Abstract
Recombinant severe acute respiratory syndrome (SARS) nucleocapsid and spike protein-based immunoglobulin G immunoassays were developed and evaluated. Our assays demonstrated high sensitivity and specificity to the SARS coronavirus in sera collected from patients as late as 2 years postonset of symptoms. These assays will be useful not only for routine SARS coronavirus diagnostics but also for epidemiological and antibody kinetic studies.
The 2003 outbreak of severe acute respiratory syndrome (SARS) lasted fewer than 9 months, yet it had a major impact on public health and socioeconomics. Since the end of the SARS outbreak, there have been 17 identified SARS-associated coronavirus (SARS-CoV) infections, 6 from direct laboratory exposure, 1 of which resulted in community transmission to seven individuals, and 4 sporadic, community-acquired infections (9). Since additional cases may occur and could, if undetected, quickly lead to another global outbreak, it is important to continue to improve our ability to reliably monitor SARS-CoV infections (3, 16, 18).
As with other coronaviruses, the spike (S) and nucleocapsid (N) proteins are abundantly expressed during virus infection and are most effective among the coronavirus structural proteins at inducing antibody responses (10, 14, 15, 23). Previous studies have demonstrated the utility of anti-N and anti-S proteins in the diagnosis of SARS-CoV infections (2, 5, 12, 21). In this study, we describe the evaluation and comparison of recombinant spike and nucleocapsid enzyme-linked immunosorbent assays (ELISAs) for specifically detecting SARS-CoV infection.
The recombinant full-length SARS N gene was amplified from SARS-CoV RNA (Urbani strain), modified to contain a C-terminal His6 tag, and cloned into the Venezuelan equine encephalitis virus replicon vector (17). Baby hamster kidney (BHK) cells were transfected with SARS N replicon RNA by electroporation. Cells were harvested, and expressed protein was purified by metal affinity chromatography and analyzed by silver staining and Western blot analysis for the appropriately sized (50-kDa) immunoreactive protein (8). The control antigen, the nontoxic 50-kDa C-terminal fragment of the botulinum neurotoxin serotype A (BoNt/HcA), was expressed as described above (7).
The soluble codon-optimized SARS-CoV S glycoprotein (170 kDa; amino acids 1 to 1190; S1190) and the control antigen, truncated angiotensin-converting enzyme 2 (120 kDa; glycosylated; tACE-2), were cloned into pcDNA3.1 Myc/His and expressed in HEK-293T/17 cells. The proteins were purified using metal-affinity chromatography and analyzed as described by Babcock et al. (1).
Recombinant SARS N and S protein indirect ELISAs were developed using a modified version of the inactivated whole-virus ELISA described by Ksiazek et al. (6). Briefly, ELISA plates (Immulon) were coated with either purified recombinant N protein (12.5 ng/well) and the control antigen BoNT/HcA or purified His6/c-myc-tagged recombinant S1190 protein (12.5 ng/well) and the control antigen tACE-2. The plates were washed and incubated with serum diluted 1:400 in phosphate-buffered saline (PBS) containing skim milk and Tween 20 (PBS-T-M) for 1 h at 37°C and washed and incubated again with horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (1:4,000; heavy plus light chain [KPL])in PBS-T-M. After washing, substrate [ABTS; 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] was added and the absorbance read at 405 nm with a 490-nm reference filter.
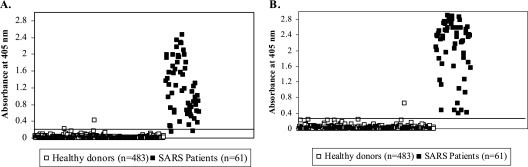
ELISA conditions were optimized using antibody-positive and -negative serum specimens, and the resultant assays were then evaluated using available serum samples collected from 61 patients from Vietnam and Taiwan with laboratory-confirmed SARS-CoV infection (2 to 150 days postonset of symptoms [dpo]), from 46 U.S. patients with non-SARS-related respiratory infections, and from 483 healthy U.S. donors with no exposure to SARS-CoV. An additional 10 serum samples were collected from non-SARS patients from Vietnam and Taiwan. Specimens used in this study were exempt from CDC Institutional Review Board review under 45 CFR 46.101(b)(4) (20). ELISA results from representative serum specimens from patients with SARS and healthy controls are shown in Fig. 1. The sums of the mean absorbance values for the healthy controls and three times the standard deviations for the S and N protein assays were 0.201 and 0.210, respectively. With these cutoff values, 7 of the 483 serum samples from healthy donors had absorbance values above the cutoff value in the N protein ELISA, and 3 samples were above the cutoff for the S protein ELISA, resulting in specificities of 98.6 and 99.4% for the N and S protein ELISAs, respectively (Table 1). Control samples reactive to either the N or S protein did not show reactivity to the alternate protein and also showed no reactivity against the inactivated SARS-CoV lysate by ELISA. In these samples, reactivity may have been due to nonspecific reactivity or cross-reacting antibodies.
FIG. 1.
Scatter chart of absorbance values with representative sera from both SARS patients and healthy controls for IgG antibodies to recombinant spike protein (A) and recombinant nucleocapsid protein (B). Specimens were obtained from 61 SARS patients at 2 to 150 days postonset of symptoms and from 384 healthy U.S. blood donors. Results are plotted as A405 values with a 490-nm reference filter. The black line indicates the cutoff values of 0.201 (A) and 0.210 (B) for each assay.
TABLE 1.
Comparison of sensitivities and specificities of recombinant nucleocapsid protein- and spike protein-based ELISAs
| Antigen | SARS patients
|
Healthy donors
|
||
|---|---|---|---|---|
| No. with positive reactivity/total | % Sensitivity | No. with negative reactivity/total | % Specificity | |
| SARS-N | 61/61 | 100 | 476/483 | 98.5 |
| SARS-S | 59/61 | 96.7 | 480/483 | 99.4 |
To further evaluate the specificity of the assays, available sera from 46 individuals infected with non-SARS-related respiratory viruses, including human coronavirus strains 229E (HCoV-229E) (n = 22 paired specimens) and HCoV-OC43 (n = 17 paired specimens), respiratory syncytial virus (n = 2), human parainfluenza virus 2 (n = 1) and 3 (n = 1), influenza B virus (n = 1), adenovirus (n = 1), and mumps virus (n = 1), were analyzed. None of the serum samples were positive by either assay (data not shown). In addition, serum samples from non-SARS patients from Taiwan and Vietnam showed no reactivity by either assay (data not shown).
All 61 sera from SARS cases were antibody positive to the N protein, and 59 were positive to the S protein. Two acute-phase specimens (≤20 dpo) were weakly reactive to the S protein and fell below the assay cutoff of 0.201. All were also positive by both immunofluorescence antibody testing and ELISA, using whole, gamma-irradiated SARS-CoV as the antigen (data not shown). The sensitivities for the N and S protein assays were 100% and 96.7%, respectively.
Persistent levels of SARS-CoV IgG have been detected in SARS cases for several months and up to 2 years after disease onset (4, 11, 12, 19, 21). In this study, serum samples from 48 SARS patients from Vietnam and the United States were collected 221 to 735 days (44 from days 221 to 250; 4 from days 633 to 735) after the onset of illness and tested for the presence of SARS-CoV- and N- and S-protein-specific IgG by ELISA. Antibodies (IgG) specific to whole virus, N protein, and S protein were detected in 40 (83.3%), 45 (93.8%), and 36 (75%) of the samples tested, respectively. Interestingly, anti-SARS-CoV and S antibodies were detected in three patients, two of whom also demonstrated a response to the N protein, almost 2 years postonset of symptoms (SARS titers = 1:400 to 1:1,600 [n = 3]; N protein titer = 1:400 [n = 2]; S protein titer = 1:400 to 1:1,600 [n = 3]).
Our evaluation of these ELISAs illustrates the value of having several assay systems to detect and then confirm a SARS-CoV infection. Although very few serum specimens from unexposed persons (<1.5%) tested positive for SARS-CoV infection, the potential for cross-reactivity between SARS-CoV and other coronaviruses, including the known human coronaviruses HCoV-OC43, HCoV-229E, and recently identified HKU1 and NL63, remains a concern (12, 13, 22). Whether these positive results are due to nonspecific reactivity to the recombinant SARS N protein or to cross-reactivity to other human CoVs requires further study. The use of protein fragments or peptides, instead of the whole recombinant N protein, for antibody detection may resolve the issue of potential cross-reactivity with proteins of other human CoVs and is the focus of further study. These false positives could present a public health dilemma as illustrated in the laboratory evaluation of four sporadic cases reported by Liang et al. (9). In those cases, it was necessary to conduct confirmatory testing using several different types of assays, because there was concern that infection with non-SARS coronaviruses may induce cross-reacting antibodies (9). Our data from that study suggest that a combination of assays may be needed to confirm the specificity of presumed SARS antibodies. Since the costs (i.e., public health interventions or outbreak response) of detecting a false-positive result and not detecting a case of SARS-CoV infection are both high, it is important to have well-characterized detection and confirmatory assays. In the absence of virus-specific control measures, e.g., a vaccine or antiviral drug, the key to controlling a reemergence of SARS is rapid diagnosis and implementation of infection control measures, i.e., isolating cases and identifying and managing contacts to prevent further transmission. The development of well-characterized detection and confirmatory serologic tests is the key to laboratory diagnostic support should SARS reemerge. These two ELISAs can be used as components of the SARS diagnostic system. These assays can also be used to study the kinetics of the protein-specific SARS antibody response and to help characterize SARS immunity and the pathogenesis of disease.
Acknowledgments
We thank Der-Yuan Wang (Bureau of Food and Drug Analysis, Department of Health, Taiwan, Republic of China), Mei-ying W. Yu (CBER/Food and Drug Administration, Bethesda, MD), and Li-Ching Hsu (Center for Disease Control, Department of Health, Taiwan, Republic of China) for providing a panel of sera from SARS patients. Thanks also to Ann Falsey (University of Rochester School of Medicine, Rochester, NY) and Dean Erdman (CDC, Atlanta, GA) for providing sera from patients with non-SARS-related respiratory infections and Debi Cannon (CDC, Atlanta, GA) for technical assistance.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Babcock, G. J., D. J. Esshaki, W. D. Thomas, Jr., and D. M. Ambrosino. 2004. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 78:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, M. S., Y. T. Lu, S. T. Ho, C. C. Wu, T. Y. Wei, C. J. Chen, Y. T. Hsu, P. C. Chu, C. H. Chen, J. M. Chu, Y. L. Jan, C. C. Hung, C. C. Fan, and Y. C. Yang. 2004. Antibody detection of SARS-CoV spike and nucleocapsid protein. Biochem. Biophys. Res. Commun. 314:931-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 4.He, Z., Q. Dong, H. Zhuang, S. Song, G. Peng, G. Luo, and D. E. Dwyer. 2004. Kinetics of severe acute respiratory syndrome (SARS) coronavirus-specific antibodies in 271 laboratory-confirmed cases of SARS. Clin. Diagn. Lab. Immunol. 11:792-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang, L. R., C. M. Chiu, S. H. Yeh, W. H. Huang, P. R. Hsueh, W. Z. Yang, J. Y. Yang, I. J. Su, S. C. Chang, and P. J. Chen. 2004. Evaluation of antibody responses against SARS coronaviral nucleocapsid or spike proteins by immunoblotting or ELISA. J. Med. Virol. 73:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 7.Lee, J. S., P. Pushko, M. D. Parker, M. T. Dertzbaugh, L. A. Smith, and J. F. Smith. 2001. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infect. Immun. 69:5709-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, S., L. Lin, H. Wang, J. Yin, Y. Ren, Z. Zhao, J. Wen, C. Zhou, X. Zhang, X. Li, J. Wang, Z. Zhou, J. Liu, J. Shao, T. Lei, J. Fang, N. Xu, and S. Liu. 2003. The epitope study on the SARS-CoV nucleocapsid protein. Genomics Proteomics Bioinformatics 1:198-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang, G., Q. Chen, J. Xu, Y. Liu, W. Lim, J. S. Peiris, L. J. Anderson, L. Ruan, H. Li, B. Kan, B. Di, P. Cheng, K. H. Chan, D. D. Erdman, S. Gu, X. Yan, W. Liang, D. Zhou, L. Haynes, S. Duan, X. Zhang, H. Zheng, Y. Gao, S. Tong, D. Li, L. Fang, P. Qin, W. Xu, and SARS Diagnosis Working Group. 2004. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg. Infect. Dis. 10:1774-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, G., S. Hu, Y. Hu, P. Chen, J. Yin, J. Wen, J. Wang, L. Lin, J. Liu, B. You, Y. Yin, S. Li, H. Wang, Y. Ren, J. Ji, X. Zhao, Y. Sun, X. Zhang, J. Fang, J. Wang, S. Liu, J. Yu, H. Zhu, and H. Yang. 2003. The C-terminal portion of the nucleocapsid protein demonstrates SARS-CoV antigenicity. Genomics Proteomics Bioinformatics 1:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, W., A. Fontanet, P. H. Zhang, L. Zhan, Z. T. Xin, L. Baril, F. Tang, H. Lv, and W. C. Cao. 2006. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome (SARS). J. Infect. Dis. 193:792-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, X., Y. Shi, P. Li, L. Li, Y. Yi, Q. Ma, and C. Cao. 2004. Profile of antibodies to the nucleocapsid protein of the severe acute respiratory syndrome (SARS)-associated coronavirus in probable SARS patients. Clin. Diagn. Lab. Immunol. 11:227-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maache, M., F. Komurian-Pradel, A. Rajoharison, M. Perret, J.-L. Berland, S. Pouzol, A. Bagnaud, B. Duverger, J. Xu, A. Osuna, and G. Paranhos-Baccalà. 2006. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid-based Western blot assay were rectified by the use of two subunits (S1 and S2) of spike for detection of antibody to SARS-CoV. Clin. Vaccine Immunol. 13:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan, K., K. H. Kim, and S. Makino. 2003. Characterization of N protein self-association in coronavirus ribonucleoprotein complexes. Virus Res. 98:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndifuna, A., A. K. Waters, M. Zhou, and E. W. Collisson. 1998. Recombinant nucleocapsid protein is potentially an inexpensive, effective serodiagnostic reagent for infectious bronchitis virus. J. Virol. Methods 70:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, K. Y. Yuen, and SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 18.Riley, S., C. Fraser, C. A. Donnelly, A. C. Ghani, L. J. Abu-Raddad, A. J. Hedley, G. M. Leung, L. M. Ho, T. H. Lam, T. Q. Thach, P. Chau, K. P. Chan, S. V. Lo, P. Y. Leung, T. Tsang, W. Ho, K. H. Lee, E. M. Lau, N. M. Ferguson, and R. M. Anderson. 2003. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science 300:1951-1966. [DOI] [PubMed] [Google Scholar]
- 19.Temperton, N. J., P. K. Chan, G. Simmons, M. C. Zambon, R. S. Tedder, Y. Takeuchi, and R. A. Weiss. 2005. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg. Infect. Dis. 11:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services. 2005. Code of Federal Regulations 46.101(b)(4).
- 21.Woo, P. C. Y., S. K. P. Lau, B. H. L. Wong, K.-H. Chan, C.-M. Chu, H.-W. Tsoi, Y. Huang, J. S. Malik Peiris, and K.-Y. Yuen. 2004. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin. Diagn. Lab. Immunol. 11:665-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo, P. C., S. K. Lau, B. H. Wong, K. H. Chan, W. T. Hui, G. S. Kwan, J. S. Peiris, R. B. Couch, and K. Y. Yuen. 2004. False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J. Clin. Microbiol. 42:5885-5888. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zhou, T., H. Wang, D. Luo, T. Rowe, Z. Wang, R. J. Hogan, S. Qiu, R. J. Bunzel, G. Huang, V. Mishra, T. G. Voss, R. Kimberly, and M. Luo. 2004. An exposed domain in the severe acute respiratory syndrome coronavirus spike protein induces neutralizing antibodies. J. Virol. 78:7217-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]