Abstract
This manuscript discusses microwave-assisted solid-phase synthesis of hydrogen-bond surrogate based α-helices and analogues by ring-closing metathesis (RCM). Microwave-mediated RCM allows access to a greater variety of amino acid residues in the macrocycles in shorter reaction times and higher yields compared to conventional heating. Surprisingly, we discovered that the Grubbs II catalyst is highly active under the influence of microwaves but catalytically dead under oil-bath conditions for the metathesis of these peptide bisolefins.
We recently described a method for the synthesis of short artificial α-helices by replacing the N-terminal hydrogen bond with a covalent carbon–carbon bond (Figure 1).1 This carbon–carbon bond was formed by a ring-closing metathesis (RCM) reaction between appropriately placed olefins on the peptide chain. A crucial feature of this design is that all side chains are available for solvent-exposed contacts with biomolecular targets. This feature is lacking in the side chain cross-linking strategies for helix stabilization, which sacrifice side chain functionality for helical nucleation.2 We have demonstrated that these hydrogen bond surrogate (HBS) α-helices target chosen protein receptors more successfully than side chain cross-linked helices.3
Figure 1.
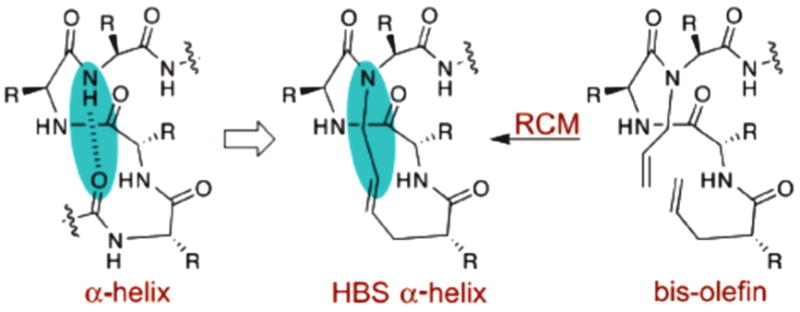
Replacement of an N-terminal hydrogen bond with a covalent carbon–carbon bond derived from an RCM reaction yielding an HBS α-helix.
The RCM reaction is the key step in our synthesis of HBS peptides, and we recently reported optimized “oil-bath” conditions for the solid-phase synthesis of 13-membered macrocycles that result in artificial α-helices.4 In principle, the HBS strategy should allow control of the structure in any oligomer stabilized through intrastrand hydrogen bonds. To test this hypothesis, we tried to prepare other helical protein secondary structures, such as the 310-helix (i, i + 3 hydrogen bond; 10-membered macrocycle) and the π-helix (i, i + 5 hydrogen bond; 16-membered macrocycle).5 However, the standard RCM conditions were found to be ineffective for the synthesis of the HBS 310-helices. Additionally, our optimized oil-bath reaction conditions provided variable yields (30–75%) for the metathesis of 13-membered and 16-membered bisolefins containing bulky tert-butyl protecting groups and often required very long reaction times (up to 72 h) for maximum conversion.4 The inability to synthesize helices with the amino acid residues that require a tert-butyl group within the putative macrocycle severely restricted the scope of our research efforts. These results suggested that further improvements for the metathesis reactions were needed to gain access to a larger range of macrocycles and to achieve greater reproducibility in the synthesis of these peptide macrocycles on the solid phase.
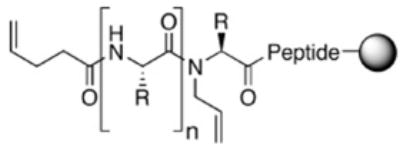
Recent reports of dramatic improvements in reaction rates and yields provided by microwave irradiation prompted us to explore this avenue.6 Here, we report studies aimed at allowing quick, high-yielding, solid-phase syntheses of these HBS helices by RCM in a microwave synthesizer. We find that microwave irradiation allows facile entry to 13- and 16-membered macrocycles in high yields independent of the peptide sequence but that the 10-membered macrocycle leading to a putative 310-helix remains inaccessible. Studies presented here were performed on five different bisolefin peptides that have proven to be the most difficult substrates in our hands, utilizing four different metathesis catalysts, Grubbs I (GI),7 Grubbs II (GII),8 Hoveyda-Grubbs II (HGII),9 and Ciba ruthenium (CR) catalysts.10 Resin-bound bisolefin peptides were synthesized as described previously.4
Table 1 shows bisolefin peptides 1–5 used in the current study to determine the potential advantages of the microwave irradiation for the synthesis of HBS helices. Bisolefin peptides 1 and 2 are designed to form two-turn α-helices, while differing only in their macrocycle amino acid sequences. Peptide 1 contains a tert-butyl-protected aspartic acid, and 2 possesses a tert-butyl-protected threonine (a β-branched amino acid). As stated earlier, the tert-butyl group is especially disruptive for the RCM step; indeed, our optimized oil-bath conditions provide respectable conversions of all bisolefins except those that feature the tert-butyl protecting group within the putative macrocycle. (Interestingly, the trityl group on asparagine or glutamine is tolerated; we speculate that the labile trityl group may not be surviving the metathesis conditions and may be cleaved before it can interfere with macrocyclization. We presume that the primary amide groups of asparagine or glutamine remain inert toward the catalyst after the trityl group is removed.) Peptide 3 is a longer peptide and would afford a 3-turn α-helix. Bisolefin 3 is similar to 1 but contains a trityl-protected histidine residue. This peptide was included because we have found trityl-histidine-containing peptides to be difficult to metathesize under our conventional heating conditions, presumably because the labile trityl group is removed under the metathesis conditions leaving a reactive histidine to interact with the catalyst. Peptides 4 and 5 form different-sized macrocycles, allowing the possibility of forming a π-helix and a 310-helix, respectively.
Table 1.
Bisolefin Peptides under Study
| bisolefin | n | size of expected macrocycle | sequencea |
|---|---|---|---|
| 1 | 2 | 13 | XDAG*NLVRA |
| 2 | 2 | 13 | XTAG*NLVRA |
| 3 | 2 | 13 | XDAA*NLVRHYA |
| 4 | 3 | 16 | XDIAA*NLVRHYA |
| 5 | 1 | 10 | XAA*DNLVRHYA |
X = pentenoic acid.
= N-allyl amino acid. The N-allyl amino acid was synthesized as a dipeptide and incorporated into the peptide during solid-phase synthesis. Each amino acid side chain was protected as follows: R, Pbf; N and H, trityl; D, E, T, and Y, t-butyl; K, Boc.
We began our studies by testing several different reaction conditions including reaction times, temperatures, and solvents in the microwave reactor11 with the HGII catalyst.12 We had previously reported that only the Hoveyda-Grubbs catalyst affords respectable yields for the metathesis reaction under conventional heating conditions; other metathesis catalysts, including GII, were ineffective for our sterically crowded bisolefin peptides.4 During the course of our microwave studies, we decided to also determine the efficacy of GI, GII, and CR along with HGII. To our surprise, GII became catalytically active under appropriate microwave conditions and activity of HGII was further enhanced in a reproducible manner; GI and CR did not afford the desired products in appreciable yields. A summary of key reactions is shown in Table 2 , and the complete list of reaction conditions surveyed is included in the Supporting Information, Table S1.
Table 2.
Summary of Key Microwave-Assisted Ring-Closing Metathesis Reactionsa
| entry | peptide | catalystb | solventb | time (min) | temp (°C) | conversion (%)c |
|---|---|---|---|---|---|---|
| 1 | 1 | HGII | DCE | 1 | 120 | trace |
| 2 | 1 | HGII | DCE | 5 | 120 | 18 |
| 3 | 1 | HGII | DCE | 10 | 120 | 24 |
| 4 | 1 | HGII | bmim | 0.5 | 200 | 0 |
| 5 | 1 | HGII | bmim | 0.5 | 300 | 0 |
| 6 | 1 | HGII | toluene | 10 | 160 | CM |
| 7 | 1 | GII | toluene | 10 | 160 | 56 |
| 8 | 1 | HGII | DCB | 5 | 200 | 81 |
| 9 | 1 | GII | DCB | 2 | 120 | 80 |
| 10 | 2 | HGII | DCE | 5 | 120 | 26 |
| 11 | 2 | HGII | DCE | 10 | 120 | 26 |
| 12 | 2 | HGII | DCE | 30 | 120 | 25 |
| 13 | 2 | HGII | DCE | 5 | 60 | trace |
| 14 | 2 | HGII | DCE | 5 | 120 | |
| 15 | 2 | HGII | DCE | 5 | 160 | 69 |
| 16 | 2 | GI | DCE | 2 | 120 | 17 |
| 17 | 2 | CR | DCE | 2 | 120 | 0 |
| 18 | 2 | GII | DCE | 2 | 60 | 20 |
| 19 | 2 | GII | DCE | 2 | 120 | 71 |
| 20 | 2 | HGII | 10% bmim/DCE | 5 | 200 | trace |
| 21 | 2 | HGII | DCB | 5 | 200 | 83 |
| 22 | 2 | GII | DCB | 2 | 120 | 84 |
| 23 | 3 | HGII | DCE | 5 | 60 | trace |
| 24 | 3 | HGII | DCE | 5 | 160 | 58 |
| 25 | 3 | HGII | DCB | 5 | 200 | 69 |
| 26 | 3 | GII | DCB | 2 | 120 | 76 |
| 27 | 3 | GII | DCB | 2 | 160 | 62 |
| 28 | 4 | HGII | DCB | 5 | 200 | 76 |
| 29 | 4 | GII | DCB | 2 | 120 | 58 |
| 30 | 5 | HGII | DCB | 5 | 200 | trace |
| 31 | 5 | GII | DCB | 2 | 120 | 0 |
The complete list of conditions surveyed is included in the Supporting Information.
Abbreviations: HGII = Hoveyda-Grubbs II; GII = Grubbs II; GI = Grubbs I; CR = Ciba-ruthenium; DCE = dichloroethane; DCB = dichlorobenzene; bmim = 1-butyl-3-methylimidazolium tetrafluoroborate, CM = significant amounts of cross-metathesis observed. All reactions were performed at 15 mol % of catalyst.
Yields are calculated from the HPLC traces at 216 nm.
We expected the reaction solvent to play an important role in these solid-phase metathesis reactions. The optimum solvent should afford efficient swelling of the resin, be inert toward the catalysts, and efficiently transfer microwave energy to the substrates. Dichloroethane (DCE) and dichlorobenzene (DCB) fit these criteria well, with DCB being the best nonprotic solvent with microwave-absorbing properties.6c After testing several different solvents, including DCB, DCE, toluene, 1-butyl-3-methylimidazolium tetrafluoroborate (bmim),12 10% bmim in DCE, and 10% DMF in DCB, at various reaction temperatures, we concluded that DCB is the most suitable solvent for the microwave-assisted metathesis of the peptide bisolefins on the solid phase. These data are reported in Table 2 and in the Supporting Information, Table S1. As expected, we found that microwaves significantly sped up the reaction rates; all microwave-mediated reactions were completed within 5 min, whereas up to 72 h was required for maximum yields with conventional heating. The yield for each reaction was calculated from HPLC peak areas after deprotection and cleavage of the peptides from the resin; representative crude HPLC traces are shown in Figure 2 and in the Supporting Information, Figure S5.
Figure 2.
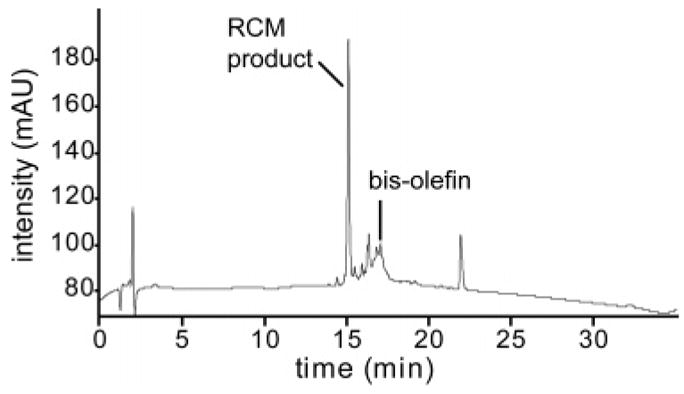
Representative crude HPLC trace after peptide synthesis, RCM in the microwave, and deprotection and cleavage with TFA monitored at 216 nm. In this specific example, bisolefin 1 was irradiated with GII for 2 min at 120 °C in dichlorobenzene.
During the course of these studies, we discovered that reaction temperature had a critical effect on the performance of HGII and GII catalysts. Both catalysts showed defined temperature dependence as illustrated in Figure 3. We find that the activity of GII peaks at 120 °C, whereas that of HGII peaks around 200 °C. This behavior is consistent with the reported observations that GII initiates at lower temperatures than the HGII catalyst.13 The RCM yields of the three 13-membered macrocycles from bisolefins 1–3 are similar from both catalysts, even though the HGII catalyst requires higher temperatures (Figure 4). No degradation of resin or increased side products were detected at these higher temperatures for the short duration of reaction times. Significantly, the high trans- to cis-alkene ratio in the products is similar between the oil-bath and the microwave-heating methods.1,4
Figure 3.
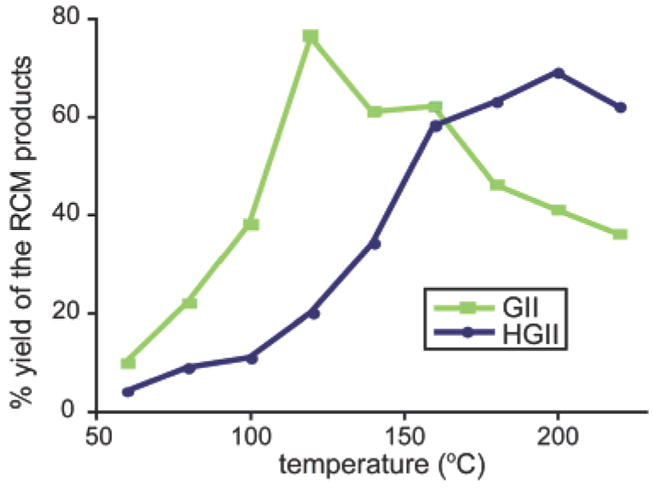
Catalytic activities of both GII and HGII being heavily influenced by temperature. Studies were carried out on peptide 3, and yields were calculated from HPLC traces at 216 nm.
Figure 4.
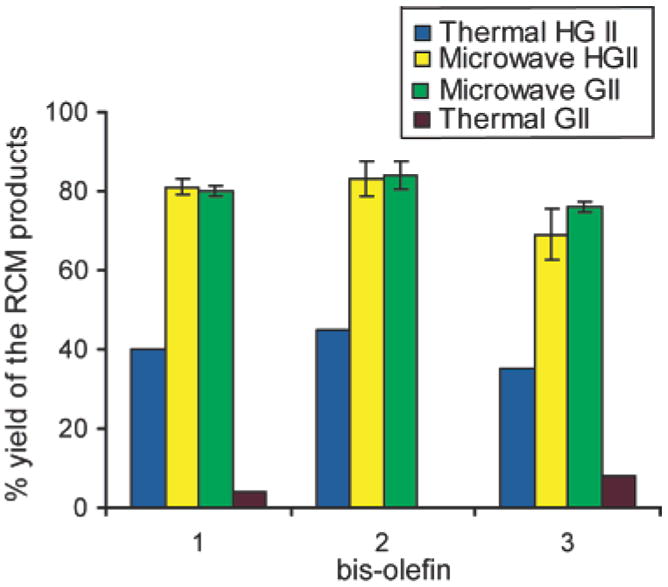
Microwave irradiation significantly improving the yield of the metathesis reactions with bisolefins 1–3 as compared to conventional heating.15
We invented the HBS strategy to prepare well-defined mimetics of protein secondary structures.1,3 In principle, this strategy should allow access to a range of peptide helices besides the α-helix. For example, deletion or addition of an amino acid residue between the two olefins in 3 affords bisolefins 4 or 5, respectively. Metathesis of bisolefins 4 and 5 would lead to 16- or 10-membered macrocycles which may provide artificial π- or 310-helices.5 We had previously found that the oil-bath RCM conditions did not provide efficient yields of the 10-membered or 16-membered rings (Figure 5). Microwave irradiation provides a high yield of the 16-membered macrocycle from 4, but our attempts to generate the 10-membered macrocycle from 5 remained unsuccessful even with microwave irradiation (Figure 5). The failed cyclization of peptide 5, while disappointing, was not unexpected, as the synthesis of small peptide macrocycles (12-membered rings or less) has proven difficult in the past.14 We are currently studying the solution conformation of HBS-peptides that contain 16-membered rings; the results of these studies will be reported in due course.
Figure 5.
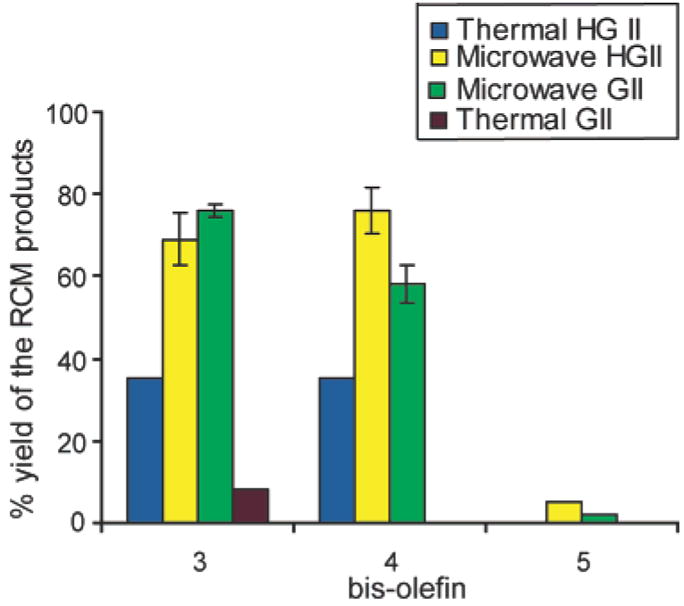
Microwave irradiation allows synthesis of 13- and 16-membered peptide macrocycles from bisolefins 3 and 4; however, the 10-membered macrocycle from 5 remains elusive.15
In conclusion, we have discovered that performing RCM on bisolefin peptides in the microwave leads to highly efficient synthesis of HBS helices on the solid phase as compared to the conventional method. Importantly, we are no longer limited in the choice of amino acid residues we may include in the macrocycles, which significantly enhances the biological relevance of the HBS helices. The optimized synthetic method affords reproducibly high yields for the 13- and 16-membered macrocycles. Significantly, the microwave irradiation has the surprising effect of reenergizing the GII catalyst that is catalytically dead when this reaction is performed on our resin-bound bisolefins in an oil-bath. Given the importance of the metathesis reaction in contemporary organic chemistry, we believe that this is a noteworthy observation that may be of considerable value to the wider community.
Supplementary Material
Acknowledgments
We are grateful for financial support from the NIH (GM073943), Research Corporation (Cottrell Scholar Award), and NYU (Whitehead Fellowship). R.N.C. thanks NYU for a GSAS Dean’s Fellowship. We thank the NSF for equipment Grants MRI-0116222 and CHE-0234863 and the NCRR/NIH for Research Facilities Improvement Grant C06 RR-16572.
Footnotes
Supporting Information Available: Synthesis, 1H NMR, 13C NMR, and HRMS of modified amino acids; synthesis, RCM, and characterization of peptides; and a complete record of all reactions performed in the microwave (Tables S1 and S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Wang D, Chen K, Kulp JK, III, Arora PS. J Am Chem Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chapman RN, DiMartino G, Arora PS. J Am Chem Soc. 2004;126:12252–12253. doi: 10.1021/ja0466659. [DOI] [PubMed] [Google Scholar]
- 2.(a) Shepherd NE, Abbenante G, Fairlie DP. J Am Chem Soc. 2005;127:2974–2983. doi: 10.1021/ja0456003. [DOI] [PubMed] [Google Scholar]; (b) Schafmeister CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]; (c) Blackwell HE, Grubbs RH. Angew Chem, Int Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Liao W, Arora PS. Angew Chem, Int Ed. 2005;117:6683–6687. [Google Scholar]
- 4.DiMartino G, Wang D, Chapman RN, Arora PS. Org Lett. 2005;7:2389–2392. doi: 10.1021/ol0506516. [DOI] [PubMed] [Google Scholar]
- 5.(a) Armen R, Alonso DOV, Daggett V. Protein Sci. 2003;12:1145–1157. doi: 10.1110/ps.0240103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fodje MN, Al-Karadaghi S. Protein Eng. 2002;15:353–358. doi: 10.1093/protein/15.5.353. [DOI] [PubMed] [Google Scholar]; (c) Bolin KA, Millhauser GL. Acc Chem Res. 1999;32:1027–1033. [Google Scholar]
- 6.(a) Bowman MD, Jacobson MM, Pujanauski BG, Blackwell HE. Tetrahedron. 2006;62:4715–4727. [Google Scholar]; (b) Murray JK, Farooqi B, Sadowsky JD, Scalf M, Freund WA, Smith LM, Chen JD, Gellman SH. J Am Chem Soc. 2005;127:13271–13280. doi: 10.1021/ja052733v. [DOI] [PubMed] [Google Scholar]; (c) Kappe CO. Angew Chem, Int Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 7.Schwab P, Grubbs RH, Ziller JW. J Am Chem Soc. 1996;118:100–110. [Google Scholar]
- 8.(a) Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]; (b) Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 9.Hoveyda AH, Gillingham DG, Van Veldhuizen JJ, Kataoka O, Garber SB, Kingsbury JS, Harrity PA. Org Biomol Chem. 2004;2:8–23. doi: 10.1039/b311496c. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Schaaf PA, Kolly R, Kirner H, Rime F, Muhlebach A, Hafner A. J Organomet Chem. 2000;606:65–74. [Google Scholar]
- 11.The microwave reactions were performed in the CEM Discover single-mode reactor with controlled power, temperature, and time settings.
- 12.(a) Garbacia S, Desai B, Lavastre O, Kappe CO. J Org Chem. 2003;68:9136–9139. doi: 10.1021/jo035135c. [DOI] [PubMed] [Google Scholar]; (b) Mayo KG, Nearhoof EH, Kiddle J. Org Lett. 2002;4:1567–1570. doi: 10.1021/ol025789s. [DOI] [PubMed] [Google Scholar]
- 13.Wakamatsu H, Blechert S. Angew Chem, Int Ed. 2002;41:794–796. doi: 10.1002/1521-3773(20020301)41:5<794::aid-anie794>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.(a) Bock VD, Perciaccante R, Jansen TP, Hiemstra H, van Maarseveen JH. Org Lett. 2006;8:919–9122. doi: 10.1021/ol053095o. [DOI] [PubMed] [Google Scholar]; (b) Meutermans WDF, Bourne GT, Golding SW, Horton DA, Campitelli MR, Craik D, Scanlon M, Smythe ML. Org Lett. 2003;5:2711–2714. doi: 10.1021/ol034907o. [DOI] [PubMed] [Google Scholar]
- 15.Optimum microwave conditions: Bisolefins in Figures 4 and 5 were irradiated with microwaves with 15 mol % of GII for 2 min at 120 °C in dichlorobenzene or with 15 mol % of HGII for 5 min at 200 °C in dichlorobenzene. Optimum oil-bath conditions: 72 h at 50 °C with 15 mol % of GII or HGII catalysts in dichloroethane.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


