Abstract
We developed a vaccine construct in which a BVP22 domain and an invariant-chain major histocompatibility complex class II-targeting motif capable of enhancing dendritic cell antigen uptake and presentation were fused to a sequence encoding a B- and T-cell antigen from the Anaplasma marginale major surface protein 1a and tested whether this construct would prime and expand immune responses in outbred calves. A single inoculation with this construct effectively primed the immune responses, as demonstrated by a significant enhancement of CD4+ T-cell proliferation compared to that in calves identically inoculated but inoculated with a DNA construct lacking the targeting domains and compared to that in calves inoculated with an empty vector. These proliferative responses were mirrored by priming and expansion of gamma interferon-positive CD4+ T cells and immunoglobulin G responses against the linked B-cell epitope. Priming by the single immunization induced memory that underwent rapid recall following reexposure to the antigen. These results demonstrate that DNA vaccines targeting key intercellular and intracellular events significantly enhance priming and expansion and support the feasibility of single-dose DNA immunization in outbred populations.
The ability to prime and expand immune responses with a single immunogen is critically important for humans, for whom vaccine coverage inversely correlates with the number of booster immunizations required, and for livestock, for which economic considerations drive vaccine delivery. Nucleic acid-based vaccination is a contemporary strategy for single-dose priming and expansion of antigen-specific B- and T-lymphocyte responses against infectious organisms in animals and humans (12, 28). Host cell transfection by a DNA vaccine construct allows prolonged intracellular antigen expression, mimicking the effect of a live vaccine (10, 35). The expressed antigen can be processed and presented by the transfected cells or released through secretion or lysis. The released antigen is available for B-cell receptor recognition and follicular dendritic cell (DC) entrapment, or it is taken up by other professional antigen-presenting cells (APCs), which present epitopes from the antigen to T lymphocytes in the context of major histocompatibility complex (MHC) and costimulatory molecules (5, 18, 31). The immunogenicity of DNA vaccines and the ability of modified vectors to enhance immunity have been clearly demonstrated in mice, but translating the results from mouse models to the outbred species that represent the actual populations to be protected by vaccination has proven to be a challenging barrier to the deployment of DNA vaccines (13, 33). Results from trials with outbred species, including humans, have shown that DNA vaccines induce suboptimal and inconsistent immune responses, even after multiple immunizations with high vaccine doses (3, 4, 9, 33, 34). While alternative approaches, such as priming with a DNA vaccine and boosting with either a protein immunogen or a viral construct, have been shown to effectively expand the immune response, these approaches negate a compelling rationale for nucleic acid vaccines: priming and expansion with a single immunization.
DCs are the key APCs required for priming of an immune response following DNA vaccination; however, they constitute <1% of the nucleated cells in any tissue, and only about 0.4% of DCs are normally transfected following DNA vaccination (8, 26). Somatic cells, which are poor APCs incapable of initiating primary immune responses, are the main transfectants following DNA vaccination (1, 14, 29). However, the DNA-encoded antigen is poorly expressed and released from the transfected somatic cells for uptake by DCs (5, 11). Consequently, a strategy that facilitates efficient intercellular trafficking of a DNA-encoded antigen at a DC-enriched immunization site (24) is predicted to significantly increase the number of DCs acquiring and processing the antigen for presentation to T lymphocytes. We focused on testing of an improved DNA vaccination strategy directed at priming and expanding B- and T-lymphocyte responses in outbred species following immunization with a single dose. Specifically, we developed a strategy to promote intercellular trafficking and intracellular targeting of a defined MHC-restricted DNA-encoded T-cell epitope and a linked B-cell epitope at a DC-enriched intradermal immunization site and tested this strategy using calves as the model population for outbred animals. The goal of this study was to test whether immunization with a single dose of a DNA vaccine capable of intercellular trafficking and targeting of the intracellular MHC class II pathway effectively primes and expands antigen-specific CD4+ T cells in an outbred animal species.
MATERIALS AND METHODS
Plasmid construction and purification.
The generation of DNA constructs expressing bovine FLT3L and granulocyte-macrophage colony-stimulating factor (GM-CSF) as molecular adjuvants in the VR-1055 eukaryotic expression vector (Vical, San Diego, CA) has been described previously (24); these constructs are designated FLT3L and GM-CSF, respectively, in this study. Five vaccine constructs were used in the present study. BVP22 was generated by linking in-frame the sequences encoding the bovine VP22 intercellular trafficking domain, the Anaplasma marginale major surface protein 1a (MSP1a) DRB3*1101-restricted F2-5 epitope (GGVSYNDGNASAARSVLETLAGHVDALGIS), a defined MSP1a B-cell epitope (QASTSS), and FLAG tag (DYKDDDDK) (Fig. 1A) (23). BVP22R is identical to BVP22, except that the bvp22 sequence is in the reverse orientation and is thus nonfunctional but retains the open reading frame (Fig. 1B) (23). The sequence encoding the adaptor protein 2 (AP-2) binding endosome/lysosome-targeting motif (MEDQRDLISNHEQLPMLGQRPGAQESKCSR) from the bovine invariant chain (25), designated IC, was added in-frame at the 5′ end of BVP22 by overlap extension PCR (22) with the following primers: ICBVP22 Fwd 1 (5′-ATAGATATCATGGAAGACCAGCGTGACCTCATCTCCAACCATG AGCAGCTG-3′), ICBVP22 Fwd 2 (5′-ATCTCCAACCATGAGCAGCTGCCCATGCTGGGCCAGCGCCCCGGAGCCCAGGA GAGCAAG-3′), ICBVP22 Fwd 3 (5′-CCCGGAGCCCAGGAGAGCAAGTGCAGTCGTATGGCCCGGTTCCACAGGCCC-3′), and FLAG Rev (5′-ATAAGATCTTACTTATCGTCATCGTCCTTGTAGTC-3′). The ICBVP22 Fwd 1 primer introduced an EcoRV restriction site (in boldface) at the 5′ end, whereas the FLAG Rev primer introduced a BglII restriction site (in italics) at the 3′ end. The EcoRV-BglII fragment was ligated into an EcoRV-BamHI-digested VR-1055 vector to generate a construct designated IC.BVP22 (Fig. 1C). The sequence encoding the lysosome-targeting motif (RKRSHAGYQTI) from the cytoplasmic domain of bovine lysosomal-associated membrane protein 1 (LAMP-1) (17), designated GpIIa, was added in-frame at the 3′ end of the BVP22F2-5FLAG chimeric gene by using the BVP22 forward primer (5′-ATAGATATCATGGCCC GGTTCCACAGGCCC-3′) and the FLAG GpIIa reverse primer (5′-ATAAGATCTTAGATGGTCTGGTAGCCGGCGTGGCTCCTCTTCCGCTTATCGTCATCGTCCTTGTAGTC-3′). The forward primer introduced an EcoRV restriction site (in boldface) at the 5′ end, and the reverse primer introduced a BglII restriction site (in italics) at the 3′ end. The EcoRV-BglII fragment was ligated into EcoRV-BamHI-digested VR-1055 to generate a construct designated BVP22.GpIIa (Fig. 1D). To test whether a combination of the IC and GpIIa motifs would have a synergistic effect on endosome-lysosome antigen targeting, the sequence encoding the GpIIa motif was added in-frame at the 3′ end of IC.BVP22 to generate a construct designated IC.BVP22.GpIIa (Fig. 1E).
FIG. 1.
DNA vaccine constructs. Eukaryotic expression constructs were generated in the VR-1055 vector, which contains the cytomegalovirus promoter (CMVP). (A) BVP22 encodes the bovine VP22 intercellular spreading domain linked in-frame to the A. marginale MSP1a DRB3*1101-restricted F2-5 epitope and the defined MSP1a B-cell epitope (23); (B) BVP22R is identical to BVP22, except that the bvp22 sequence is in the reverse orientation and is nonfunctional; (C) IC.BVP22 encodes the bovine invariant-chain endosome/lysosome targeting motif, the IC motif, at the 5′ end of BVP22; (D) BVP22.GpIIa encodes the LAMP-1 lysosome-targeting motif, the GpIIa motif, at the 3′ end of BVP22; (E) IC.BVP22.GpIIa contains the IC motif at the 5′ end and the GpIIa motif at the 3′ of BVP22. A FLAG tag was added to each construct for analysis of protein expression by immunocytochemistry and intercellular spreading.
To generate a recombinant protein for enzyme-linked immunosorbent assay (ELISA) measurement of antibody and for testing of recall responses, the human CD5 secretory signal sequence was linked in-frame to the sequence encoding the chimera, described above, containing the F2-5 epitope, the MSP1a B-cell epitope, and the FLAG tag epitope (DYKDDDDK) by overlap extension PCR. The resultant chimeric gene was subcloned into VR-1055 as an EcoRV-BamHI fragment to generate a construct designated MSP1aBT. The constructs described above and the unmodified VR-1055 vector were amplified in Escherichia coli DH5α cells (Invitrogen, Carlsbad, CA), and large-scale plasmid DNA was purified with Endo-Free Plasmid Gigaprep kits (QIAGEN, Valencia, CA). The DNA was ethanol precipitated and resuspended in clinical-grade normal saline (Abbott Laboratories, North Chicago, IL). The endotoxin content in the plasmid DNA preparations was measured with a Limulus amebocyte assay kit (BioWhittaker, Walkersville, MD) and was <5 endotoxin units/mg in all samples.
Protein expression and intercellular spreading.
The expression and in vivo adjuvant activities of the FLT3L and GM-CSF constructs have been reported previously (24). Protein expression by the BVP22, BVP22R, IC.BVP22, BVP22.GpIIa, and IC.BVP22.GpIIa constructs was confirmed by immunocytometric analysis of transfected human embryonic kidney (HEK) 293-F cells (Invitrogen), as described previously (23). Briefly, 293-F cell monolayers at ∼60% confluence were transfected with 0.2 μg of the DNA constructs by using Lipofectamine 2000 (Invitrogen). The 293-F cells transfected with the BVP22 construct served as a positive control, whereas the VR-1055 empty vector served as a negative control (23). The transfected cells were incubated with a 1/1,000 dilution of a mouse anti-FLAG M2-alkaline phosphatase conjugate (Sigma, St. Louis, MO) in blocking buffer (1× phosphate-buffered saline [PBS] with 5% fetal bovine serum). Duplicate transfected monolayers were reacted with an isotype control monoclonal antibody (MAb), and following washes in blocking buffer, the monolayers were incubated with a 1/1,000 dilution of alkaline phosphatase-conjugated goat anti-mouse MAb (Jackson ImmunoResearch Laboratories, West Grove, PA) in blocking buffer. Following washes in blocking buffer, the alkaline phosphatase activity was detected by using the Fast Red TR-Naphthol AS-MX substrate (Sigma). Stained cells were visualized and photographed with an inverted phase-contrast microscope (model CK-2; Olympus Optical, Tokyo, Japan). Recombinant MSP1aBT was expressed as a FLAG-tagged protein in 293 Free-Style cells (Invitrogen) and was affinity purified with anti-FLAG M2 agarose beads (Sigma).
BVP22-directed intercellular spreading was evaluated in 293-F cells transfected with 0.05 μg DNA, followed by immunocytometric analysis with the mouse anti-FLAG M2-alkaline phosphatase conjugate (Sigma); and spreading was quantified by flow cytometry with a mouse anti-FLAG M2-fluorescein isothiocyanate conjugate (Sigma) following intracellular staining of cells transfected with 0.2 μg DNA, as described previously (23).
Effect of endosome/lysosome-targeting motifs on antigen presentation.
The influence of the endosome/lysosome-targeting IC and GpIIa motifs on MHC class II epitope presentation by DCs following BVP22-directed intercellular trafficking was evaluated by proliferation assays with monocyte-derived DCs and F2-5-specific CD4+ T cells, as described previously (23). Briefly, 293-F cells were transfected with 0.2 μg of BVP22, BVP22R, IC.BVP22, BVP22.GpIIa, or IC.BVP22.GpIIa DNA. The VR-1055 vector served as a negative control (23). One day posttransfection, the HEK 293-F cell transfectants were harvested, washed with PBS, and then mixed at a ratio of 3:1 with monocyte-derived DCs at the fifth day of differentiation. The cell mixture was plated on poly-d-lysine (Sigma-Aldrich)-coated 100-mm petri dishes and left overnight to establish a mixed cell monolayer with maximum cell-to-cell contact (23). The DCs were labeled with mouse anti-bovine DEC-205 MAb CC98 (15), followed by positive selection with goat anti-mouse immunoglobulin G (IgG) microbeads (Miltenyi Biotec, Auburn, CA). The purities and the phenotypes of the isolated DCs were confirmed by flow cytometry, as described previously (23). Irradiated (3,000-rad) DCs (2.5 × 103, 5 × 103, or 104 cells per well) were cultured in triplicate wells with 2 × 104 MSP1a F2-5 epitope-specific short-term CD4+ T cells generated from MSP1-immunized calf 87 (7). The cells were radiolabeled for the last 18 h of culture with 0.25 μCi [3H]thymidine, harvested with an automated cell harvester (Tomtec, Orange, CT), and counted with a liquid scintillation counter. The results are presented as the mean counts per minute (±1 standard deviation [SD]) of [3H]thymidine incorporation. The significance of the differences in proliferation of the F2-5-specific CD4+ T cells among the DC treatments was determined by analysis of variance (ANOVA), followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant.
Immunization of calves.
Typing for bovine MHC class I and class II (DRB3) was done by microarray hybridization, as described previously (27), and 14 DRB3*1101-positive male Holstein calves (age, 6 months) seronegative for A. marginale were selected for use in this study. Peripheral blood mononuclear cells (PBMCs) from these calves were tested in a proliferation assay with A. marginale antigens and were found to be nonresponsive. The calves were allocated to three groups, and all were inoculated intradermally with a mixture of 2 mg of DNA encoding the molecular adjuvants bovine FLT3L and GM-CSF to recruit DCs to the intradermal immunization site (24) (Table 1). Three days later, group 1 calves (n = 5) were inoculated with 3 mg of the IC.BVP22 construct at the same intradermal site where the cytokine constructs were injected. Group 2 calves (n = 5) were similarly inoculated but were inoculated with the BVP22R construct, in which the bvp22 sequence is present but in the reverse orientation and is thus nonfunctional (23). The control calves, which were in group 3 (n = 4), were inoculated with the VR-1055 empty vector. To test whether the primed immune responses could undergo rapid recall upon antigen reexposure, the calves were inoculated with 20 μg of the recombinant MSP1aBT protein emulsified in alum (Pierce, Rockford, IL) on day 105 postimmunization, and the recall responses were evaluated within 96 h.
TABLE 1.
Immunization protocola
| Group no. | Calf no. | DRB3 alleleb | DNA vaccinec |
|---|---|---|---|
| 1 | 3892 | 1101/1201 | IC.BVP22 |
| 3937 | 1001/1101 | IC.BVP22 | |
| 3943 | 1501/1101 | IC.BVP22 | |
| 3945 | 0101/1101 | IC.BVP22 | |
| 3959 | 1001/1101 | IC.BVP22 | |
| 2 | 3939 | 1101/1201 | BVP22R |
| 3982 | 1001/1101 | BVP22R | |
| 3985 | 1501/1101 | BVP22R | |
| 3992 | 0101/1101 | BVP22R | |
| 4000 | 1001/1101 | BVP22R | |
| 3 | 3960 | 1101/1201 | VR-1055 |
| 3962 | 1101/0201 | VR-1055 | |
| 3989 | 1101/1501 | VR-1055 | |
| 3991 | 1401/1101 | VR-1055 |
Calves were inoculated intradermally with the DNA molecular adjuvant constructs encoding FLT3L and GM-CSF at 2 mg each (24). The calves were then reexposed to antigen by inoculation of recombinant MSP1aBT protein at 20 μg, and recall responses were evaluated within 96 h.
MHC class II DRB3 alleles were defined by microarray analysis of exon 2 (27).
Calves were inoculated with the DNA construct encoding the antigen or the VR-1055 vector alone (each at 3 mg) at the same site where the cytokine constructs were injected.
Detection of F2-5-specific lymphocytes by ELISPOT assay.
To detect F2-5-specific T lymphocytes, gamma interferon (IFN-γ)-positive (IFN-γ+) T cells in CD8− γδ− PBMCs were quantified by an enzyme-linked immunospot (ELISPOT) assay 11 days postvaccination and weekly thereafter. Depletion of CD8+ and γδ+ T lymphocytes was done by incubating PBMCs with CD8-specific MAb 7C2B and γδ T-cell receptor-specific MAb GB21A (Monoclonal Antibody Center, Washington State University), followed by immunomagnetic separation with goat anti-mouse IgG micro beads (Miltenyi Biotec). The efficiency of depletion was confirmed by flow cytometry with the CD8+- and γδ+-specific MAbs. Whole PBMCs were used for all assays after day 46 postimmunization, as direct comparison of PBMCs and CD4+ T cells isolated from PBMCs revealed no differences. Bovine IFN-γ-specific ELISPOT assays were conducted in triplicate wells of MultiScreen-HA plates (Millipore), as described previously (36). Briefly, 0.5 × 106 CD8− γδ− PBMCs or PBMCs were added in 100-μl volumes containing complete RPMI 1640 medium with 10 μg/ml F2-5 peptide or a negative control peptide, MSP2-P1 (6). The positive control was medium containing 10 μg/ml concanavalin A, whereas medium alone served as a negative control. After incubation for 36 h at 37°C, the plates were washed, developed, and dried overnight. The spots were quantified with an ELISPOT reader (Cell Technology) and AID 2.9 software (AutoImmun Diagnostika). For each sample, the mean number of spots in the negative control wells was subtracted from the mean number of spots in the test wells to determine the mean number of F2-5-specific IFN-γ-secreting T lymphocytes or spot-forming cells. The results are presented as the mean number of spot-forming cells per 106 CD8− γδ− PBMCs or PBMCs. The significance of the differences in F2-5-specific IFN-γ+ T-cell responses among the groups was analyzed by ANOVA, followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant.
Proliferation assays.
Proliferation assays were conducted essentially as described previously (6). Briefly, CD8− γδ− PBMCs or PBMCs (2 × 105 cells) were cultured in triplicate wells of round-bottom 96-well plates (Costar) for 3 days in a total volume of 100 μl of complete medium containing 10 μg/ml F2-5 peptide or the negative control peptide, MSP2-P1(6). The positive control was medium containing 2.5 μg/ml concanavalin A, whereas medium alone served as a negative control. Cells were radiolabeled, harvested, and counted as described above. The results are presented as the mean counts per minute (±1 SD) of [3H]thymidine incorporation. The significance of the differences in proliferation among groups was determined by ANOVA, followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant.
MSP1aBT-specific IgG titers.
ELISA was used to determine the MSP1aBT-specific IgG titers, as described previously (36). Briefly, ELISA microplates were coated with 100 μl of 50 ng/ml affinity-purified rMSP1aBT in coating buffer (0.05 M Na2CO3, pH 9.6) overnight at 4°C, washed, and then blocked with 5% milk in PBS with 0.1% Tween 20. The serum was diluted (1:100 to 1:100,000) in blocking buffer, and 100 μl/well was added to duplicate wells. Following the washes, 100 μl/well of 1:500 recombinant protein G-horseradish peroxidase (Invitrogen) was added, and the plates were incubated for 1 h at room temperature. After the plates were washed, 100 μl/well tetramethylbenzidine substrate (Invitrogen) were added to the plates, the plates were incubated for 1 h, and 100 μl/well STOP buffer (1 N hydrochloric acid) was added. The optical density at 450 nm was determined with a Titertek Multiscan MCC/340 microplate reader (MTX Lab Systems). The significance of the difference in MSP1aBT-specific IgG titers among the groups was determined by ANOVA, followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant.
RESULTS
Invariant chain AP-2 binding motif enhances DC epitope presentation.
The BVP22 chimeric construct (Fig. 1A) (23) was modified by adding the sequence encoding the bovine invariant-chain endosome/lysosome-targeting (IC) motif (25) at the 5′ end or the sequence encoding the lysosome-targeting motif (17) from bovine LAMP-1 (GpIIa) at the 3′ end to generate chimeric constructs designated IC.BVP22 (Fig. 1C) and BVP22.GpIIa (Fig. 1D), respectively. In addition, a single construct with both the IC and the GpIIa motifs, designated IC.BVP22.GpIIa (Fig. 1E), was developed and tested to determine if there was an additive or synergistic effect from use of the combination of the two targeting motifs. Each construct expressed protein at equivalent levels; and each construct with the BVP22 in the correct orientation similarly directed intercellular trafficking at equivalent levels, as confirmed by immunocytometric analysis (Fig. 2) and flow cytometric analysis (data not shown) of transfected 293-F cells (23). The BVP22R construct (Fig. 1B) served as a negative control for intercellular spreading (23). The effect of the targeting motifs on antigen presentation was determined by proliferation assays with F2-5-specific CD4+ T cells and graded numbers of DCs positively selected from overnight monolayer cocultures of nontransfected DCs and 293-F cells transfected with each construct. The addition of the IC motif at the N terminus of the BVP22/antigen chimera directed significantly greater DC presentation of F2-5 to CD4+ T cells compared to that achieved with all other constructs (Fig. 3), including those with the GpIIa-targeting motif at the C terminus or the combination of the N-terminal IC motif and the C-terminal GpIIa motif. The impact of the IC motif on DC F2-5 presentation to CD4+ T cells (P < 0.001) was most evident with lower numbers of DCs (Fig. 3).
FIG. 2.
In vitro protein expression following transfection with the vaccine constructs. Monolayers of HEK 293-F cell transfectants were evaluated for protein expression by in situ immunocytochemistry with a mouse anti-FLAG-alkaline phosphatase conjugate. (A) Cells transfected with the VR-1055 vector; (B) cells transfected with the IC.BVP22 construct; (C) cells transfected with the BVP22.GpIIa construct; (D) cells transfected with the IC.BVP22.GpIIa construct. Protein expression by the BVP22 and the BVP22R constructs has been reported previously (23).
FIG. 3.
Invariant chain AP-2 binding motif enhances DC epitope presentation. The effects of the endosome/lysosome-targeting IC and GpIIa motifs on epitope presentation to F2-5-specific CD4+ T cells were tested by proliferation assays with irradiated DCs positively selected from overnight monolayer cocultures of nontransfected DCs and transfected 293-F cells. Proliferation of the F2-5-specific CD4+ T cells is presented as the mean counts per minute (±1 SD) of [3H]thymidine incorporation. The significance of the differences in proliferation was determined by ANOVA, followed by Bonferroni's multiple-comparison test, and a P value of ≤0.05 was considered significant. The mean response to IC.BVP22 was significantly different from that for all other groups when a DC/T-cell ratio of 1:2 (*, P < 0.05), 1:4 (†, P < 0.01), or 1:8 (‡, P < 0.001) was used in the proliferation assay. The symbols represent BVP22 (⋄), BVP22R (□), IC.BVP22 (Δ), BVP22.GpIIa (•), IC.BVP22.GpIIa (▴), and the VR-1055 vector (○).
A single immunization primes CD4+ T-cell and B-cell responses.
On the basis of the ability of the IC.BVP22 construct to enhance presentation of the antigen to T cells in vitro, this construct was used to immunize calves and to test its ability to prime cells following a single immunization. Priming of F2-5-specific T cells was tested by an IFN-γ-specific ELISPOT assay and T-cell proliferation. B-cell priming was evaluated by measuring the titers of antibody to the linked B-cell epitope (2). Inoculation of calves with a single dose of the IC.BVP22 construct (Fig. 1C) at a DC-enriched intradermal site effectively primed F2-5-specific CD4+ T-cell responses (Fig. 4A). All five IC.BVP22-immunized calves had significant (P < 0.05) increases in the number of IFN-γ-secreting CD4+ T cells within 11 days following a single DNA inoculation compared both to their preimmunization numbers and to the numbers in the five calves immunized on an identical schedule with the VR-1055 vector (Fig. 4A). This priming was also evident when the level of proliferation of CD4+ T cells (Fig. 4B) and the IgG titer against the linked B-cell epitope (Fig. 4C) were compared to their preimmunization levels and titers and to those of the control calves inoculated with the VR-1055 vector. Compared to the results of immunization with the control BVP22R construct lacking the functional BVP22 trafficking domain (the bvp22-coding sequence is in the reverse orientation) and the IC-targeting motif (Fig. 1B), a single immunization with the IC.BVP22 construct induced significantly greater mean numbers of IFN-γ+ CD4+ T cells (P < 0.05; Fig. 4A), higher mean levels of proliferation of CD4+ T cells (P < 0.001; Fig. 4B), and higher IgG titers (P < 0.01; Fig. 4C).
FIG. 4.
A single dose of IC.BVP22 DNA vaccine primes T- and B-lymphocyte responses. (A) F2-5-specific IFN-γ+ CD4+ responses. An asterisk denotes statistically significant differences between the IC.BVP22 group mean and both the BVP22R and the VR-1055 vector group means, whereas a dagger denotes a lack of a statistically significant difference between the BVP22R and the VR-1055 vector group means. (B) F2-5-specific CD4+ T-cell proliferation. The double dagger denotes statistically significant differences between the IC.BVP22 vector group mean and both the BVP22R and the VR-1055 vector group means, whereas double vertical lines denote a lack of a statistically significant difference between the BVP22R and the VR-1055 vector group means. All responses were measured 11 days following a single DNA vaccine immunization. The bars represent group means, and the arrows point to the responses detected in calves 3937 and 3959.
Maintenance and expansion of IC.BVP22-primed responses.
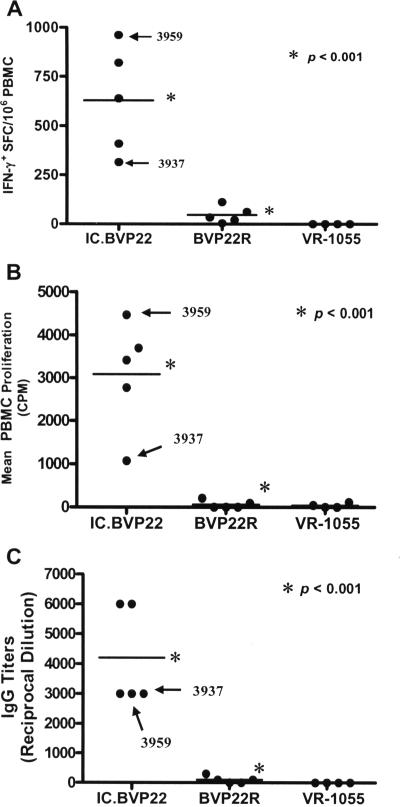
The kinetics of the F2-5-specific IFN-γ+ CD4+ T-cell precursor frequency postimmunization were evaluated weekly for 3 months by an IFN-γ-specific ELISPOT assay, whereas T-cell proliferation was evaluated by determination of the level of [3H]thymidine incorporation and IgG titers by ELISA. The F2-5-specific IFN-γ+ CD4+ T-cell responses primed by the single-dose IC.BVP22 DNA vaccine immunization continued to increase and remained significantly greater (P < 0.001) throughout the postpriming period compared to those in calves identically inoculated but inoculated with the DNA construct lacking the targeting domains or to those in calves inoculated with the VR-1055 vector (Fig. 5A). The proliferation of F2-5-specific CD4+ T cells diminished slightly over time but remained significantly greater (P < 0.001) in the postimmunization period compared to the proliferation of CD4+ T cells for control vaccinees inoculated with the construct lacking the targeting domains or for calves inoculated with the VR-1055 vector (Fig. 5B). Consistent with the CD4+ T-cell responses in the IC.BVP22 vaccinees, the IgG titer against the linked B-cell epitope increased and remained significantly greater (P < 0.001) than that for the controls (Fig. 5C). Most importantly, there was a significant expansion of F2-5 specific IFN-γ+ CD4+ T-cell and IgG responses by the IC.BVP22 vaccinee (calf 3937) that initially had the lowest responses 11 days postimmunization (Fig. 5A,C).
FIG. 5.
IC.BVP22 DNA vaccine-primed immune responses are maintained and expanded following a single immunization. The kinetics of the postimmunization responses were evaluated weekly; and the results from day 95, representative of the response throughout the post-single-immunization period, are shown. (A) F2-5-specific IFN-γ+ PBMC responses; (B) F2-5-specific PBMC proliferation; (C) IgG titers against the defined MSP1a B-cell epitope linked to F2-5. The bars represent group means, and asterisks denote a statistically significant difference between the IC.BVP22 vaccinees and calves identically vaccinated but vaccinated with the control BVP22R. Arrows point to the responses of the IC.BVP22 vaccinee (calf 3937) that initially had the lowest responses 11 days postimmunization and the responses of calf 3959.
IC.BVP22 primed B- and T-cell responses undergo rapid recall.
To determine the magnitude of the memory responses induced by the single immunization with the DNA vaccine, the vaccinees were inoculated with recombinant MSP1aBT protein on day 95 postimmunization, and the recall responses were evaluated within 96 h. The IC.BVP22-primed T-cell and B-cell responses underwent rapid recall, with increases in the number of F2-5 specific IFN-γ+ CD4+ T cells (Fig. 6A), the level of F2-5-specific T-cell proliferation (Fig. 6B), and the titer of antibody to the linked B-cell epitope (Fig. 6C) compared to the levels and titers prior to reexposure in the same animals (Fig. 5A to C). There were no increases in F2-5-specific IFN-γ+ CD4+ T cells, T-cell proliferation, or antibody titers in the calves initially inoculated with the VR-1055 vector and then given MSP1aBT protein (Fig. 5A to C), clearly demonstrating that these were indeed recall responses rather than de novo induction. The recall responses for the IC.BVP22-primed calves were most dramatic for F2-5-specific T-cell proliferation (20-fold increase), with more modest increases in both the mean antibody titer (fivefold) and the mean number of F2-5-specific IFN-γ+ CD4+ T cells (two- to threefold). In contrast, there was no to a minimal recall response in the calves initially immunized with the BVP22R DNA construct lacking the functional targeting domains (Fig. 5A to C), demonstrating that the differences in the recall responses were attributable to the effect of the targeting and trafficking domains on the initial priming.
FIG. 6.
IC.BVP22 DNA vaccine-primed memory responses undergo rapid recall. (A) F2-5-specific IFN-γ+ PBMC responses; (B) F2-5-specific PBMC proliferation; (C) IgG responses against the MSP1a B-cell epitope linked to F2-5. All responses were measured 96 h after the calves were reexposed by inoculation of MSP1aBT on day 105. The bars represent group means, and asterisks denote statistically significant differences between the IC.BVP22 vaccinees and the calves inoculated with the control BVP22R construct. Arrows point to the responses of the IC.BVP22 vaccinee (calf 3937) that initially had the lowest responses 11 days postimmunization.
DISCUSSION
Incorporation of the invariant-chain endosome/lysosome-targeting (IC) motif to the N terminus of BVP22 significantly improved DC presentation of a CD4+ T-cell epitope to cognate T cells. This finding is consistent with those from earlier studies, in which the IC endosome/lysosome-sorting motif directed endogenously synthesized proteins to efficiently enter the MHC class II presentation pathway (30), and demonstrates that it similarly targets antigens encoded by DNA vaccines. The impact of the IC motif on DC F2-5 presentation to CD4+ T cells was most evident with lower numbers of DCs, and this is most representative of the in vivo situation, in which DCs constitute <1% of nucleated cells in any tissue (8, 26). Compared to the effects of BVP22 alone and to BVP22 containing individual targeting motifs, a combination of the N-terminal IC motif and the C-terminal GpIIa motif had a negative effect, but not the expected synergistic effect, on DC antigen presentation to CD4+ T cells. This is probably due to the utilization of different adaptor proteins and endosome/lysosome-targeting pathways by these two targeting motifs. Endosome/lysosome targeting of MHC class II invariant-chain complexes is mediated by a dileucine-based signal in the IC motif that binds to the AP-2 complex, which is a component of the clathrin coats, which are involved in vesicle formation and cargo sorting. Apparently, AP-2 is associated only with the plasma membrane, and thus, a significant pool of MHC class II molecules traffic to the endosomal/lysosomal system by means of the cell surface (21). In contrast, the lysosome-targeting motif from LAMP-1 binds to AP-3, and the resultant complex is directly transported from the trans-Golgi network to the endosome/lysosome compartments (20). Consequently, a combination of the two targeting motifs could have been antagonistic rather than synergistic in directing the antigen targeting the endosome/lysosome compartments.
Immunization of calves at a DC-enriched intradermal site with a single dose of the IC.BVP22 DNA vaccine targeting key intercellular and intracellular events significantly enhanced the priming and expansion of antigen-specific CD4+ T-cell and B-cell responses. This response to a single DNA immunization suggests that the inclusion of the intercellular trafficking BVP22 domain and the IC intracellular targeting motif significantly enhanced priming. This is consistent with our demonstration that IC.BVP22 significantly enhanced greater DC presentation of a CD4+ T-cell epitope to cognate T cells than BVP22 alone and BVP22.GpIIa in vitro. This is also consistent with the previous demonstration that IC motif-directed endosome/lysosome targeting of an endogenously expressed antigen enhances MHC class II presentation (30, 32). Whether this enhanced priming of immune responses in IC.BVP22 vaccinees is attributable to the BVP22-directed intercellular spreading alone or the IC motif-dependent endosome/lysosome targeting alone, the additive or synergistic effects of the two signals cannot be determined by use of the present study design, in which only the optimal in vitro construct was tested in vivo. Priming was detected in all the IC.BVP22 vaccinees, but there was clear heterogeneity in the responses, with the same individual (calf 3937) having the fewest IFN-γ-secreting CD4+ T cells; the lowest level of T-cell proliferation; and, along with another cohort, the lowest antibody titer. This cannot be attributed simply to the MHC haplotype, as a second vaccinee in the IC.BVP22 group, calf 3959, shared identical haplotypes yet had a higher-magnitude response (Table 1 and Fig. 4A to C). This heterogeneity among outbred animals, even when they share MHC haplotypes, is consistent with previous observations of marked variation in the responses of human vaccinees expressing a relevant MHC allele capable of presenting a specific epitope (33). It is important to emphasize that the goal is not to generate a uniform maximal response in all vaccinees, which is an unlikely result, given the genetic heterogeneity of outbred animals and humans, but to increase the percentage of vaccinees that are primed with a single immunization. The priming in all vaccinees following a single immunization with IC.BVP22 fulfills this criterion.
An attractive feature of the original DNA vaccine concept was that continued antigen expression would result in expansion and maintenance of the immune response without the need for an additional booster. The immune responses primed by the single IC.BVP22 DNA vaccine immunization increased and were maintained throughout the postpriming period. Importantly, there was a significant expansion of immune responses in calf 3937, which initially had the lowest responses. Vaccine-induced protection from infectious diseases relies on the effective generation and preservation of specific immunological memory. Priming by the single IC.BVP22 DNA vaccine immunization induced memory T- and B-cell responses that underwent rapid recall in all the vaccinees, which demonstrated that effective memory had been induced, regardless of the heterogeneity in the magnitude of the initial responses. These results demonstrate that DNA vaccines targeting key intercellular and intracellular events significantly enhance priming and expansion and support the feasibility of single-dose DNA immunization in outbred populations.
Priming is the critical event that determines whether a specific immunological memory is established, and thus, an efficacious vaccine must consistently prime significant immune responses in the target vaccine population. Achieving this with a single immunization is a critical need in terms of both human and animal vaccine programs. In addition to the issue of decreased compliance associated with the need for repeated boosters and the economic considerations of repeated vaccination of livestock, the efficacy of mass vaccination campaigns in response to disease outbreaks will be enhanced by an efficacious single immunization. In the present study, we used a first inoculation of DNA-encoded FLT3L and GM-CSF as molecular adjuvants to create a DC-rich intradermal site, followed 72 h later by the single immunization of the DNA vaccine. A clear next step is to coadminister the DNA-encoded molecular adjuvants and the IC.BVP22 vaccine at the same time, a strategy that should be effective, as DNA constructs encoding BVP22 have been shown to enhance intercellular trafficking and the uptake of linked antigen for greater than 6 days (23). The development of a single-dose DNA vaccine for the codelivery of DC recruitment factors and immunogen would represent a substantial step forward for the actual deployment of DNA vaccines for use in outbred animals. Finally, while the DNA vaccine vector modifications tested in the present study are unique and clearly meet the priming criteria, there are additional targeting modifications reported in the literature that have been shown to be efficacious in murine models (11, 16, 19). We support the testing of novel vectors in outbred species, such as the calf model used in this study, to identify those modifications that enhance the priming and expansion of immune responses in a genetically heterogeneous vaccinee population. These studies are necessary to bridge the gap between the mouse models of immunization and the need for improved vaccines for animal and human populations.
Acknowledgments
We thank David L. Christie (School of Biological Sciences, The University of Auckland, Auckland, New Zealand) for providing bovine GpIIa cDNA. We also thank Kim Kegerreis, Victoria Hulubei, Emma Karel, and Jennifer Eldridge for excellent technical assistance.
USDA-NRI 2004-35204-14206, BARD US-3315-02C, NIH R01 GM060986, and NIH R01 AI053692 grants supported this work. Waithaka Mwangi was supported by grant T32-AI07025.
Footnotes
Published ahead of print on 10 January 2007.
REFERENCES
- 1.Akbari, O., N. Panjwani, S. Garcia, R. Tascon, D. Lowrie, and B. Stockinger. 1999. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J. Exp. Med. 189:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allred, D. R., T. C. McGuire, G. H. Palmer, S. R. Leib, T. M. Harkins, T. F. McElwain, and A. F. Barbet. 1990. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc. Natl. Acad. Sci. USA 87:3220-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulkanthan, A., W. C. Brown, T. C. McGuire, and D. P. Knowles. 1999. Biased immunoglobulin G1 isotype responses induced in cattle with DNA expressing msp1a of Anaplasma marginale. Infect. Immun. 67:3481-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiuk, L. A., R. Pontarollo, S. Babiuk, B. Loehr, and S. van Drunen Littel-van den Hurk. 2003. Induction of immune responses by DNA vaccines in large animals. Vaccine 21:649-658. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, J. S., C. Koniaras, and A. M. Lew. 1997. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int. Immunol. 9:1897-1906. [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., T. C. McGuire, W. Mwangi, K. A. Kegerreis, H. Macmillan, H. A. Lewin, and G. H. Palmer. 2002. Major histocompatibility complex class II DR-restricted memory CD4+ T lymphocytes recognize conserved immunodominant epitopes of Anaplasma marginale major surface protein 1a. Infect. Immun. 70:5521-5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casares, S., K. Inaba, T. D. Brumeanu, R. M. Steinman, and C. A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox, G. J., T. J. Zamb, and L. A. Babiuk. 1993. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J. Virol. 67:5664-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danko, I., and J. A. Wolff. 1994. Direct gene transfer into muscle. Vaccine 12:1499-1502. [DOI] [PubMed] [Google Scholar]
- 11.Deliyannis, G., J. S. Boyle, J. L. Brady, L. E. Brown, and A. M. Lew. 2000. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc. Natl. Acad. Sci. USA 97:6676-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly, J. J., A. Friedman, D. Martinez, D. L. Montgomery, J. W. Shiver, S. L. Motzel, J. B. Ulmer, and M. A. Liu. 1995. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat. Med. 1:583-587. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly, J. J., B. Wahren, and M. A. Liu. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633-639. [DOI] [PubMed] [Google Scholar]
- 14.Dupuis, M., K. Denis-Mize, C. Woo, C. Goldbeck, M. J. Selby, M. Chen, G. R. Otten, J. B. Ulmer, J. J. Donnelly, G. Ott, and D. M. McDonald. 2000. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 165:2850-2858. [DOI] [PubMed] [Google Scholar]
- 15.Gliddon, D. R., J. C. Hope, G. P. Brooke, and C. J. Howard. 2004. DEC-205 expression on migrating dendritic cells in afferent lymph. Immunology 111:262-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser, H., L. Shen, Q. L. Gu, S. Krueger, and S. Y. Chen. 2004. Secretory heat-shock protein as a dendritic cell-targeting molecule: a new strategy to enhance the potency of genetic vaccines. Gene Ther. 11:924-932. [DOI] [PubMed] [Google Scholar]
- 17.Hieber, A. D., and D. L. Christie. 1993. Characterization of glycoprotein II from bovine adrenal medullary chromaffin granules. Identification of components representing the secretory vesicle counterparts of the lysosomal-associated membrane glycoproteins (lamp-1 and lamp-2). J. Biol. Chem. 268:11073-11078. [PubMed] [Google Scholar]
- 18.Inchauspe, G., L. Vitvitski, M. E. Major, G. Jung, U. Spengler, M. Maisonnas, and C. Trepo. 1997. Plasmid DNA expressing a secreted or a nonsecreted form of hepatitis C virus nucleocapsid: comparative studies of antibody and T-helper responses following genetic immunization. DNA Cell Biol. 16:185-195. [DOI] [PubMed] [Google Scholar]
- 19.Kim, J. W., C. F. Hung, J. Juang, L. He, T. W. Kim, D. K. Armstrong, S. I. Pai, P. J. Chen, C. T. Lin, D. A. Boyd, and T. C. Wu. 2004. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 11:1011-1018. [DOI] [PubMed] [Google Scholar]
- 20.Le Borgne, R., A. Alconada, U. Bauer, and B. Hoflack. 1998. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 273:29451-29461. [DOI] [PubMed] [Google Scholar]
- 21.McCormick, P. J., J. A. Martina, and J. S. Bonifacino. 2005. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc. Natl. Acad. Sci. USA 102:7910-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitani, Y., T. Nakayama, M. Harbers, and Y. Hayashizaki. 2004. Aptamer-dependent full-length cDNA synthesis by overlap extension PCR. BioTechniques 124-126:128-129. [DOI] [PubMed] [Google Scholar]
- 23.Mwangi, W., W. C. Brown, G. A. Splitter, Y. Zhuang, K. Kegerreis, and G. H. Palmer. 2005. Enhancement of antigen acquisition by dendritic cells and MHC class II-restricted epitope presentation to CD4+ T cells using VP22 DNA vaccine vectors that promote intercellular spreading following initial transfection. J. Leukoc. Biol. 78:401-411. [DOI] [PubMed] [Google Scholar]
- 24.Mwangi, W., W. C. Brown, H. A. Lewin, C. J. Howard, J. C. Hope, T. V. Baszler, P. Caplazi, J. Abbott, and G. H. Palmer. 2002. DNA-encoded fetal liver tyrosine kinase 3 ligand and granulocyte macrophage-colony-stimulating factor increase dendritic cell recruitment to the inoculation site and enhance antigen-specific CD4+ T cell responses induced by DNA vaccination of outbred animals. J. Immunol. 169:3837-3846. [DOI] [PubMed] [Google Scholar]
- 25.Niimi, M., Y. Nakai, and Y. Aida. 1996. Identification of bovine invariant chain (Ii) gene by nucleotide sequencing. Biochem. Biophys. Res. Commun. 222:7-12. [DOI] [PubMed] [Google Scholar]
- 26.Nussenzweig, M. C., R. M. Steinman, M. D. Witmer, and B. Gutchinov. 1982. A monoclonal antibody specific for mouse dendritic cells. Proc. Natl. Acad. Sci. USA 79:161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park, Y. H., Y. S. Joo, J. Y. Park, J. S. Moon, S. H. Kim, N. H. Kwon, J. S. Ahn, W. C. Davis, and C. J. Davies. 2004. Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J. Vet. Sci. 5:29-39. [PubMed] [Google Scholar]
- 28.Polack, F. P., S. H. Lee, S. Permar, E. Manyara, H. G. Nousari, Y. Jeng, F. Mustafa, A. Valsamakis, R. J. Adams, H. L. Robinson, and D. E. Griffin. 2000. Successful DNA immunization against measles: neutralizing antibody against either the hemagglutinin or fusion glycoprotein protects rhesus macaques without evidence of atypical measles. Nat. Med. 6:776-781. [DOI] [PubMed] [Google Scholar]
- 29.Porgador, A., K. R. Irvine, A. Iwasaki, B. H. Barber, N. P. Restifo, and R. N. Germain. 1998. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 188:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson, S., K. Frauwirth, and N. Shastri. 1995. Expression of endogenous peptide-major histocompatibility complex class II complexes derived from invariant chain-antigen fusion proteins. Proc. Natl. Acad. Sci. USA 92:7217-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrijver, R. S., J. P. Langedijk, G. M. Keil, W. G. Middel, M. Maris-Veldhuis, J. T. Van Oirschot, and F. A. Rijsewijk. 1998. Comparison of DNA application methods to reduce BRSV shedding in cattle. Vaccine 16:130-134. [DOI] [PubMed] [Google Scholar]
- 32.Thomson, S. A., S. R. Burrows, I. S. Misko, D. J. Moss, B. E. Coupar, and R. Khanna. 1998. Targeting a polyepitope protein incorporating multiple class II-restricted viral epitopes to the secretory/endocytic pathway facilitates immune recognition by CD4+ cytotoxic T lymphocytes: a novel approach to vaccine design. J. Virol. 72:2246-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, R., D. L. Doolan, T. P. Le, R. C. Hedstrom, K. M. Coonan, Y. Charoenvit, T. R. Jones, P. Hobart, M. Margalith, N. Jennifer, W. R. Weiss, M. Sedegah, C. de Taisne, J. A. Norman, and S. L. Hoffman. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476-480. [DOI] [PubMed] [Google Scholar]
- 34.Wang, R., J. Epstein, F. M. Baraceros, E. J. Gorak, Y. Charoenvit, D. J. Carucci, R. C. Hedstrom, N. Rahardjo, T. Gay, P. Hobart, R. Stout, T. R. Jones, T. L. Richie, S. E. Parker, D. L. Doolan, J. Norman, and S. L. Hoffman. 2001. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 98:10817-10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff, J. A., J. J. Ludtke, G. Acsadi, P. Williams, and A. Jani. 1992. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1:363-369. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Y., G. H. Palmer, J. R. Abbott, C. J. Howard, J. C. Hope, and W. C. Brown. 2003. CpG ODN 2006 and IL-12 are comparable for priming Th1 lymphocyte and IgG responses in cattle immunized with a rickettsial outer membrane protein in alum. Vaccine 21:3307-3318. [DOI] [PubMed] [Google Scholar]