bands detected in the silver stain are indicated on the right.
Serological reactivity in the ELISA.
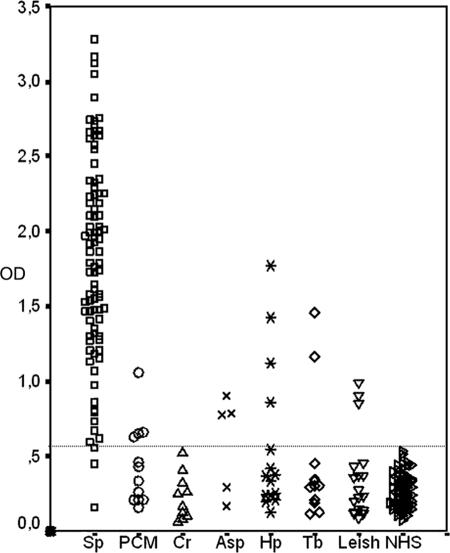
The serological reactivity against the mycelial-phase exoantigen was investigated by the described ELISA in the sera of patients with sporotrichosis or other infections and with control serum samples from healthy individuals. The cutoff point for the ELISA was established as the mean absorbance plus three standard deviations of the healthy controls. Accordingly, samples with absorbances higher than 0.582 were considered positive (Fig. 2). With this cutoff, none of control sera were positive. Table 1 summarizes the results obtained with each serum group. Of 90 sporotrichosis patient sera, only three samples gave negative results. Chi-square analysis of sera from individuals with sporotrichosis and the negative controls showed a statistically significant difference between the antibody responses (χ2 = 154.37, P < 0.0001). A total of 72 heterologous sera were also tested in the ELISA, and 16 were found to be positive. Also, chi-square analysis between the positive and negative heterologous sera showed a statistically significant difference (χ2 = 95.73, P < 0.0001), as did the analysis between sera from healthy controls and heterologous sera (χ2 = 18.94, P < 0.0001).
FIG. 2.
Detection by ELISA of IgG responses to S. schenckii exoantigens in sera from sporotrichosis patients, from patients with other proven infectious diseases, and from healthy controls. The dashed horizontal line shows the cutoff point. Sp, sporotrichosis; PCM, paracoccidioidomycosis; Cr, cryptococcosis; Asp, aspergillosis; Hp, histoplasmosis; Tb, tuberculosis; Leish, leishmaniasis; NHS, normal healthy sera (controls).
TABLE 1.
ELISA results in IgG detection against S. schenckii exoantigens
| Patient group (no. of patients) | No. of positive patients (%) | Mean absorbance ± SD |
|---|---|---|
| Sporotrichosis (90) | 87 (97) | 1.824 ± 0.681 |
| Paracoccidioidomycosis (12) | 4 (33) | 0.440 ± 0.267 |
| Cryptococcosis (10) | 0 (0) | 0.230 ± 0.153 |
| Aspergillosis (5) | 3 (60) | 0.585 ± 0.330 |
| Histoplasmosis (15) | 4 (27) | 0.567 ± 0.499 |
| Tuberculosis (12) | 2 (17) | 0.441 ± 0.421 |
| Leishmaniasis (18) | 3 (17) | 0.353 ± 0.283 |
| Healthy individuals (76) | 0 (0) | 0.261 ± 0.107 |
ROC analysis.
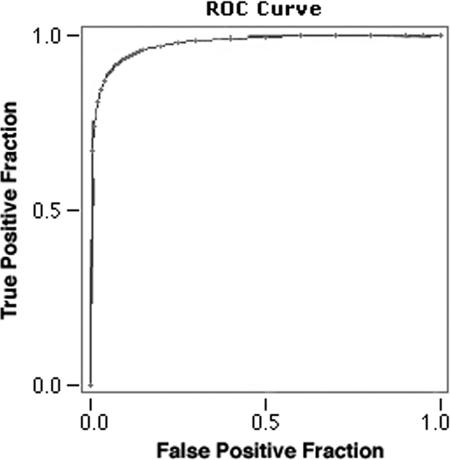
Figure 3 shows the ROC curve for the ELISA described in the present study. The area under the curve was 0.9767 ± 0.0085, and the chosen cutoff (mean absorbances plus three standard deviations of the healthy controls) achieved the maximum efficacy of the test. The described ELISA had a 97% sensitivity (95% confidence interval [95%CI] = 90.57 to 99.31), a 89% specificity (95%CI = 83.04 to 93.69), a 92% global efficiency (95%CI = 87.81 to 95.13), a 84% positive predictive value (95%CI = 76 to 90.85), and a 98% negative predictive value (95%CI = 93.64 to 99.54).
FIG. 3.
ROC curve of the described ELISA. The area under the curve is 0.9767 ± 0.0085.
Serological evaluation of patients with different clinical forms of sporotrichosis.
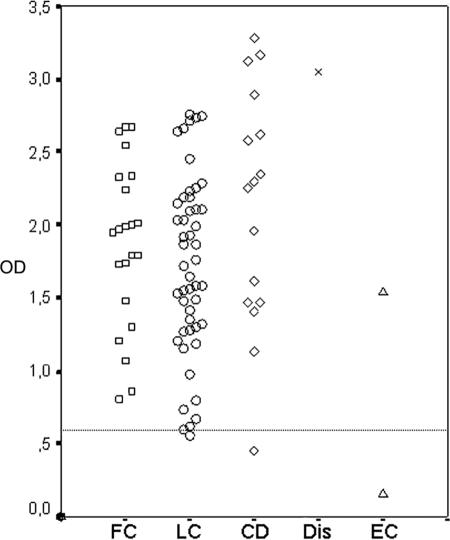
A total of 90 sera from patients with sporotrichosis were analyzed in this ELISA, and 3 of them presented negative results: one from a patient with disseminated cutaneous disease, one with lymphocutaneous infection, and the last patient had the extracutaneous form (infection in the central nervous system) of sporotrichosis. All sera obtained from patients with the fixed cutaneous forms of sporotrichosis gave positive results. Figure 4 shows the absorbance values of each single serum according to the clinical form of sporotrichosis. Student unpaired t test analysis showed that there is no statistically significant difference between the mean absorbances among distinct sporotrichosis clinical forms.
FIG. 4.
IgG antibodies responses from patients with different clinical forms of sporotrichosis by ELISA. The dashed horizontal line shows the cutoff point. FC, fixed cutaneous sporotrichosis; LC, lymphocutaneous sporotrichosis; CD, cutaneous disseminated sporotrichosis; Dis, disseminated sporotrichosis; EC, extracutaneous sporotrichosis.
Cross-reaction analysis.
In order to evaluate cross-reactions in the assay, 72 heterologous sera from patients with selected infectious diseases were used in the present study. A specificity of 78% was obtained for the ELISA using the S. schenckii mycelial exoantigens with the heterologous sera. Of the 16 false-positive results, 4 were from the sera of patients with paracoccidioidomycosis, 4 were from patients with histoplasmosis, 3 were from patients with aspergillosis, 3 were from patients with leishmaniasis, and 2 were from patients with tuberculosis. Sera collected from 10 patients infected with Cryptococcus neoformans were all negative (Table 1). Chi-square analysis revealed statistical differences between the number of positive and negative sera from the tested diseases and sporotrichosis (χ2 = 95.73; P < 0.0001).
The mean absorbances obtained from the sera from the 90 sporotrichosis cases compared to the 76 serum samples from healthy individuals were statistically different as determined by the Student unpaired t test (t = 22.69, P < 0.0001). Similarly, the absorbance values from sera of patients with sporotrichosis and heterologous sera were statistically different (t = 19.19, P < 0.0001), as were the values from the heterologous sera and sera from healthy controls (t = 3.13, P = 0.002).
ELISA reproducibility.
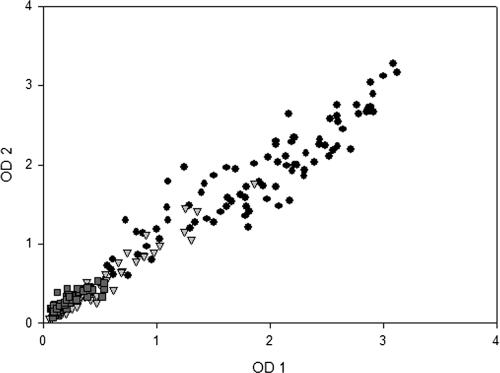
To ensure the reproducibility of the described ELISA, the optical density values for each serum obtained in two different assays were compared. The reproducibility of the test is shown in Fig. 5. The correlation coefficient (r2) was 0.9608, showing uniformity throughout the results for the ELISA described.
FIG. 5.
Distribution and reproducibility of ELISA results in two distinct experiments. Symbols: •, homologous sera; ▾, heterologous sera; ▪, healthy control sera. The correlation coeficient (r2) between these two experiments was 0.9608.
DISCUSSION
The detection of antibodies in the sera of sporotrichosis patients by immunoelectrophoresis and agglutination techniques has historically been very useful in the serodiagnosis of this mycosis (1, 16). A limitation of these tests is their lack of sensitivity in cutaneous sporotrichosis (1, 28). Immunoassays are a very interesting potential solution for this problem because of their high sensitivity. Whereas there have been several described immunoassays for detecting infections due to some dimorphic fungi, such as histoplasmosis, paracoccidioidomycosis, and penicilliosis (2, 10, 12, 21, 26), to our knowledge there are only two publications of immunoassays for sporotrichosis (6, 30). The recently published ELISA for sporotrichosis (6) utilizes a single cell wall antigen, SsCBF, prepared by affinity chromatography with concanavalin A that is purported to provide less batch-to-batch variation and resulted in 90% sensitivity and 86% global efficiency. Importantly, our new ELISA has a collection of antigens that are easily isolated, especially in laboratories in areas with limited financial resources, and is another alternative for the diagnosis of this life-threatening disease.
The antigenic composition of S. schenckii is poorly understood, and there are few described antigens, which complicates the development of specific and sensitive immunoassays. Mendoza et al. described the antigenic composition of mycelial-phase S. schenckii exoantigens and showed that the protein profile of exoantigens produced in Sabouraud dextrose broth did not cross-react with a single serum specimen of coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis (22). The mycelial-phase S. schenckii exoantigens have also been used in immunodiffusion and immunoelectrophoresis assays in which there were no cross-reactions with chromoblastomycosis and leishmaniasis sera (1). Therefore, we decided to standardize and to evaluate the mycelial-phase exoantigens in an ELISA format.
Our ELISA had a 97% sensitivity. All serum samples from patients with different clinical forms of disease showed high absorbance values, suggesting that this test could be broadly applied for the serodiagnosis of sporotrichosis. The mean absorbances of the various cutaneous forms of sporotrichosis were not statistically significantly different, which was also observed by Bernardes-Engemann et al. (6). This is in contrast to what occurs in chromoblastomycosis, another subcutaneous mycosis, where patients with multiple lesions had higher titers of specific IgG compared to patients with localized lesions (9). Although humoral immune responses can participate in the control and prevention of sporotrichosis (25), antibody titers to S. schenckii antigens do not appear to be related to the severity of the disease, since patients with a fixed cutaneous lesion have absorbances similar to those of patients with multiple lesions.
Albornoz et al. found that when the mycelial-phase S. schenckii exoantigens are used in immunoelectrophoresis, all positive sera present an anodic arc, called the “S arc” (1). The protein involved in the formation of this precipitation arc may be responsible for the high sensitivity observed in our test because we also used mycelial-phase S. schenckii exoantigens in our ELISA. Since this antigen has not been purified and characterized before now, more studies are necessary to verify this hypothesis.
The measurement of assay performance by ROC analysis indicated a statistically significant difference between the mean absorbances observed in sporotrichosis sera and those achieved in healthy controls and heterologous sera. However, of mean absorbances heterologous sera were statistically different from those encountered in the healthy control group. The S. schenckii antigens are composed mainly of glycoproteins with a high degree of glycosylation, especially with mannosis, rhamnosis, and galactosis (32). Cross-reactivity in serodiagnostic assays for other mycoses has been attributed to such glycosidic moieties (35, 36). S. schenckii cross-reactions have been documented with several pathogenic fungi, such as Exophiala werneckii, Fonsecaea pedrosoi, Histoplasma capsulatum, Coccidioides immitis, Aspergillus fumigatus, and Trichophyton mentagrophytes because of glycosylated epitopes present in antigens from these fungi (13). Cross-reactions between environmental fungi (14, 27) and bacterial species, such as group B streptococcus and Klebsiella pneumoniae (24, 32), have also been described. Nonspecific recognition of glycosylated epitopes could explain the cross-reactions observed in our ELISA (22%) and also the differences between the heterologous and healthy control sera. In addition, we could not exclude the possibility of previous exposure to S. schenckii in our false-positive patients because all sera used in the present study were collected in an area where sporotrichosis is endemic (4).
In an ELISA using a crude antigen extract from P. brasiliensis, cross-reactions were observed with sera from patients with other mycoses, such as histoplasmosis, lobomycosis, cryptococcosis, candidiasis, and sporotrichosis (21). Cross-reactivity could be eliminated by absorbing the sera with H. capsulatum antigens before their use in the immunoassay (21). With our S. schenckii ELISA, we also observed cross-reactions with some infectious diseases, which similarly occurred in the recently reported ELISA developed by Bernardes-Engemann et al. utilizing the single cell wall antigen (6). In order to reduce cross-reactions, an absorption method could be applied to sera before sporotrichosis ELISA. Another strategy is the deglycosylation of S. schenckii antigens, as is currently done in histoplasmosis immunoassays, which increases the specificity of the assay (10, 26). Studies are currently being carried out in our laboratory to verify whether this approach can be applied to S. schenckii antigens.
Cutaneous sporotrichosis and American tegumentary leishmaniasis share various clinical and epidemiological characteristics, and some patients infected with S. schenckii report that that the disease had first appeared as an insect bite, which can lead to a misdiagnosis of the infection by clinicians, especially those with limited laboratory conditions for a correct diagnosis (20). A recent study has shown that up to 48% of sporotrichosis patients react to the Montenegro skin test, and 23% of them are positive in an ELISA using Leishmania antigens (5). In our ELISA, 17% of the sera from patients with leishmaniasis were positive, showing that cross-reactions between S. schenckii and Leishmania occurred. Although cross-reactions in our ELISA were less common than in the Leishmania ELISA, it is necessary to identify antigens that discriminate these two diseases, since they have distinct treatments.
The use of this antigen in ELISA gave 97% sensitivity, 89% specificity, and 92% efficiency, showing that this assay can be used as a conjunctive tool for the diagnosis of sporotrichosis. The mycelial exoantigen ELISA's high negative predictive value (98%) suggests that this immunoassay might serve as a useful screen to eliminate sporotrichosis from the differential diagnosis of dermatological lesions in an outbreak setting. Notably, our immunoassay was highly reproducible, which again favors its application in the serodiagnosis of sporotrichosis. Therefore, the mycelial exoantigen ELISA is an important tool for the diagnosis of sporotrichosis, especially in its cutaneous forms that occur in the majority of patients infected with S. schenckii (18, 23, 28).
Acknowledgments
R.A.-P. was supported in part by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty Internacional Center (NIH D43-TW007129). J.D.N. was supported in part by NIH AI52733 and AI056070-01A2, a Wyeth Vaccine Young Investigator Research Award from the Infectious Disease Society of America, and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519). R.M.Z.-O. was in part supported by CNPq 306288/2006-0.
We thank Maria Clara Gutierrez Galhardo and Armando de Oliveira Schubach for providing information about the medical status of the patients included in this study.
Footnotes
Published ahead of print on 10 January 2007.
REFERENCES
- 1.Albornoz, M. B., E. Villanueva, and E. D. Torres. 1984. Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis. Mycopathologia 85:177-183. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque, C. F., S. H. Silva, and Z. P. Camargo. 2005. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 43:1944-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Tawfiq, J. A., and K. K. Wools. 1998. Disseminated sporotrichosis and Sporothrix schenckii fungemia as the initial presentation of immunodeficiency virus infection. Clin. Infect. Dis. 26:1403-1406. [DOI] [PubMed] [Google Scholar]
- 4.Barros, M. B. L., A. O. Schubach, A. C. F. do Valle, M. C. Gutierrez-Galhardo, F. Conceição-Silva, T. M. Schubach, R. S. Reis, B. Wanke, K. B. Marzochi, and M. J. Conceição. 2004. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin. Infect. Dis. 38:529-535. [DOI] [PubMed] [Google Scholar]
- 5.Barros, M. B. L., A. O. Schubach, A. C. F. do Valle, M. C. Gutierrez-Galhardo, T. M. P. Schubach, F. Conceição-Silva, M. Matos-Salgueiro, E. Mouta-Confort, R. S. Reis, M. F. Madeira, T. Cuzzi, L. P. Quintella, J. P. S. Passos, M. J. Conceição, and M. C. A. Marzochi. 2005. Positive Montenegro skin test among patients with sporotrichosis in Rio de Janeiro. Acta Trop. 93:41-47. [DOI] [PubMed] [Google Scholar]
- 6.Bernardes-Engemann, A. R., R. C. Orofino, B. P. Miguens, C. V. L. Penha, E. Neves, B. A. S. Pereira, C. M. P. Dias, M. Mattos, M. C. Gutierrez-Galhardo, M. S. Lazéra, A. O. Schubach, M. P. Oliveira-Neto, and L. M. Lopes-Bezerra. 2005. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 43:487-493. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, D. M., I. F. Salkin, R. A. Duncan, N. J. Hurd, J. H. Haines, M. E. Kemna, and F. B. Coles. 1991. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J. Clin. Microbiol. 29:1106-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esterre, P., M. Jahevitra, and A. Adriantsimahavandy. 2000. Humoral immune response in chromoblastomycosis during and after therapy. Clin. Diagn. Lab. Immunol. 7:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guimarães, A. J., C. V. Pizzini, H. L. M. Guedes, P. C. Albuquerque, J. M. Peralta, A. J. Hamilton, and R. M. Zancopé-Oliveira. 2004. ELISA for early diagnosis of histoplasmosis. J. Med. Microbiol. 53:509-514. [DOI] [PubMed] [Google Scholar]
- 11.Hajjeh, R., S. McDonnell, S. Reef, C. Licitra, M. Hankins, B. Toth, A. Padhye, L. Kaufman, L. Pasarell, C. Cooper, L. Hutwagner, R. Hopkins, and M. McNeil. 1997. Outbreak of sporotrichosis among tree nursery workers. J. Infect. Dis. 176:499-504. [DOI] [PubMed] [Google Scholar]
- 12.Hammilton, A. J. 1998. Serodiagnosis of histoplasmosis, paracoccidioidomycosis, and penicilliosis marneffei; current status and future trends. Med. Mycol. 36:351-364. [PubMed] [Google Scholar]
- 13.Ishizaki, H., Y. Nakamura, and R. W. Wheat. 1981. Serological cross-reactivity between Sporothrix schenckii and various unrelated fungi. Mycopathologia 73:65-68. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki, H., Y. Nakamura, H. Kariya, T. Iwatsu, and R. W. Wheat. 1976. Delayed hypersensitivity cross-reactions between Sporothrix schenckii and Ceratocystis species in sporotrichotic patients. J. Clin. Microbiol. 3:545-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajiwara, H., M. Saito, S. Ohga, T. Uenotsuchi, and S. Yoshida. 2004. Impaired host defense against Sporothrix schenckii in mice with chronic granulomatous disease. Infect. Immun. 72:5073-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlin, J. V., and H. S. Nielsen. 1970. Serologic aspects of sporotrichosis. J. Infect. Dis. 121:316-327. [DOI] [PubMed] [Google Scholar]
- 17.Kostiala, A. A., and I. Kostiala. 1981. Enzyme-linked immunosorbent assay (ELISA) for IgM, IgG and IgA class antibodies against Candida albicans antigens: development and comparison with other methods. Sabouraudia 19:123-134. [DOI] [PubMed] [Google Scholar]
- 18.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA.
- 19.Laemmili, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:280-285. [DOI] [PubMed] [Google Scholar]
- 20.Lober, C., R. Kaplan, and C. Herron. 1980. Sporothrix schenckii inoculation on the abdomen. South. Med. J. 73:1637-1638. [DOI] [PubMed] [Google Scholar]
- 21.Mendes-Giannini, M. J. S., M. E. Camargo, C. S. Lacaz, and A. W. Ferreira. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidmycosis. J. Clin. Microbiol. 20:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza, M., A. M. Diaz, M. B. Hung, E. A. Zambrano, E. Díaz, and M. C. de Albornoz. 2002. Production of culture filtrates of Sporothrix schenckii in diverse culture media. Med. Mycol. 40:447-454. [DOI] [PubMed] [Google Scholar]
- 23.Morris-Jones, R. 2002. Sporotrichosis. Clin. Dermatol. 27:427-431. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura, Y., H. Ishizaki, and R. W. Wheat. 1977. Serological cross-reactivity between group B Streptococcus and Sporothrix schenckii, Ceratocystis species, and Graphium species. Infect. Immun. 16:547-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nascimento, R. C., and S. R. Almeida. 2005. Humoral immune response against soluble and fractionate antigens in experimental sporotrichosis. FEMS Immunol. Med. Microbiol. 43:241-247. [DOI] [PubMed] [Google Scholar]
- 26.Pizzini, C. V., R. M. Zancopé-Oliveira, E. Reiss, R. Hajjeh, L. Kaufman, and J. M. Peralta. 1999. Evaluation of a Western blot test in an outbreak of acute histoplasmosis. Clin. Diagn. Lab. Immunol. 6:20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polonelli, L., and G. Morace. 1982. Exoantigen studies of Sporothrix schenckii, Ceratocystis minor, and Graphium penicillioides cultures. J. Clin. Microbiol. 15:362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rippon, J. W. 1988. Sporotrichosis, p. 325-352. In J. W. Rippon (ed.), Medical mycology. The W. B. Saunders Company, Philadelphia, PA.
- 29.Sanchez-Aleman, M. A., J. Araiza, and A. Bonifaz. 2004. Aislamiento y caracterización de cepas silvestres de Sporothrix schenckii y investigación de reactores a la esporotricina. Gac. Med. Mex. 140:507-512. [PubMed] [Google Scholar]
- 30.Scott, E. N., and H. G. Muchmore. 1989. Immunoblot analysis of antibody responses to Sporothrix schenckii. J. Clin. Microbiol. 27:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma, S., R. Choudhary, M. Juneja, C. Grover, and B. S. Nagi-Reddy. 2005. Cutaneous tuberculosis mimicking sporotrichosis. Indian J. Pediatr. 72:86. [DOI] [PubMed] [Google Scholar]
- 32.Takata, M., and H. Ishizaki. 1983. Correlation among culture times, sugar composition, and biological activities of Sporothrix schenckii antigens. Mycopathologia 84:31-39. [DOI] [PubMed] [Google Scholar]
- 33.Tobin, E. H., and W. W. Jih. 2001. Sporotrichoid lymphocutaneous infections: etiology, diagnosis, and therapy. Am. Fam. Physician 63:326-332. [PubMed] [Google Scholar]
- 34.Vidal, M. S. M., L. G. M. Castro, S. C. Cavalcante, and C. S. Lacaz. 2003. Immunoprecipitation techniques and ELISA in the detection of anti-Fonsecaea pedrosoi antibodies in chromoblastomycosis. Rev. Inst. Med. Trop. S. Paulo 45:315-318. [DOI] [PubMed] [Google Scholar]
- 35.Wheat, L. J., L. V. F. Morris, S. Kamel, and R. P. Tewari. 1986. Evaluation of cross-reactions in Histoplasma capsulatum serologic tests. J. Clin. Microbiol. 23:493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zancopé-Oliveira, R. M., S. L. Bragg, E. Reiss, B. Wanke, and J. M. Peralta. 1994. Effects of histoplasmin M antigen chemical and enzymatic deglycosylation on cross-reactivity in the enzyme-linked immunoelectrotransfer blot method. Clin. Diagn. Lab. Immunol. 1:390-393. [DOI] [PMC free article] [PubMed] [Google Scholar]