Abstract
A single-platform volumetric flow cytometer, the Partec Cyflow SL_3, was evaluated against a BD FACSCalibur/Sysmex XT1800i dual platform for measuring CD4+ lymphocytes, total lymphocytes, and the percentage of CD4 lymphocytes in whole-blood samples for monitoring the immune systems of human immunodeficiency virus (HIV)/AIDS patients. Statistical analyses for precision, correlation, and agreement were performed. Coefficients of variation (CV) of 5.8, 4.6, and 3.9% were obtained for low, medium, and high CD4+ cell counts, respectively, using the SL_3, and CV of 3.7, 4.0, and 0.94 were obtained for the same categories, using the BD FACSCalibur. Significant correlations (P < 0.005) between the two assays for CD4 counts, total lymphocyte counts, and percentages of CD4 were obtained, with correlation coefficients of 0.99, 0.96, and 0.99, respectively (n = 229). Using the Bland-Altman plot, mean biases of −18 cell/μl (95% confidence interval (CI); −91 to 54 cells/μl), −0.8% (95% CI; −3.6 to 2%), and −36.8 cells/μl (95% CI; −477 to 404 cells/μl) were obtained for comparisons of CD4 counts, percentages of CD4 cells, and total lymphocyte counts, respectively. The effects of the age of the samples on the three parameters were also analyzed by comparing results from the same samples analyzed at 6, 24, and 48 h after collection. The correlation coefficients for comparisons among different time points for the same machine and among all the time points for the two different machines were greater than 0.90. These data showed that the Partec Cyflow SL_3 assay is comparable to the BD FACSCalibur/Sysmex XT1800i dual-platform method for measuring the amount of CD4+ cells and total lymphocytes and the percentages of CD4 cells in blood samples for the purpose of monitoring HIV/AIDS patients.
Of the 40 million people that are infected with human immunodeficiency virus (HIV) globally, approximately 95% live in severely resource-constrained settings. HIV/AIDS mainly affects adults in their productive primes, leaving the very young and old to cope alone (15). This severely hampers economic growth and development in the societies concerned. The use of highly active antiretroviral therapy in the management of HIV/AIDS has proven remarkably effective in controlling the progression of HIV infection to AIDS, thereby improving the quality of life of HIV/AIDS patients and ultimately prolonging their survival (2, 4, 16, 18). In Zimbabwe, the estimated HIV/AIDS prevalence in adults (aged 15 to 49 years) was 20.1% in 2005; an estimated 1,700 000 Zimbabweans were living with HIV/AIDS, and approximately 8% of HIV-infected persons were receiving antiretroviral therapy (ART). The numbers of AIDS-related deaths during 2005 were estimated to be 139,950 among adults and 28,720 among children (15).
CD4 lymphocytes (T-helper cells) are the major target cells of HIV infection. Progressive decline in CD4 levels caused by various pathological processes associated with HIV infection, including destruction by other immunological cells and virus-induced cell lysis, has been shown to be associated with increased risk of developing opportunistic infections and with mortality (6, 8). Thus, CD4 counts serve as the major clinical indicators of immune competence in patients with HIV infections. Where testing is available, the CD4 count is the most important consideration in the decision to initiate therapy. The World Health Organization recommends the use of CD4 counts as a monitoring tool in the management of ART for HIV/AIDS patients (17).
While the introduction of generic antiretroviral drugs significantly dropped the prices of ART and increased access to treatment, laboratory monitoring of patients on ART has emerged as one of the major limiting factors in the development of strategies in the fight against HIV/AIDS, especially in developing countries. In most resource-limited settings, measurement of CD4 levels is centralized, mostly being done at provincial and central hospitals. ART programs are significantly improved when the availability of antiretroviral drugs is complemented by easily accessible, reliable, and affordable laboratory diagnostic services for monitoring the patients on therapy, especially through CD4 counts. This, in turn, is determined by the accuracy, sensitivity, selectivity, robustness, and throughput of equipment and by the costs of reagents and instruments. The standard method for determining CD4 counts, using flow cytometers, sometimes complemented by hematology instruments which provide comprehensive lymphocyte subset analyses, is costly. Hence, it is imperative that new, affordable, and reliable tests for the enumeration of CD4 cells in peripheral blood be developed to reduce the overall costs of managing therapy for HIV/AIDS patients.
The purpose of this study was to evaluate a method for counting CD4/CD45 lymphocytes and determining percentages of CD4 lymphocytes in the blood, using a simple, single-platform volumetric flow cytometer, the Cyflow green (Partec GmbH, Munster, Germany) (3, 7), as an alternative for the determination of absolute CD4 counts and percentages by comparing the results obtained from the Cyflow with results from a BD FACSCalibur/Sysmex XT1800i dual-platform system (Becton Dickinson).
MATERIALS AND METHODS
Patients and blood samples.
Two hundred twenty nine whole-blood samples were collected by venipuncture into K2EDTA tubes from patients attending the Connaught Clinic, the Parirenyatwa Central Hospital Opportunistic Infections Clinic, or the Harare Central Hospital Opportunistic Infections clinic. The Connaught Clinic is a private HIV/AIDS-care center, and all the samples from there were HIV-positive, as were all the samples from the opportunistic infections clinics of the two central hospitals. The evaluation was carried out in the National Reference Microbiology Laboratory's hematology department. The first tests were done within 6 h of sample collection, and the samples were divided into two aliquots for further analysis after 24 and 48 h, using the FACS Calibur/Sysmex dual-platform system, which is the predicate model, and the Cyflow SL_3. After the first analyses, samples for further analyses using the Cyflow were stored at 4°C, while samples analyzed by the FACSCalibur/Sysmex XT1800i dual-platform system were stored at room temperature (25°C) in accordance with recommendations by the manufacturers of the instruments.
Equipment used in the study. (i) Partec Cyflow SL_3.
The Partec Cyflow SL_3 is a single-platform, three-parameter (SSC plus two-color fluorescence) desktop flow cytometer. It contains a solid-state laser for green excitation. It analyzes concentrations of any particle or cell subpopulation of interest, using true volumetric absolute counting. For data analysis, the analyzer uses Flomax software. The Partec CD4% Reagent kit used contained direct immunofluorescence reagents for enumeration of mature CD4+ T lymphocytes and, simultaneously, of CD45+ cells in peripheral blood. The kit consists of a monoclonal antibody, MEM-241, which recognizes the human CD4 antigen, a transmembrane glycoprotein (59 kDa) of the immunoglobulin supergene family, present on a subset of T lymphocytes (“helper/inducer” T cells) and expressed at lower levels on monocytes and granulocytes. Approximately 20 to 60% of human peripheral blood mononuclear cells and a subpopulation of monocytes were stained, albeit with a weaker signal. For internal quality control, count check beads of a known concentration were run every day to make sure that the laser was properly aligned and the analyzer was functioning optimally.
(ii) BD FACSCalibur/Sysmex dual-platform system.
Absolute CD4 counts were obtained as part of comprehensive T-cell profiles from a combination of results from a hematology analyzer (Sysmex Europe Gmbh, Norderstedt, Germany) and flow cytometry on a BD FACSCalibur cytometer (Becton Dickinson, San Jose, CA). The Sysmex XT1800i is a compact, high-performance hematology analyzer. It provides accurate and precise full-blood count results, including fully automated white blood cell five-part differential reticulocyte counts and fluorescent optical platelet counts.
The BD FACSCalibur used is an automated, multicolor, bench top flow cytometer. It uses BD Multiset software for data analysis. The reagents employed were BD Multiset CD3/CD4/CD8/CD45 reagents (Becton Dickinson TriTEST immunofluorescence reagent package insert; Becton Dickinson, San Jose, CA). When coupled to total lymphocyte counts from the hematological analyzer, the flow cytometric analyses produced comprehensive T-cell profiles. For quality control, BD Multi-Check controls were used with the BD FACSCalibur and e-check controls were used with the Sysmex XT1800i. The NMRL participates in an external quality assurance program through the UK NEQAS immunophenotyping external quality assurance program.
Analysis.
The study was divided into three components, part I, part II, and part III. Part I assessed the precision of the two methods at low, medium, and high CD4 counts, as determined by the reference method. CD4 counts of less than 300 cells per μl were considered low, counts between 301 and 600 were considered medium, and counts greater than 600 were considered high. To measure the inherent instrument or method variation, three samples were selected, one from each of the three categories. Ten replicate preparations were then made and analyzed by the two methods. Large single preparations, also from each of the three categories, were also made and analyzed consecutively ten times.
Part II involved analyzing samples using the two methods in parallel. All samples were stained and analyzed within 6 h of collection. Forty-eight samples with sufficient volumes from the first day of the study were aliquoted. They were then stained and analyzed again at 24 and 48 h after being collected. The sample aliquots for analysis on the Cyflow SL_3 were stored at 4°C, while those for analysis on the FACS Calibur/Sysmex dual-platform model were stored at room temperature per the manufacturers' instructions. For comparison of the precision of the two methods, their coefficients of variation (CVs) in the three different CD4 strata were determined. Student's t test was also used to compare the results obtained from the two systems. For part II, correlation studies and the Bland-Altman method were used to assess correlation and agreement, respectively, between the two methods. The Bland-Altman plot is a statistical method to compare two measuring techniques. In this graphical method, the differences between the two techniques are plotted against the average results of the two techniques (1). The accuracy of the Partec Cyflow SL_3 was also calculated using the results from the FACSCalibur as the true values. Cross correlations were done for results obtained from the 49 samples which were analyzed within 6 h or 24 or 48 h after the samples were collected.
The effect of the order in which samples of different CD4 counts were analyzed was evaluated in the carry-over analysis which constituted part III of the study. Four replicate samples for a specimen with a medium CD4 cell count and three each for specimens with high and low CD4 counts were prepared and analyzed in the following order: middle, high, low, middle, middle, low, low, high, high, middle. The results were then analyzed for any significant differences attributable to the position of the sample in the analysis sequence.
RESULTS AND DISCUSSION
High accuracy and precision are critical attributes of any analytical method used as an aid in the laboratory management of patients. Monitoring of the immune status of HIV/AIDS patients through CD4 counts requires accurate and precise methods for attaining results to manage patient therapies, especially with the introduction of ART. At the moment, flow cytometry is considered the gold standard in immune phenotyping, and because of the urgent need for these instruments in the fight against HIV/AIDS, there are a number of flow cytometry instruments which are being introduced into the market. It is of paramount importance that any instrument introduced for clinical purposes must first be thoroughly evaluated and shown to be giving results which are accurate and consistent with the methods currently considered the gold standards in that area.
In the present study comparing the Partec Cyflow SL_3 and the BD FACSCalibur/Sysmex XT1800i dual-platform system, both methods were required to pass an acceptability check for precision, which was set at a CV of not more than 10%. Both systems showed good precision in both method and instrument, having CVs of less than 6% for low, medium, and high CD4 counts. As determined by the Student t test (P = 0.05), there were no significant differences between the means for the CD4 counts, total lymphocyte counts, and percentages of CD4 lymphocytes in the blood for 10 repetitions of the process with the three samples analyzed by the two methods (Tables 1 and 2).
TABLE 1.
Raw data for method precision, with measurements by the Partec Cyflow SL_3 and the BD FACSCalibur/Sysmex XT1800i
| Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partec Cyflow SL_3
|
BD FACSCalibur/Sysmex XT1800i
|
|||||||||||
| CD4 lymphocyte count
|
% of CD4 lymphocytes in whole blood
|
CD4 lymphocyte count
|
% of CD4 lymphocytes in whole blood
|
|||||||||
| Low | Medium | High | Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| 1 | 163 | 455 | 1,537 | 13 | 20 | 41 | 169 | 453 | 1,434 | 11 | 20 | 42 |
| 2 | 184 | 475 | 1,447 | 13 | 20 | 41 | 181 | 467 | 1,454 | 12 | 21 | 42 |
| 3 | 178 | 497 | 1,481 | 13 | 20 | 41 | 179 | 418 | 1,450 | 12 | 19 | 42 |
| 4 | 171 | 474 | 1,380 | 13 | 20 | 41 | 166 | 468 | 1,445 | 11 | 21 | 42 |
| 5 | 168 | 466 | 1,458 | 12 | 19 | 41 | 176 | 482 | 1,451 | 12 | 21 | 42 |
| 6 | 193 | 436 | 1,379 | 14 | 19 | 41 | 180 | 461 | 1,438 | 12 | 20 | 42 |
| 7 | 161 | 445 | 1,439 | 14 | 19 | 41 | 180 | 476 | 1,411 | 12 | 21 | 41 |
| 8 | 185 | 433 | 1,355 | 13 | 20 | 41 | 186 | 477 | 1,440 | 13 | 21 | 42 |
| 9 | 176 | 440 | 1,460 | 12 | 19 | 39 | 187 | 476 | 1,440 | 13 | 21 | 42 |
| 10 | 176 | 473 | 1,387 | 13 | 19 | 41 | 179 | 462 | 1,421 | 12 | 21 | 41 |
| Mean | 176 | 459 | 1,432 | 13.0 | 20.0 | 40.8 | 178 | 464 | 1,438 | 12 | 20.6 | 41.8 |
| SD | 10 | 21 | 56 | 0.6 | 0 | 0.6 | 7 | 18 | 14 | 0.7 | 1.0 | 0.4 |
| CV | 5.76 | 4.56 | 3.94 | 4.57 | 1.83 | 1.55 | 3.70 | 3.97 | 0.94 | 5.56 | 3.39 | 1.01 |
Ten replicate samples from the same specimens were stained and analyzed for each of the low-, medium-, and high-count samples. SD, standard deviation.
TABLE 2.
Raw data for instrument precision, with measurements by the Partec Cyflow SL_3 and the BD FACSCalibur/Sysmex XT1800i
| Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Partec Cyflow SL_3
|
BD FACSCalibur/Sysmex XT1800i
|
|||||||||||
| CD4 lymphocyte count
|
% of CD4 lymphocytes in whole blood
|
CD4 lymphocyte count
|
% of CD4 lymphocytes in whole blood
|
|||||||||
| Low | Medium | High | Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| 1 | 88 | 483 | 1,016 | 7 | 30 | 34 | 92 | 472 | 1,055 | 8 | 32 | 35 |
| 2 | 91 | 486 | 961 | 8 | 31 | 33 | 87 | 470 | 1,043 | 8 | 32 | 35 |
| 3 | 85 | 491 | 1,005 | 7 | 30 | 34 | 89 | 482 | 1,070 | 8 | 33 | 35 |
| 4 | 94 | 463 | 1,018 | 8 | 29 | 34 | 92 | 506 | 1,073 | 8 | 34 | 36 |
| 5 | 94 | 470 | 1,067 | 8 | 30 | 35 | 87 | 499 | 1,064 | 8 | 34 | 35 |
| 6 | 93 | 493 | 1,037 | 8 | 30 | 34 | 92 | 466 | 1,059 | 8 | 31 | 35 |
| 7 | 92 | 484 | 1,034 | 8 | 30 | 34 | 105 | 456 | 1,084 | 9 | 31 | 36 |
| 8 | 86 | 476 | 996 | 7 | 29 | 34 | 84 | 479 | 1,036 | 7 | 32 | 34 |
| 9 | 96 | 466 | 986 | 8 | 29 | 33 | 96 | 464 | 1,057 | 8 | 31 | 35 |
| 10 | 92 | 469 | 1,012 | 8 | 29 | 34 | 94 | 478 | 1,057 | 8 | 32 | 35 |
| Mean | 91 | 478 | 1,013 | 7.7 | 29.7 | 33.9 | 92 | 477 | 1,060 | 8.0 | 32.2 | 35.1 |
| SD | 4 | 11 | 29 | 0.5 | 0.7 | 0.6 | 6 | 15 | 14 | 0.5 | 1.1 | 0.6 |
| CV | 3.99 | 2.25 | 2.90 | 6.27 | 2.27 | 1.67 | 6.41 | 3.25 | 1.32 | 5.89 | 3.53 | 1.62 |
Single large preparations were made for each of the three samples and analyzed 10 consecutive times. SD, standard deviation.
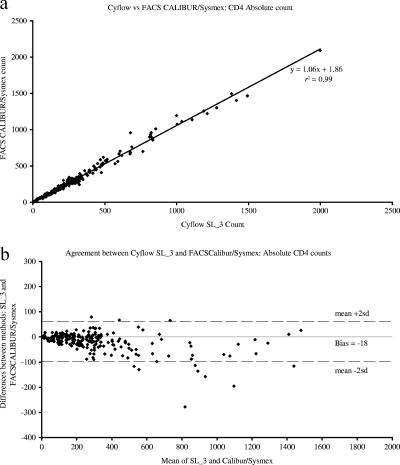
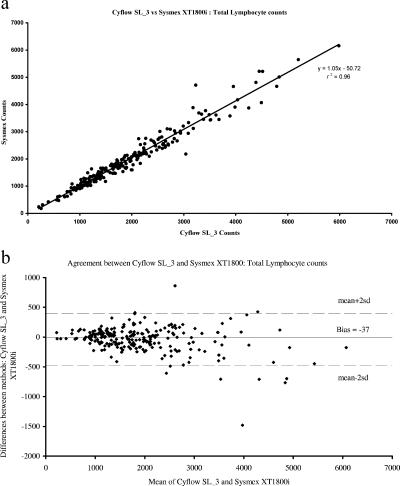
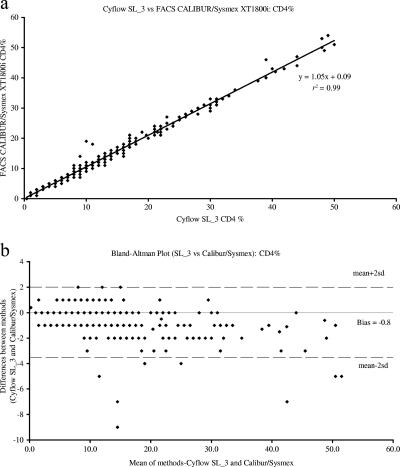
When the 229 samples were run in parallel, the two methods showed good correlation for all three parameters analyzed, with correlation coefficients of 0.99, 0.96, and 0.99 for the absolute CD4 count, total lymphocyte count, and percentage of CD4 lymphocytes, respectively. Bland-Altman analyses demonstrated close agreement between the methods, with biases of −18 ± 37, −37 ± 220, and −0.8 ± 1.4 for the absolute CD4 count, total lymphocyte count, and percentage of CD4 lymphocytes, respectively (Fig. 1, 2, and 3).
FIG. 1.
A comparison of absolute CD4 counts, as determined by the Partec Cyflow SL_3 and BD FACSCalibur/Sysmex XT1800i dual-platform system through (a) correlation analyses of the results and (b) Bland-Altman bias plots illustrating a bias of −18 cells/μl (95% CI, −91 to 55). r2, correlation coefficient; sd, 0.007.
FIG. 2.
A comparison of total lymphocyte counts, as determined by the Partec Cyflow SL_3 and BD FACSCalibur/Sysmex XT1800i dual-platform system through (a) correlation analyses of the results and (b) Bland-Altman bias plots illustrating a bias of −37 cells/μl (95% CI, −477 to 404). r2, correlation coefficient; sd, 0.013.
FIG. 3.
A comparison the percentages of CD4 lymphocytes in the blood, as determined by the Partec Cyflow SL_3 and BD FACSCalibur/Sysmex XT1800i dual-platform system through (a) correlation analyses of the results and (b) Bland-Altman bias plots illustrating a bias of −0.8% (95% CI, −3.6 to 2.0). r2, correlation coefficient; sd, 0.007.
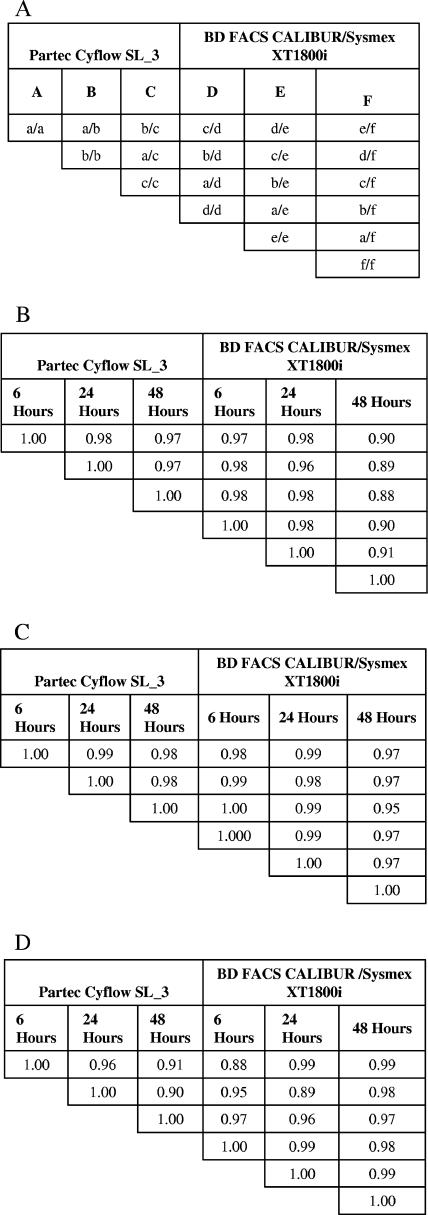
In the analyses of the effect of sample age on the results, the SL_3 showed good correlation of results obtained from samples analyzed 6 h, 24 h, and 48 h after the samples were collected, as shown by the correlation coefficients of the comparisons between samples analyzed after 6 h and 24 h, 6 h and 48 h, and 24 and 48 h, which were 0.98, 0.97, and 0.97, respectively. For the FACSCalibur/Sysmex system, the correlation between results obtained from samples analyzed within 6 h and 24 h was good at 0.98. However, after 24 h the correlation was lower, with a correlation coefficient of 0.89 and 0.90 for the 6 and 48 h and 24 and 48 h comparisons, respectively. This poor correlation was due to a number of outlying values from the 48-h results. However, for the Cyflow SL_3, the data suggest that there is no difference in results obtained after 48 h (Fig. 4).
FIG. 4.
Effect of sample age on analyses by the two methods. The tables show correlation coefficients from multiple cross-correlation analyses of data from the analysis of sample age on the results from the two machines. Results for each time point from each analyzer were compared with all the other time points from both machines. Figure 3A shows the key to the cross correlations in Fig. 4B, C, and D for absolute CD4 counts, total lymphocyte counts, and percentages of CD4 lymphocytes, respectively.
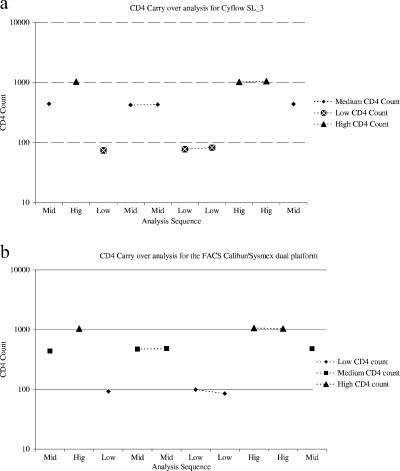
In the carry-over analysis, there were no significant differences among all the samples with low CD4 counts and all those with medium CD4 counts, regardless of their positions in the sequence of analysis for both systems. This is clearly illustrated in Fig. 5 and implies that particles or cells from previous runs are not carried over to subsequent runs.
FIG. 5.
Carry-over analyses for the Partec Cyflow SL_3 (a) and the FACSCalibur/Sysmex XT1800i dual-platform system (b). These analyses assessed the effects of residual samples on subsequent runs.
Our results are in agreement with other comparisons of the SL_3 with other flow cytometers. Pattanapanyasat et al. (12) carried out a comparative study with two reference methods, the single-platform, microbead-based, three-color TruCOUNT tube with the FACScan FCM and a two-color FACSCount system (Becton Dickinson Biosciences). A high correlation of absolute CD4 counts was shown between those obtained with the Cyflow and those obtained with the bead-based, three-color TruCOUNT system (12).
Conclusion.
The study comparing the volumetric Partec Cyflow SL_3 cytometer, using CD4% reagents from Cytecs, and the BD FACSCalibur/Sysmex XT1800i dual-platform system, using MultiTest reagents, for the determination of absolute CD4 counts and percentages of CD4 lymphocytes in the blood for monitoring the immune status of HIV/AIDS patients demonstrated very good correlation and agreement between the two methods. The Partec Cyflow SL_3 has been registered as an in vitro diagnostic product in the European Union, while the BD FACSCalibur has been approved by the FDA (5). Therefore, the two methods can be safely and confidently used for clinical applications, since there are no significant differences in the results produced from these instruments. These findings confirm other results published from studies in South America, Asia, and West Africa (9, 10, 11, 13, 14). The SL_3 has therefore now passed several evaluations carried out by different laboratories and has been evaluated against various standard methods, thus confirming it to be a reliable alternative in immune status monitoring of HIV/AIDS patients.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Bland, J., and D. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 2.Carrieri, F., B. Spire, S. Duran, C. Katlama, D. Peyramond, C. Francois, G. Chene, J. Lang, J. Moatti, C. Leport, and the APROCO study group. 2003. Health related quality of life after 1 year of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 32:38-47. [DOI] [PubMed] [Google Scholar]
- 3.Cassens, U., W. Gohde, G. Kuling, A. Groning, P. Schlenke, L. Lehman, Y. Taore, J. Servais, Y. Hemin, D. Reichelt, and B. Greve. 2004. Simple volumetric flow cytometry allows feasible and accurate determination of CD4 T-lymphocytes in immunodeficient patients worldwide. Antivir. Ther. 9:395-405. [PubMed] [Google Scholar]
- 4.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2006. Guidelines for the use of the antiretroviral agents in HIV-1 infected adults and adolescents. Office of AIDS Research Advisory Council, Bethesda, MD. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 5. Federal Register. 1996. Notice of specific list for categorization of laboratory test systems, assays, and examinations by complexity; notice of additional waived laboratory test systems, assays, and examinations; and notice of announcement of boards approved by HHS. Fed. Regist. 61:35736-35762. http://www.fda.gov/cdrh/CLIA/fr/61fr35736.html. [Google Scholar]
- 6.Gougeon, M. L., H. Lecoeur, A. Dulloust, M. G. Enouf, M. Crouvoiser, C. Goujard, et al. 1996. Programmed cell death in peripheral lymphocytes from HIV infected persons: increased susceptibility to apoptosis of CD4 and CD8 cells correlated with lymphocyte activation and with disease progression. J. Immunol. 156:35009-35020. [PubMed] [Google Scholar]
- 7.Greve, B., U. Cassens, C. Westerberg, W. Gohdejun, W. Sibrowski, D. Reichelt, and W. Gohde. 2002. A new no-lyse, no-wash flow cytometric method for the determination of CD4 T cells in blood samples. Transfus. Med. Hemother. 30:8-13. [Google Scholar]
- 8.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 9.Imade, G. E., B. Badung, S. Pam, O. Agbaji, D. Egah, A. S. Sagay, J.-L. Sankalé, S. Kapiga, J. Idoko, and P. Kanki. 2005. Comparison of a new, affordable flow cytometry method and the manual magnetic bead technique for CD4 T-lymphocyte counting in a Northern Nigerian setting. Clin. Diagn. Lab. Immunol. 12:224-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karcher, H., D. Bohning, R. Downing, S. Mashate, and G. Harms. 2006. Comparison of two alternative methods for CD4+ T-cell determination (Coulter manual CD4 count and Cyflow) against standard dual platform flow cytometry in Uganda. Clin. Cytom. 70B:163-169. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson, J. K. A. 1989. Use of flow cytometry in the evaluation and diagnosis of primary and secondary immunodeficiency diseases. Arch. Pathol. Med. 113:598-605. [PubMed] [Google Scholar]
- 12.Pattanapanyasat, K., S. Lerdwana, S. Noulsri, T. Chaowanachan, P. Wansinrapee, N. Sakulploy, V. Pobopkeeree, O. Suksripanich, S. Thanpransertsuk, J. T. Spiral, W. J. Tappero, and W. C. Levine. 2005. Evaluation of a new single-parameter volumetric flow cytometer (Cyflowgreen) for enumeration of absolute CD4+ T lymphocytes in human immunodeficiency virus type 1-infected Thai patients. Clin. Diagn. Lab. Immunol. 12:1416-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimann, K. A., M. R. G. O'Gorman, J. Spritzler, C. L. Wilkening, D. E. Sabath, K. Helm, D. E. Campbell, and the NIAID DAIDS New Technologies Evaluation Group. 2000. Multisite comparison of CD4 and CD8 T-lymphocyte counting by single- versus multiple-platform methodologies: evaluation of Beckman Coulter Flow-Count fluorospheres and the tetraONE System. Clin. Diagn. Lab. Immunol. 7:344-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnizlein-Bick, C. T., J. Spritzler, C. L. Wilkening, J. K. A. Nicholson, M. R. G. O′Gorman, Site Investigators, and The NIAID DAIDS New Technologies Evaluation Group. 2000. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Clin. Diagn. Lab. Immunol. 7:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS. 2006. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland.
- 16.Weinberg, M. 2005. Generic HIV drugs—enlightened policy for global health. N. Engl. J. Med. 352:747-750. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2004. Guidelines for HIV diagnosis and monitoring of antiretroviral therapy. WHO project no. ICP BCT 001. World Health Organization, Geneva, Switzerland.
- 18.World Health Organization. 1997. The implications of antiretroviral treatments: informal consultations. World Health Organization, Geneva, Switzerland.