Abstract
Systemic low-grade inflammation is recognized in an increasing number of chronic diseases. With the aim of establishing an experimental human in vivo model of systemic low-grade inflammation, we measured circulating inflammatory mediators after intravenous administration of Escherichia coli endotoxin (0.3 ng/kg of body weight) either as a bolus injection or as a 4-h continuous intravenous infusion, as well as after saline administration, in 10 healthy male subjects on three separate study days. Only bolus endotoxin caused an increase in heart rate, whereas a slight increase in rectal temperature was observed in both endotoxin groups. Tumor necrosis factor alpha (TNF-α), interleukin-6, and neutrophil responses were earlier and more pronounced in the bolus trial compared with the infusion trial results, whereas lymphocytes increased after endotoxin bolus injection as well as infusion without any difference between groups. Finally, endotoxin activated the hypothalamo-pituitary-adrenal axis slightly earlier in the bolus compared to the infusion trial. The continuous endotoxin infusion model may be more representative of human low-grade inflammation than the bolus injection model due to a less dynamic and more sustained increase in circulating levels of inflammatory mediators over time. In conclusion, low-dose endotoxin infusion elicits an inflammatory response, as assessed by a rise in TNF-α, and the responses are significantly different according to whether low-dose endotoxin is applied as a bolus injection or as a continuous infusion.
Systemic low-grade inflammation, defined by a 2- to 3-fold increase in plasma concentrations of cytokines and acute phase proteins (16), is associated with chronic disease such as atherosclerosis, the metabolic syndrome, and type 2 diabetes mellitus (3, 6, 14, 24). Moreover, systemic low-grade inflammation may be of significance in age-associated cognitive decline, including Alzheimer's disease and vascular dementia (9, 12, 25). However, the molecular and physiological significance of systemic low-grade inflammation in chronic disease is not yet fully understood.
A human experimental model for sepsis has been developed using an intravenous bolus injection of purified Escherichia coli endotoxin (4). The human endotoxemia model is used for the study of sepsis pathophysiology by the use of large doses of endotoxin (2 to 4 ng/kg body weight), which elicits fever and flu-like symptoms as well as a dramatic increase in circulating levels of both pro- and anti-inflammatory cytokines (5). However, the extent and duration of endotoxin-induced host responses are dose dependent, and endotoxin models have also been applied to study systemic inflammation not associated with overt physiological stress (19).
In addition to an increase in systemic plasma concentrations of cytokines (13) and acute phase proteins in response to the presence of endotoxin, activation of the hypothalamo-pituitary-adrenal (HPA) axis has been previously described, as represented by a rise in plasma levels of cortisol (4) as well as of adrenocorticotropic hormone (13). The degree of HPA activation appears to depend on both type and dose of endotoxin applied and is probably mediated by cytokines released in response to endotoxin; thus, interleukin-6 (IL-6) stimulates cortisol production in humans (2, 21), and a reduced cortisol response is observed after endotoxin administration to IL-6-deficient mice (23). Conversely, the baseline plasma level of cortisol may in itself influence the cortisol response to endotoxin in humans (17).
Reichenberg et al. (18) found that administration to humans of Salmonella abortus equi endotoxin (0.8 ng/kg) induced an approximately 100-fold increase in levels of circulating cytokines (tumor necrosis factor alpha [TNF-α], IL-6, and IL-1ra), an increase in rectal temperature of 0.5°C, and activation of the HPA axis. In contrast, Mullington et al. (15) administered low-dose Salmonella abortus equi endotoxin bolus injections (0.2 ng/kg) to healthy volunteers but found neither activation of the HPA axis nor an increase in body temperature. Moreover, our laboratory applied Escherichia coli endotoxin bolus injections (0.2 ng/kg) and found a modest but significant increase in cytokine levels and a shift in leukocyte subpopulation concentrations but no activation of the HPA axis (9).
We have implemented low-dose intravenous administration of Escherichia coli endotoxin (0.06 to 0.2 ng/kg) with the aim of studying low-grade inflammation. This dose induces a 2- to 100-fold increase in circulating levels of plasma TNF-α and IL-6 (9, 10, 20). As mentioned above, however, low-grade systemic inflammation may be a chronic condition, and the aim of the present study was to develop a more representative model of the pathophysiology of this condition. We hypothesized that bolus injection and continuous infusion of a low-dose Escherichia coli endotoxin would induce equal increases in circulating levels of inflammatory mediators represented by TNF-α and IL-6 but with different release profiles over time.
MATERIALS AND METHODS
Subjects.
Ten healthy male volunteers (mean age, 24 years ± 5 years standard deviation [SD]) participated in the study after giving oral and written informed consent. The volunteers had an unremarkable previous medical history, including no signs of infection within 4 weeks ahead of the first trial day, and used no medication. Subjects underwent a thorough physical examination as well as blood sample analysis for hemoglobin, hematocrit, white blood cell count, electrolytes, renal and hepatic function, plasma glucose, and thyroid-stimulating hormone, all of which were within a normal range. The study was approved by the Scientific Ethical Committee of Copenhagen and Frederiksberg Municipalities, Denmark (journal no. KF 11-032/02).
Study design.
The study was performed in a randomized, cross-over, single-blind fashion. On three separate study days, subjects received (i) an intravenous bolus injection of Escherichia coli endotoxin (batch G2 B274; The United States Pharmacopeial Convention, Inc., Rockville, MD) (0.3 ng/kg) followed by a 4-h infusion of saline (bolus trial); (ii) an intravenous saline bolus followed by a 4-h intravenous infusion of endotoxin (total dose, 0.3 ng/kg) (infusion trial); and (iii) an intravenous bolus injection of saline followed by a 4-h intravenous infusion of saline (placebo trial).
The volumes of saline were identical to the volumes of endotoxin. The placebo trial was always placed on the second trial day. The first trial day was at least 2 weeks ahead of the second trial day to allow for a complete restitution in inflammatory mediators upon endotoxin infusion. The study period was 8 h from the start of infusion in all trials.
On study days subjects reported to our research unit at 7:00 a.m. after an overnight fast. A peripheral venous catheter was placed in one antecubital vein for infusion and in the contralateral antecubital vein for blood sampling. Noninvasive blood pressure (mean arterial pressure), heart rate, and rectal temperature were monitored continuously and recorded at baseline and every hour throughout all trials. Blood samples were drawn at baseline and hourly until 8 h after bolus injection.
Cytokines.
Blood samples were drawn into tubes containing EDTA and centrifuged instantly hereafter. Plasma was kept at −80°C until analyzed. Plasma concentrations of TNF-α, IL-6, and cortisol were measured using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Samples were analyzed in duplicate against standards of a known dilution; mean concentrations were calculated from these duplicate measurements for each sample.
Full-blood hemoglobin, hematocrit, white blood cell and differential counts, glucose, and lactate.
Blood samples were analyzed using standard laboratory methods.
Statistical analysis.
Since data showed normal distribution by Kolmogorov-Smirnov analysis, parametric methods were used; P < 0.05 was considered statistically significant.
Data were analyzed using two-way repeat-measure analysis of variance (ANOVA), applying epsilon corrections as indicated by Mauchly's test of sphericity. Analyses were done in a general linear model analyzing the effect of time and intervention as well as the interaction between time and intervention. This enabled us to identify significant differences between interventions (bolus versus placebo, infusion versus placebo, and bolus versus infusion) as well as differences between interventions with regard to changes to specific time points (using values at 0 h as a reference).
The time-averaged mean value (TAM) for each variable during the entire study period was calculated as the area under the curve divided by the study duration, i.e., 8 h, to provide a measure of the total inflammatory response triggered during each of the three interventions. Moreover, it was part of the prespecified objective to test whether the bolus injection produced a more dynamic response than the continuous infusion. Accordingly, we calculated the ratio between the peak and the TAM values as a measure of the dynamic effect of the intervention. Mean values and peak/TAM ratios for different interventions were compared using a one-way ANOVA followed by Bonferroni-corrected t tests as appropriate to identify significant differences. All analysis was done using SPSS Base and Advanced Models version 11.0 for Windows (SPSS Inc., Chicago, IL). All values are presented as means ±SD.
RESULTS
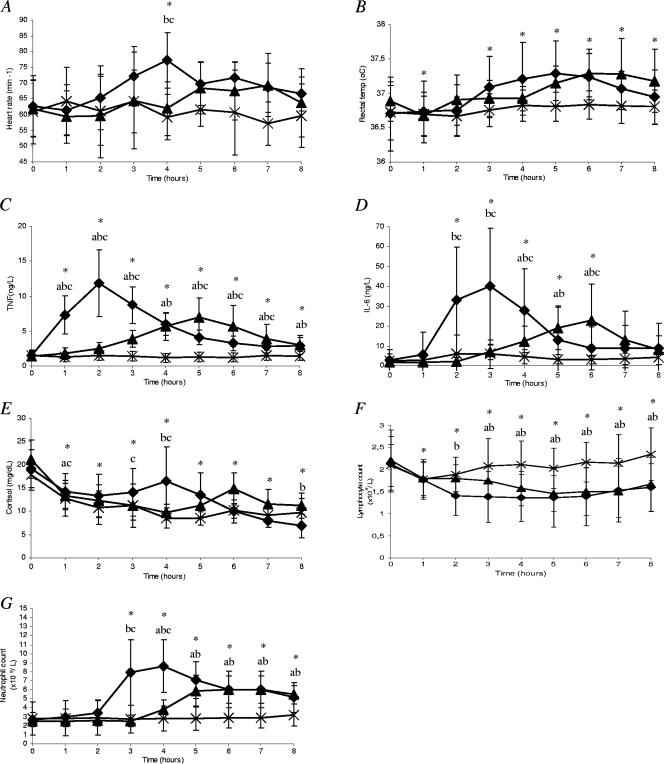
No complications occurred during trials; all subjects were well and discharged home as planned after 8 h. The time courses of selected variables are presented in Fig. 1, and the TAM values are given in Table 1.
FIG. 1.
Time course of clinical and biochemical variables after a bolus injection of endotoxin (⧫), a 4-h infusion of endotoxin (▴), or administration of a placebo (×) in young healthy subjects (n = 10). (A) Heart rate; (B) rectal temperature; (C) plasma tumor necrosis factor alpha; (D) plasma interleukin-6; (E) plasma cortisol; (F) whole-blood lymphocyte count; (G) whole-blood neutrophil count. Data represent means ± SD. Statistical analysis was carried out using two-way ANOVA followed by post hoc t tests to identify significant differences. Asterisks indicate significant differences in overall ANOVA results between the value at 0 h and the specific time point for the three groups. Significant differences between groups according to the post hoc comparisons of changes from baseline at the specific time point between groups are flagged as follows: a, infusion versus placebo; b, bolus versus placebo; c, infusion versus bolus.
TABLE 1.
Time-averaged mean resultsa
| Group | TAM (SD)
|
||||||
|---|---|---|---|---|---|---|---|
| Heart rate (min−1) | Rectal temp (°C) | TNF-α (ng/liter) | IL-6 (ng/liter) | Cortisol (mg/dl) | Lymphocyte count (109/liter) | Neutrophil count (109/liter) | |
| Placebo | 61 (7) | 36.8 (0.2) | 1.36 (0.68) | 4.03 (0.77) | 0.61 (0.14) | 2.1 (0.5) | 2.9 (1.5) |
| Infusion | 64 (7) | 37.0 (0.2) | 3.88 (1.46)b | 9.82 (6.26) | 0.62 (0.13) | 1.7 (0.4) | 4.2 (0.9) |
| Bolus | 68 (6) | 37.0 (0.3) | 5.37 (1.36)b,c | 16.8 (14.4)b | 0.69 (0.18) | 1.5 (0.5) | 5.5 (1.6)b |
Time-averaged means, representing total inflammatory responses, were calculated as the area under the curve (using absolute values of variables over time) divided by the duration of the study, i.e., 8 h.
Significantly different compared to placebo result.
Significantly different compared to infusion result.
Clinical observations. (i) Heart rate (Fig. 1A).
The two-way ANOVA revealed a significant effect on heart rate of intervention (P = 0.01) and time (P < 0.05) and the interaction between time and intervention (P < 0.001). The difference between groups with regard to the effect of intervention was significant between the bolus and placebo groups (P < 0.05) as well as between the bolus and infusion groups (P < 0.05) but not between the infusion and placebo groups. A significant difference between groups with regard to time points was found only at 4 h, when the change in the bolus group (from baseline) differed from that in both the placebo and the infusion groups. The TAM did not differ between groups (Table 1). Taken together, the findings indicate that heart rate increased slightly after the endotoxin bolus, with a peak at 4 h; however, the overall change in the endotoxin groups (as assessed by the TAM) was nonsignificant compared to placebo results.
(ii) Rectal temperature (Fig. 1B).
There were significant effects of both intervention and time (P < 0.01 for both); no interaction was found. The difference in tissue effect of intervention was significant between the bolus and placebo groups (P < 0.01) and between the infusion and placebo groups (P = 0.01) but not between the bolus and infusion groups. No differences between the results for the three trial groups were observed at any specific time points. The TAMs did not differ between groups. Taken together, these data indicate that endotoxin induced an overall slight increase in rectal temperature that was too small to be significant in point-by-point comparisons to the placebo trial and that did not differ with respect to time course between the bolus and infusion groups.
(iii) Mean arterial pressure.
No effect was found on this variable (data not shown).
Cytokines. (i) TNF-α (Fig. 1C).
There was a significant effect of both intervention (P < 10−7) and time (P < 0.001) as well as an interaction between intervention and time (P < 0.001). The effect of intervention was significant for all comparisons (bolus versus placebo, P < 10−5; infusion versus placebo, P < 0.001; bolus versus infusion, P < 0.01), and there were significant differences between groups with regard to changes at most time points. Finally, the TAMs differed in all comparisons between groups (Table 1). The peak/TAM ratio was higher in the bolus trial than in the infusion trial (2.2 ± 0.5 versus 1.8 ± 0.3; P = 0.028). These findings suggest that endotoxin induced a marked increase in plasma levels of TNF-α which was faster and larger in the bolus than in the infusion group, with a peak that occurred at 2 h in the former and at 5 h in the latter group.
(ii) IL-6 (Fig. 1D).
There was a significant effect of intervention (P < 0.05) and time (P < 0.001) as well as an interaction between intervention and time (P < 0.01). The effect of intervention differed between the bolus and placebo trials as well as between the infusion and placebo trials (P < 0.05 for both) but not between the bolus and infusion trials. The changes over time differed between the bolus and placebo groups from 2 through 6 h, between the infusion and placebo groups at 4 through 6 h, and between the bolus and the infusion groups at 2, 3, 4, and 6 h. The TAMs differed significantly only between the bolus and placebo trials; the peak/TAM ratios for IL-6 did not differ between trials. Taken together, the findings suggest that endotoxin induced an increase in IL-6 which was faster and more pronounced after the bolus injection than during the infusion, with a peak at 3 h after the bolus injection and at 6 h after start of the infusion. However, the overall production of IL-6 differed only between the bolus and placebo groups, possibly because of the large variability in data.
(iii) Cortisol (Fig. 1E).
There were effects of both intervention (P < 0.05) and time (P < 0.01) as well as an interaction between intervention and time (P < 0.05). The differences in results with regard to the effects of intervention were significant between the bolus and placebo trials as well as between the infusion and placebo trials (P < 0.05 for both), whereas no difference was found between the bolus and infusion trials. Significant differences between groups with regard to changes over time were found at 1 h (infusion versus bolus and infusion versus placebo), 3 h (bolus versus infusion), 4 h (bolus versus placebo and bolus versus infusion), and 8 h (bolus versus placebo). The TAMs did not differ between groups. These data suggest that plasma cortisol levels increased following endotoxin administration to a peak at 4 h after bolus injection and at 6 h after the start of infusion, although the latter result did not attain statistical significance.
Immune cells. (i) Lymphocyte count (Fig. 1F).
Significant effects of intervention (P < 10−6) and time (P < 0.01) as well as an interaction between intervention and time (P < 0.05) were found. The differences in results with regard to the effects of intervention were significant for the bolus versus placebo trials (P < 10−4) and for the infusion versus placebo trials (P < 10−5) but not the for bolus versus infusion trials. The changes in lymphocyte counts over time differed between the bolus and placebo trials from 2 through 8 h and between the infusion and placebo trials from 3 through 8 h but did not differ between the bolus and infusion trials at any time. The TAMs did not differ between trials. Together, the results suggest that endotoxin induced a depression of circulating lymphocyte counts and that the effects were largely similar in the bolus and the infusion trials.
(ii) Neutrophil count (Fig. 1G).
Significant effects of intervention and time as well as an interaction between these two were found (P < 0.001 for all three analyses). The effects of intervention were significant for all comparisons (bolus versus placebo, P < 0.01; infusion versus placebo, P < 0.05; bolus versus infusion, P < 0.05). The changes at specific time points differed between groups at 3 h (bolus versus placebo and bolus versus infusion), 4 h (all three comparisons), and 5 through 8 h (bolus versus placebo and infusion versus placebo). The TAMs were significantly higher in the bolus than in the placebo trial but did not differ between the bolus and infusion trials or between the infusion and placebo trials. Thus, endotoxin induced an increase in the neutrophil count that peaked earlier in the bolus trial (at 4 h) than in the infusion trial (at 7 h); however, the results with regard to total activation of neutrophils did not appear to differ between these two trials.
(iii) Hemoglobin, glucose, and lactate.
Endotoxin administration did not affect whole-blood hemoglobin, blood glucose, or lactate concentrations (data not shown).
DISCUSSION
The main finding of the present study was that the mode of endotoxin administration was decisive for the dynamics of the TNF-α response. Thus, a bolus injection of endotoxin induced a more pronounced and dynamic TNF-α response than that elicited by a continuous infusion of an identical dose of endotoxin, as assessed by the repeat-measure ANOVA, the TAM, and the peak/TAM ratio. The neutrophil response followed the same pattern, being more pronounced in response to a bolus of endotoxin than to an infusion of endotoxin. Regarding the IL-6 response, a similar tendency was detected, although the result was less distinct.
In the high-dose (2 to 4 ng/kg of body weight) endotoxin model, which has been regarded as a model of human sepsis, both increased heart rate (5) and decreased blood pressure (8) have been described previously. In the present low-dose endotoxin models, an increase in heart rate was found only when endotoxin was administered as a bolus, suggesting that the presence of tachycardia depends upon, e.g., the peak level of TNF-α or the total inflammatory response. In the present and other studies using low-dose endotoxin, blood pressure was not affected, suggesting that cardiovascular changes are dose dependent, being more pronounced in the high-dose endotoxin model.
TNF-α and IL-6 levels measured in the present study were comparable to levels observed with certain chronic diseases recently associated with low-grade systemic inflammation, i.e., the metabolic syndrome, diabetes mellitus, atherosclerosis, Alzheimer's disease, and vascular dementia (16). It would have been of interest to study IL-1 levels as well; IL-1 is a major pyrogen as well as a mediator of lipopolysaccharide-induced HPA activation (22). Human IL-1 baseline levels are, however, below the detection limit of current laboratory methods. We elected not to include measurement of this particular cytokine in the study because of concerns that the accuracy of the measurement would be insufficient to allow for strong conclusions regarding this cytokine, given the low anticipated level of inflammatory activation.
The HPA axis is activated in high-dose endotoxin models (11) but was not activated in a recent study in which we applied a bolus of 0.2 ng/kg endotoxin (9). In the present study, we found an increase in plasma cortisol levels in response to a bolus of 0.3 ng/kg endotoxin, with a peak that occurred earlier in the bolus than in the infusion group. The difference between groups at 1 h is difficult to interpret in the present context but may be related to the relatively high baseline levels measured in all three groups. Endotoxin induced a slight increase in rectal temperature without any difference between the results of the two endotoxin trials. Thus, it appears that the present endotoxin doses were just sufficient to activate the HPA axis and that the results with regard to this activation were largely similar during both endotoxin trials, even though the inflammatory loads as assessed by the TNF-α response clearly differed between trials.
Chronic inflammation has been linked with altered metabolism such as insulin resistance, which can be induced by cortisol (1). This study was not designed to determine metabolic changes induced by endotoxin, however. Moreover, we included only male volunteers, because inclusion of female volunteers would have presented the logistical challenge of defining the time of the menstrual cycle for study participants; in effect, extrapolation of our findings to apply to women may not be justified.
We consider it a strength that a paired-samples approach was used, but a low number of volunteers was included, leading to low statistical power and the risk of type II errors in data analysis due to the variability of the methods used for cytokine and cortisol measurements. This is a potential explanation of the finding, e.g., that the IL-6 responses did not differ significantly between infusion and bolus trials in the face of a significant difference in the TNF responses between these trials.
The present study attempted to further develop the human low-grade inflammation model by endotoxin infusion with the aim of transforming it into a more representative model of human systemic low-grade inflammation in chronic disease. The results confirm that continuous low-dose infusion of endotoxin is safe in healthy humans. Additionally, the infusion model induces a less dynamic and more sustained release of systemic inflammatory mediators over time. The model may be applied in future studies as a model of chronic diseases associated with systemic low-grade inflammation.
In conclusion, an intravenous low-dose endotoxin infusion elicits an inflammatory response, represented by a rise in TNF-α, which is less pronounced and more sustained than the rise in TNF-α induced by intravenous bolus injection of an identical dose of endotoxin.
Acknowledgments
Bettina Starup Mentz, Ruth Rousing, and Hanne Villumsen are acknowledged for their technical assistance.
This study was conducted at The Centre of Inflammation and Metabolism (supported by a grant from the Danish National Research Foundation—DG 02-512-555) and at The Copenhagen Muscle Research Centre (supported by grants from The Faculties of Science and of Health Sciences of The University of Copenhagen). The study was, furthermore, supported by grants from The Copenhagen Hospital Corporation, The Danish National Research Council (grant 504-14), and the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS).
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Bessey, P. Q., J. M. Watters, T. T. Aoki, and D. W. Wilmore. 1984. Combined hormonal infusion simulates the metabolic response to injury. Ann. Surg. 200:264-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethin, K. E., S. K. Vogt, and L. J. Muglia. 2000. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc. Natl. Acad. Sci. USA 97:9317-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan, B. B., M. I. Schmidt, J. S. Pankow, C. M. Ballantyne, D. Couper, A. Vigo, R. Hoogeveen, A. R. Folsom, and G. Heiss. 2003. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52:1799-1805. [DOI] [PubMed] [Google Scholar]
- 4.Elin, R. J., S. M. Wolff, K. P. McAdam, L. Chedid, F. Audibert, C. Bernard, and F. Oberling. 1981. Properties of reference Escherichia coli endotoxin and its phthalylated derivative in humans. J. Infect. Dis. 144:329-336. [DOI] [PubMed] [Google Scholar]
- 5.Fijen, J. W., A. C. M. Kobold, P. de Boer, C. R. Jones, T. S. van der Werf, J. W. C. Tervaert, J. G. Zijlstra, and J. E. Tulleken. 2000. Leukocyte activation and cytokine production during experimental human endotoxemia. Eur. J. Intern. Med. 11:89-95. [DOI] [PubMed] [Google Scholar]
- 6.Hansson, G. K. 2005. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685-1695. [DOI] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Krabbe, K. S., H. Bruunsgaard, J. Qvist, C. M. Hansen, K. Møller, L. Fonsmark, P. L. Madsen, G. Kronborg, U. Frandsen, H. O. Andersen, P. Skinhøj, and B. K. Pedersen. 2001. Hypotension during endotoxemia in aged humans. Eur. J. Anaesthesiol. 18:572-575. [DOI] [PubMed] [Google Scholar]
- 9.Krabbe, K. S., A. Reichenberg, R. Yirmiya, A. Smed, B. K. Pedersen, and H. Bruunsgaard. 2005. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 19:453-460. [DOI] [PubMed] [Google Scholar]
- 10.Krogh-Madsen, R., K. Møller, F. Dela, G. Kronborg, S. Jauffred, and B. K. Pedersen. 2004. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-α, and FFAs to low-dose endotoxemia in humans. Am. J. Physiol. Endocrinol. Metab. 286:E766-E772. [DOI] [PubMed] [Google Scholar]
- 11.Kumar, A., S. Zanotti, G. Bunnell, K. Habet, R. Anel, A. Neumann, M. Cheang, C. A. Dinarello, D. Cutler, and J. E. Parrillo. 2005. Interleukin-10 blunts the human inflammatory response to lipopolysaccharide without affecting the cardiovascular response. Crit. Care Med. 33:331-340. [DOI] [PubMed] [Google Scholar]
- 12.Licastro, F., S. Pedrini, L. Caputo, G. Annoni, L. J. Davis, C. Ferri, V. Casadei, and L. M. Grimaldi. 2000. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J. Neuroimmunol. 103:97-102. [DOI] [PubMed] [Google Scholar]
- 13.Michie, H. R., K. R. Manogue, D. R. Spriggs, A. Revhaug, S. Odwyer, C. A. Dinarello, A. Cerami, S. M. Wolff, and D. W. Wilmore. 1988. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 318:1481-1486. [DOI] [PubMed] [Google Scholar]
- 14.Moller, D. E., and K. D. Kaufman. 2005. Metabolic syndrome: a clinical and molecular perspective. Annu. Rev. Med. 56:45-62. [DOI] [PubMed] [Google Scholar]
- 15.Mullington, J., C. Korth, D. M. Hermann, A. Orth, C. Galanos, F. Holsboer, and T. Pollmächer. 2000. Dose-dependent effects of endotoxin on human sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278:R947-R955. [DOI] [PubMed] [Google Scholar]
- 16.Petersen, A. M., and B. K. Pedersen. 2005. The anti-inflammatory effect of exercise. J. Appl. Physiol. 98:1154-1162. [DOI] [PubMed] [Google Scholar]
- 17.Pollmächer, T., J. Mullington, C. Korth, W. Schreiber, D. Hermann, A. Orth, C. Galanos, and F. Holsboer. 1996. Diurnal variations in the human rest response to endotoxin. J. Infect. Dis. 174:1040-1045. [DOI] [PubMed] [Google Scholar]
- 18.Reichenberg, A., R. Yirmiya, A. Schuld, T. Kraus, M. Haack, A. Morag, and T. Pollmächer. 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58:445-452. [DOI] [PubMed] [Google Scholar]
- 19.Santos, A. A., and D. W. Wilmore. 1996. The systemic inflammatory response: perspective of human endotoxemia. Shock 6:S50-S56. [PubMed] [Google Scholar]
- 20.Starkie, R., S. R. Ostrowski, S. Jauffred, M. Febbraio, and B. K. Pedersen. 2003. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 17:884-886. [DOI] [PubMed] [Google Scholar]
- 21.Steensberg, A., C. P. Fischer, C. Keller, K. Møller, and B. K. Pedersen. 2003. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285:E433-E437. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull, A. V., and C. L. Rivier. 1999. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 79:1-71. [DOI] [PubMed] [Google Scholar]
- 23.van Enckevort, F. H., C. G. Sweep, P. N. Span, P. N. Demacker, C. C. Hermsen, and A. R. Hermus. 2001. Reduced adrenal response to bacterial lipopolysaccharide in interleukin-6-deficient mice. J. Endocrinol. Investig. 24:786-795. [DOI] [PubMed] [Google Scholar]
- 24.Wellen, K. E., and G. S. Hotamisligil. 2005. Inflammation, stress, and diabetes. J. Clin. Investig. 115:1111-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaffe, K., A. Kanaya, K. Lindquist, E. M. Simonsick, T. Harris, R. I. Shorr, F. A. Tylavsky, and A. B. Newman. 2004. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292:2237-2242. [DOI] [PubMed] [Google Scholar]