Abstract
The objective of the present study was to investigate the maturation of immunoglobulin G (IgG) avidity after Toxoplasma gondii seroconversion during pregnancy and the factors that affect IgG avidity over time. The study used 309 serum samples from 117 women and a multiple linear mixed regression analysis to show the patterns of variation of IgG avidity throughout gestation. The IgG avidity ratios and the patterns of their evolution with time were quite diverse among the women and were statistically heterogeneous (P = 0.011); however, the trend was toward a statistically significant increase (P < 0.0001). On average, a 1.0167-fold increase was observed for each additional gestational week after the putative date of infection. At 12 weeks after putative infection (the expected IgG avidity maturation time), the mean avidity ratio was 16.6% (95% confidence interval, 15.4 to 17.9%). At all times, the avidity ratio remained significantly heterogeneous among the women (P < 0.05); for 95% of them, that ratio ranged from 7.8 to 35.3% at 12 weeks after putative infection. Maternal age at the putative time of infection did not influence the maturation of IgG avidity. However, on average, a 1.009-fold decrease (P = 0.03) in that avidity was observed for each additional week of gestational age before infection and a 1.03-fold increase (P = 0.0003) was observed for each additional week of delay to the onset of spiramycin treatment. The rate of increase in the avidity ratio was lower if infection occurred late in pregnancy and higher if the delay to treatment was long. This information cannot allow accurate determination of the delay since the time of infection. The present results provide support for interpretation of the assay and caution against overinterpretation.
Acute Toxoplasma gondii infection can have serious consequences on fetal development, including abortion, neurological and ocular fetal lesions, and subclinical infections (26). Moreover, some toxoplasma-linked ocular lesions appear or relapse during childhood or adolescence (25, 33). This is why, in France, a monthly serological follow-up during pregnancy has been mandatory for seronegative women since 1992 (32).
The risk of mother-to-child transmission of Toxoplasma gondii was shown to be inversely proportional to the stage of pregnancy at which maternal infection occurs (9, 17). Thus, dating of maternal toxoplasma infection during pregnancy is of great importance because it allows determination of the fetal risk. Today, various serological markers may be used to estimate the time that has elapsed since Toxoplasma seroconversion, including measurement of immunoglobulin G (IgG) avidity (16). Although it is well recognized that a high IgG avidity ratio can rule out acute infection, a low IgG avidity ratio is insufficient to infer a recent infection (21). Moreover, data on the evolution of the IgG avidity ratio over time after Toxoplasma infection are lacking. Although the heterogeneity of that evolution has already been reported (13), studies on the effect of treatment on that evolution have reported contradictory findings (13, 18, 24, 28), and studies on the effects of other factors are still awaited.
The aim of this study was to investigate the patterns of maturation of IgG avidity after Toxoplasma seroconversion during pregnancy and to determine factors that could influence the evolution of the IgG avidity ratio.
MATERIALS AND METHODS
Inclusion criteria for pregnant women.
The evolution of the IgG avidity ratio over time was studied retrospectively in a cohort of pregnant women in whom Toxoplasma seroconversion was confirmed in our laboratory between April 2001 and November 2003.
For each woman, the time of conception was obtained from the medical records. In this cohort, in accordance with the law, all women had monthly follow-up visits. Thus, the putative date of infection between the time of the last negative IgG test and the time of the first positive IgG test was first determined. Then, a set of other clinical and serological criteria (IgM and IgG kinetics) allowed determination of the putative date of infection to an accuracy of within a week (3, 20, 30).
Data on monthly IgG titers from the time of seroconversion until the end of pregnancy were extracted from our laboratory database. Pregnant women with IgG titers that were too low for determination of the IgG avidity ratio were excluded from the analysis.
All pregnant women who seroconverted were treated with spiramycin (9 MIU/day) until the end of pregnancy. The delay between Toxoplasma seroconversion and the onset of spiramycin treatment was calculated. Women who received treatments other than spiramycin were excluded.
Finally, the set of data kept for analysis concerned 117 pregnant women and 309 avidity ratio values. Each pregnant woman who participated in the study provided one to seven consecutive serum samples (mean, 2.6 serum samples per woman; median, 2 serum samples per woman). These serum samples were drawn 1.3 to 41 weeks after putative infection, and half of them were drawn within 9.6 weeks. The length of follow-up of the women ranged from 3 to 41.6 weeks; half of the follow-up times were longer than 13.7 weeks.
Avidity assay.
Toxoplasma-specific IgG was detected with an Enzygnost Toxoplasmosis/IgG enzyme-linked immunosorbent assay kit (Dade Behring, Marburg, Germany). After the samples were appropriately diluted, they were divided into two aliquots, which were incubated and then washed: one aliquot was washed with the wash solution provided with the kit, and the other was washed with a homemade wash solution with 6 M urea. This was followed by washing, labeling with peroxidase-conjugated anti-human IgG, addition of substrate for revelation of the peroxidase activity, reading of the A450, and calculation of IgG titers (for more details, see reference 3). The percent IgG avidity ratio was the ratio of the absorbance with and without urea treatment × 100. This value is referred to as the “IgG avidity ratio” or the “avidity ratio,” according to the context. The cutoff for a high avidity ratio was set at 38% to exclude infections acquired at 12 weeks or less before sampling, with 12 weeks being the theoretical time of IgG avidity maturation.
The reproducibility of the avidity ratio was estimated by replicate measurements with two serum samples known to have low and high avidity ratios, respectively. The coefficients of variation of the intra-assay reproducibility (n = 40) were 10.8% and 15.0%, respectively, and the coefficients of variation of the interassay reproducibility (n = 30) were 10.4% and 12.8%, respectively.
Factors assumed to influence maturation of IgG avidity.
Various factors (covariates) likely to influence the maturation of IgG avidity over time were analyzed: gestational age at the putative time of infection, delay to spiramycin treatment after the estimated date of infection, and maternal age at the putative time of infection.
Statistical analysis. (i) Distribution of IgG avidity ratios.
The histogram that represented the distribution of the original percent IgG avidity ratios was found to be skewed to the left. After a neperian logarithmic transformation, the distribution of the log-transformed avidity ratios, noted as the ln(IgG avidity ratio), showed a distribution close to normal. This step was needed for the subsequent modeling step.
(ii) Modeling.
A multiple linear mixed regression analysis (14) was used to model the evolution of the percent IgG avidity ratios after Toxoplasma seroconversion. The ln(IgG avidity ratio) was used as the dependent variable.
The mixed regression model, including both fixed and random effects, allowed the individual evolutions of the avidity ratio to be modeled and the heterogeneity among the women to be quantified and explained. The random effects on the intercept and the slope of the regression line were used to quantify the heterogeneity of these two parameters around the mean ln(IgG avidity ratio) at any given time and around the mean rate of increase of this ratio over time, respectively.
The factors assumed to influence the maturation of IgG avidity were introduced in the model as fixed effects to explain heterogeneity.
In all tests, the level of statistical significance was set at a P value of 0.05. All analyses were performed with R 2.0.1 software (Free Software Foundation, Boston, MA).
RESULTS
Evolution of IgG avidity ratio over time.
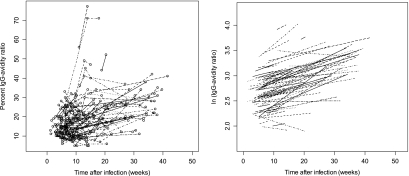
A plot of the original percent IgG avidity ratios versus time after putative infection showed that the original ratios and the patterns of evolution were quite diverse among the women (Fig. 1, left panel). A plot of the model-predicted evolution by use of the log-transformed avidity ratios showed that the slopes of the different lines ranged from −0.017 to +0.051 for 95% of the lines. These slopes were significantly heterogeneous among the women (P = 0.011). However, the general evolution trend was that of an increase in the avidity ratios over time (Fig. 1, right panel). That increase was statistically significant (P < 0.0001). On average, an increase in the log-transformed ratios of 0.0167 was observed for each additional week after putative infection, which corresponds to a 1.017-fold increase with the original percent IgG avidity ratio values.
FIG. 1.
Consecutive measurements of the percent IgG avidity ratios during pregnancy versus the putative time after Toxoplasma infection in 117 pregnant women (309 serum samples) (left panel) and predictions of the multiple linear mixed regression model after logarithmic transformation of the IgG avidity ratios (right panel).
In addition, with this model, a mean avidity ratio could be determined at any given time after putative infection. For example, 12 weeks after putative infection, which is the expected time of maturation for IgG avidity, the mean avidity ratio was 2.811 (95% confidence interval, 2.735 to 2.887) with the log-transformed values, which corresponds to 16.6% (95% confidence interval, 15.4 to 17.9%) with the original values. However, the avidity ratio remained significantly heterogeneous among the women (P < 0.05). For example, at 12 weeks, for 95% of the women, that ratio ranged from 2.059 to 3.563 with the log-transformed values, which corresponds to a range from 7.8 to 35.3% with the original values.
Factors that influence maturation of IgG avidity.
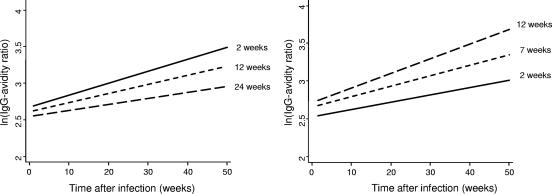
The influence of gestational age at the time of putative infection on the IgG avidity ratio was significant (P = 0.03). On average, the ln(IgG avidity ratio) decreased by 0.009 for each additional week of pregnancy before infection, which corresponds to a 1.009-fold decrease with the original percent IgG avidity ratios (see Fig. 2, left panel, for three selected gestational ages at the time of infection).
FIG. 2.
Predicted increase in the ln(IgG avidity ratio) with time after infection for three gestational ages at the time of putative infection, with the delay to treatment being fixed (left panel), and the predicted increase in the ln(IgG avidity ratio) with time after infection for three delays of treatment, with the gestational age at infection being fixed (right panel).
The influence of the delay to spiramycin treatment on the avidity ratio was also significant (P = 0.0003). On average, the ln(IgG avidity ratio) increased by 0.03 for each additional week of delay to the onset of spiramycin treatment, which corresponds to a 1.03-fold increase with the original percent IgG avidity ratios (see Fig. 2, right panel, for three selected delays to treatment).
In addition, working with the log-transformed IgG avidity ratios, we found that the rate of increase in the avidity ratio fell by 0.0003 for each additional week of pregnancy at the time of putative infection but increased by 0.0009 for each additional week of delay to treatment (Fig. 2, left and right panels, respectively). However, these trends were not statistically significant.
Interestingly, even after adjustment of the gestational age at the time of putative infection and the delay to treatment, the percent avidity ratios remained heterogeneous among the women. For example, at 12 weeks after putative infection it varied from 9 to 30% for 95% of the women.
The mean age of the pregnant women at the putative time of seroconversion was 31 years (standard deviation, 9.9 years; range, 19 to 43 years). Maternal age at the putative time of infection was not found to influence the maturation of IgG avidity (data not shown).
DISCUSSION
We showed that the effect of gestational age on the IgG avidity ratio was significant and independent of the effect of the delay to spiramycin treatment; the avidity ratio was even lower if maternal infection occurred later in pregnancy and if the delay to spiramycin treatment was short.
The significant increase in the IgG avidity ratio over time that we found adds to previous results on (i) the evolution of immunoglobulin affinity during immune response (11); (ii) the persistence of low IgG avidity ratios, even 11 years after infection in some immunocompetent patients and pregnant women (21); and (iii) the slow increase in IgG avidity over 2 years in congenitally infected children (2). Although we report here a constant increase in the IgG avidity ratio with time after putative infection, this increase was so heterogeneous among women that it could not be used for accurate determination of the time that has elapsed since infection. This heterogeneity was partially explained by two factors: gestational age at the putative time of infection and the delay to the onset of treatment. However, even when these two factors were taken into account, the great heterogeneity in IgG avidity persisted. In addition, we found that maternal age is not likely to be involved in IgG avidity. The reproducibility of our tests was also sufficiently high to exclude technical problems. According to reports on experimental infections, the maturation of IgG avidity may be affected by some factors related to the parasite, such as the size of the inoculum (12) or the parasitic stage at the time of infection (7). Such factors cannot be monitored in human infections. Host-related factors, such as the dendritic cell response (8) or genetic susceptibility to infection (31), could also interfere with the maturation of IgG avidity; but such factors were not addressed in this study.
The effect of gestational age at the time of seroconversion on the maturation of IgG avidity has not been reported previously. This could be explained by the fact that the T-cell immune response is modified during pregnancy to avoid rejection of the fetus (34). The role of CD4+ CD25+ regulatory T cells during pregnancy has recently been outlined (35). These cells seem to control the production and maturation of the affinity of antigen-specific antibodies (10); they proliferate during the second and the third trimesters of pregnancy (29). This could partially explain the delay to the time of maturation of Toxoplasma-specific avidity until the late phase of pregnancy. In addition, some Th3 cytokines (such as transforming growth factor β) produced by the placenta during the third trimester of pregnancy could decrease the maturation of IgG avidity (19).
Studies on the influence of treatment on the maturation of IgG avidity have shown conflicting results. Our results agree with those of Sensini et al. (28) and Petersen et al. (24). Sensini et al. (28) first hypothesized that treatment slowed the maturation of avidity and compared the mean IgG avidity values between treated and untreated women, but they did not specify the type of treatment or its length. In two other studies, treatment was reported not to influence the maturation of avidity. However, Jenum et al. (18) compared the mean IgG avidities of 85 serum samples before and after treatment of 23 pregnant women with Toxoplasma seroconversion, but they did not provide information on the time interval between the two blood draws or the type or the length of treatment. Flori et al. (13) compared the IgG avidities of four groups of women treated for different lengths of time and a control group but found no significant differences. The length of spiramycin treatment cannot be taken into account in the regression analysis, as it is dependent on the gestational age at the time of infection and is highly correlated with the putative time of infection. In fact, the regression analysis did not consider the length of treatment but considered the delay to the time of treatment, which is not related to the gestational age.
Although the role of treatment is not yet well understood, it is highly probable that treatment, especially spiramycin treatment, plays a role in the maturation of IgG avidity. The actions of macrolides on Toxoplasma gondii have not yet been elucidated, but they possibly act through the inhibition of protein synthesis (1). Maturation of avidity is the result of different mechanisms, such as somatic hypermutation of rearranged Ig genes and the selection of B cells with high-avidity surface Ig by deletion of low-avidity B cells (23). Somatic hypermutation is a T-cell-dependent, antigen-driven immune response that occurs in the germinal centers of secondary lymphoid tissues. As high-affinity B cells require small amounts of antigen to be selected and to survive (15), it is conceivable that spiramycin delays the maturation of avidity by reducing the amounts of Toxoplasma antigen that select high-affinity B cells (4, 5, 6). Another hypothesis is that spiramycin acts on the immune maturation of lymphocytes and the secretion of cytokines but has no direct effect on Toxoplasma (22, 27).
In conclusion, we have demonstrated that the maturation of Toxoplasma IgG avidity in pregnant women is highly variable and that its evolution over time is influenced by gestational age at the time of maternal infection and by the delay to the onset of treatment. Such variability should be kept in mind when toxoplasma serology in pregnant women is interpreted. Further studies are needed to determine others factors that could influence the maturation of IgG avidity and whether the kinetics of maturation of avidity could influence the transmission of the parasite from the mother to the child or play a role in the severity of congenital toxoplasmosis. Finally, our attempt to bring to light a reliable link between the IgG avidity ratio and the time since infection in patients with toxoplasmosis was largely negative. Indeed, although the avidity ratio generally increases during the follow-up period, its heterogeneity between individuals is such that it cannot be used to accurately date toxoplasma infections. The present results do not indicate any additional usefulness of IgG avidity testing, but they provide support for interpretation of the assay and caution against overinterpretation.
Acknowledgments
We thank the laboratory technicians of Laboratoire de Parasitologie, Mycologie Médicale et Pathologie Exotique Equipe d'Accueil EA 3732, Université Lyon 1, UFR Médecine Lyon Nord, Lyon, France, for technical assistance and Jane Mitchell and Jean Iwaz for editing and revising the manuscript.
Footnotes
Published ahead of print on 3 January 2007.
REFERENCES
- 1.Blais, J., C. Tardif, and S. Chamberland. 1993. Effect of clindamycin on intracellular replication, protein synthesis, and infectivity of Toxoplasma gondii. Antimicrob. Agents Chemother. 37:2571-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buffolano, W., M. Lappalainen, L. Hedman, F. Ciccimarra, M. Del Pezzo, R. Rescaldani, N. Gargano, and K. Hedman. 2004. Delayed maturation of IgG avidity in congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 23:825-830. [DOI] [PubMed] [Google Scholar]
- 3.Cozon, G. J., J. Ferrandiz, H. Nebhi, M. Wallon, and F. Peyron. 1998. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 17:32-36. [DOI] [PubMed] [Google Scholar]
- 4.Derouin, F. 2001. Anti-toxoplasmosis drugs. Curr. Opin. Investig. Drugs 2:1368-1374. [PubMed] [Google Scholar]
- 5.Derouin, F., and C. Chastang. 1988. Enzyme immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue culture. Antimicrob. Agents Chemother. 32:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derouin, F., and C. Chastang. 1989. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 33:1753-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derouin, F., C. Bultel, S. Roze, et al. 2005. Toxoplasmose: état des connaissances et évaluation du risque lié à l'alimentation, p. 1-318. Rapport du Groupe de Travail “Toxoplasma gondii” Agence Française de Sécurité Sanitaire des Aliments. http://www.afssa.fr/ftp/afssa/34487-34488.pdf.
- 8.Diana, J., C. Vincent, F. Peyron, S. Picot, D. Schmitt, and F. Persat. 2005. Toxoplasma gondii regulates recruitment and migration of human dendritic cells via different soluble secreted factors. Clin. Exp. Immunol. 141:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn, D., M. Wallon, F. Peyron, E. Petersen, C. Peckham, and R. Gilbert. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829-1833. [DOI] [PubMed] [Google Scholar]
- 10.Eddahri, F., G. Oldenhove, S. Denanglaire, J. Urbain, O. Leo, and F. Andris. 2006. CD4+ CD25+ regulatory T cells control the magnitude of T-dependent humoral immune responses to exogenous antigens. Eur. J. Immunol. 36:855-863. [DOI] [PubMed] [Google Scholar]
- 11.Eisen, H. N., and G. W. Siskind. 1964. Variations in affinities of antibodies during the immune response. Biochemistry 3:996-1008. [DOI] [PubMed] [Google Scholar]
- 12.Eyles, D. E., and N. Coleman. 1956. Relationship of size of inoculum to time to death in mice infected with Toxoplasma gondii. J. Parasitol. 42:272-276. [PubMed] [Google Scholar]
- 13.Flori, P., L. Tardy, H. Patural, B. Bellete, M. N. Varlet, J. Hafid, H. Raberin, and R. T. Sung. 2004. Reliability of immunoglobulin G antitoxoplasma avidity test and effects of treatment on avidity indexes of infants and pregnant women. Clin. Diagn. Lab. Immunol. 11:669-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein, H. 1995. Multilevel statistical models, 2nd ed., p. 15-40. Kendall's Library of Statistics, London, United Kingdom.
- 15.Gray, D., and H. Skarvall. 1988. B-cell memory is short-lived in the absence of antigen. Nature 336:70-73. [DOI] [PubMed] [Google Scholar]
- 16.Hedman, K., M. Lappalainen, I. Seppaia, and O. Makela. 1989. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 159:736-740. [DOI] [PubMed] [Google Scholar]
- 17.Hohlfeld, P., F. Daffos, J. M. Costa, P. Thulliez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 18.Jenum, P. A., B. Stray-Pedersen, and A. G. Gundersen. 1997. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 35:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehrl, J. H., A. B. Roberts, L. M. Wakefield, S. Jakowlew, M. B. Sporn, and A. S. Fauci. 1986. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J. Immunol. 137:3855-3860. [PubMed] [Google Scholar]
- 20.Lebech, M., D. H. Joynson, H. M. Seite, P. Thulliez, R. E. Gilbert, G. N. Dutton, B. Ovlisen, E. Petersen, et al. 1996. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. Eur. J. Clin. Microbiol. Infect. Dis. 15:799-805. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre-Pettazzoni, M., S. Le Cam, M. Wallon, and F. Peyron. 2006. Delayed maturation of immunoglobulin G avidity: implication for the diagnosis of toxoplasmosis in pregnant women. Eur. J. Clin. Microbiol. Infect. Dis. 25:687-693. [DOI] [PubMed]
- 22.Li, S. Y., and D. S. Nelson. 1986. Acetylspiramycin and the immune system. II. Effects on lymphocyte proliferation, lymphokine production, delayed-type hypersensitivity and antibody production. Int. J. Immunopharmacol. 8:657-664. [DOI] [PubMed] [Google Scholar]
- 23.Longo, N. S., and P. E. Lipsky. 2006. Why do B cells mutate their immunoglobulin receptors? Trends Immunol. 27:374-380. [DOI] [PubMed] [Google Scholar]
- 24.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 43:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyron, F., M. Wallon, and C. Bernardoux. 1996. Long-term follow-up of patients with congenital ocular toxoplasmosis. N. Engl. J. Med. 334:993-994. [DOI] [PubMed] [Google Scholar]
- 26.Remington, J. S., R. McLeod, and G. Desmonts. 2000. Toxoplasmosis, p. 205-346. In J. S. Remington, and J. O. Klein (ed.), Infectious diseases of fetus and newborn infant, 5th ed. Saunders, Philadelphia, PA.
- 27.Rencuzogullari, E., H. B. Ila, M. Topaktas, A. Kayraldiz, S. Budak, and M. Arslan. 2002. No significant increase in chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with spiramycin. Teratog. Carcinog. Mutagen. 22:51-58. [DOI] [PubMed] [Google Scholar]
- 28.Sensini, A., S. Pascoli, D. Marchetti, R. Castronari, M. Marangi, G. Sbaraglia, C. Cimmino, A. Favero, M. Castelletto, and A. Mottola. 1996. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: a multicenter study. Clin. Microbiol. Infect. 2:25-29. [DOI] [PubMed] [Google Scholar]
- 29.Somerset, D. A., Y. Zheng, M. D. Kilby, D. M. Sansom, and M. T. Drayson. 2004. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112:38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stronati, M., L. Bollani, C. Vigano, P. Lanzarini, and G. Rondini. 1998. Application and evaluation of a classification system and case definitions of Toxoplasma gondii infection. Eur. J. Clin. Microbiol. Infect. Dis. 17:67-68. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, Y., S. Y. Wong, F. C. Grumet, J. Fessel, J. G. Montoya, A. R. Zolopa, A. Portmore, F. Schumacher-Perdreau, M. Schrappe, S. Koppen, B. Ruf, B. W. Brown, and J. S. Remington. 1996. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. J. Infect. Dis. 173:265-268. [DOI] [PubMed] [Google Scholar]
- 32.Thulliez, P. 1992. Screening programme for congenital toxoplasmosis in France. Scand. J. Infect. Dis. Suppl. 84:43-45. [PubMed] [Google Scholar]
- 33.Wallon, M., L. Kodjikian, C. Binquet, J. Garweg, J. Fleury, C. Quantin, and F. Peyron. 2004. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 113:1567-1572. [DOI] [PubMed] [Google Scholar]
- 34.Wegmann, T. G., H. Lin, L. Guilbert, and T. R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14:353-356. [DOI] [PubMed] [Google Scholar]
- 35.Zenclussen, A. C. Regulatory T cells in pregnancy. Semin. Immunopathol., in press. [DOI] [PubMed]